Determination of the Elemental Impurity Levels in Different Mushroom Species and Probabilistic Risk Assessment
Funding: The authors received no specific funding for this work.
ABSTRACT
Mushroom cultivation and traditional mushroom procurement are widespread worldwide. Therefore, it is important to determine the extent of the risk of heavy metal exposure by consuming wild edible mushrooms. The aim of this study was to determine the levels of four elemental impurities (Cd, Pb, As, and Hg) in edible mushroom species (Macrolepiota mastoidea, Bovista aestivalis, Tricholoma fracticum, Helvella sp., and Rhizopogon roseolus) collected from Çanakkale and Van regions of Türkiye and to investigate potential health risks. For elemental impurity analysis, 0.3 g dry samples of mushrooms collected in Çanakkale and Van provinces between 2022 and 2023 were weighed and analyzed by inductively coupled plasma-mass spectrometry (ICP-MS). Target hazard quotient (THQ) was calculated for non-carcinogenic risk assessment in children and adults, followed by hazard index (HI) for each mushroom. Carcinogenic risk (CR) was determined. Probabilistic total THQ and total CR in children and adults were calculated using the Monte Carlo simulation method. Cd levels in five mushroom species ranged between 11.19–4838.71 μg/kg, Pb levels between 495.43 and 2836.81 μg/kg, As levels between 3267.52 and 25823.07 μg/kg, and Hg levels between 0.00 and 1594.50 μg/kg. The non-carcinogenic risk potential of As level was observed in all mushroom samples. THQ value was found to be greater than 1. These values also affected the HI value in all mushrooms; the latter was also greater than 1. Since As was detected in all mushrooms in the study and the CR value of this risk element was at a level that may pose a potential carcinogenic hazard, a potential health risk in adults and children can be noted. Furthermore, the Monte Carlo simulation showed that the health risks due to elemental impurity accumulation in mushrooms may increase significantly in children and adults, causing serious health problems. Children are more severely affected by elemental impurity concentrations than adults, especially considering their body weight, which makes it necessary to take protective measures.
1 Introduction
Mushrooms have been consumed as foodstuffs since ancient times, and there is an increasing demand for their cultivation and consumption worldwide. Mushroom consumption is particularly important culturally and economically in Asia (e.g., China, Japan, and South Korea), Eastern Europe (e.g., Poland, Russia, and the Balkan countries), Türkiye, and some Latin American countries. The intensive consumption of mushrooms in these regions, both cultivated and wild-harvested, demonstrates the high demand for this food group. Edible mushrooms have been recognized as valuable healthy foodstuffs due to their high nutrient content, such as proteins, vitamins, and minerals, leading to increased consumption worldwide [1-3]. However, pollutants generated by human activities, fuel combustion, urban growth, and agricultural chemicals can cause elemental impurity accumulation in mushrooms by mixing with soil, water, and air [4]. The released risk elements accumulate in the environment and may alter the microbial processes, which can cause an increase in their availability and their toxicity to higher plants, mushrooms, and other organisms, as well as physicochemical properties of soils, leading to loss of fertility, disturbing plant metabolism, and reducing biomass production and crop yields. Saprophytic mushrooms have high decomposition ability and increased activity of catalase that multiplies the concentration of these elements [5]. Wild edible mushrooms easily absorb and accumulate some harmful elements from the environment. Some metallic and metalloid elements, such as cadmium (Cd), lead (Pb), arsenic (As), and mercury (Hg) can be well bio-concentrated by many mushrooms collected from nature or farms [6]. Anthropogenic activities also contribute to the bioaccumulation of such potentially toxic elements in wild edible mushrooms [5, 7]. These elemental impurities are well known to cause serious disorders, especially in physiological processes such as neuropathy, liver and kidney diseases, and cancer [4]. Food safety and health risk assessment of elemental impurities has received considerable attention worldwide due to their toxic nature and possible transfer into the human food chain. Risk assessment calculations of elemental impurities based on critical exposure limits and other recommended parameters vary [8].
Methodologies for assessing health risks from exposure to multiple chemicals have been developed by the European Commission Services, World Health Organization (WHO)/International Programme on Chemical Safety (IPCS). These guidelines include Cd, Pb, As, and Hg as potentially toxic elements. Therefore, it is especially important to conduct carcinogenic and non-carcinogenic risk assessments of these four elemental impurities [9, 10]. According to previous monitoring results and literature reports, Cd, Pb, As, and Hg concentrations in edible mushrooms were higher and more toxic [10, 11]. Therefore, the risk assessment of contamination factors in edible mushrooms is very important for food safety assessment. The level and variety of elemental impurities in mushrooms may vary according to the type of mushrooms [12]. Mushrooms are bioaccumulators that can absorb elemental impurities, especially from the soil.
This feature may vary according to mushroom species. Therefore, the diversity in mushroomspecies studied in the literature in the last decade shows the importance of the subject once again. When previous studies were examined, the toxic element risk assessment of Helvella sp., Macrolepiota mastoidea, Bovista aestivalis, Rhizopogon roseolus, and Tricholoma fracticum, which are edible wild mushroom species, was not performed or not performed adequately.
Mushrooms collected and consumed by humans in nature generally belong to Basidiomycota and Ascomycota [13, 14]. Ascomycota (marsupial fungi), the largest subdivision of the fungal kingdom, has about 65 000 described species that have evolved as saprotrophs, which are parasites of plants, animals, and other fungi that form symbiotic relationships in lichens [15]. Helvellaceae Fr., one of the important classes of the Ascomycota subdivision, harbors some epigeous apothecial forms and hypogeous ascomata-producing taxa. According to the recent systematic classification, Helvellaceae in Ascomycota includes six genera: Balsamia Vittad. (syn. Barssia Gilkey), Dissingia K. Hansen, X.H. Wang & T. Schumach., Helvella L., Pindara Velen., Midotis Fr. (syn. Wynnella Boud.) and Underwoodia Peck sensu stricto [16, 17].
Helvella L. (saddle mushroom) is the largest genus of the Helvellaceae family, with a worldwide distribution. The ascomata (edible fleshy part) are usually white, creamy white, gray, and brown to black in color. Members of the genus often form ectomycorrhizal symbioses with plants from various families, such as Fagaceae, Pinaceae, and Salicaceae. In addition, many species of Helvella are edible due to their high nutritional value. Therefore, the genus has a high economic value [17, 18].
Another important subdivision is Basidiomycota, which includes about 35% (40 000 species) of the fungal kingdom. The largest order of this subdivision, Agaricales, includes four of our study specimens (Macrolepiota mastoidea, Bovista aestivalis, Rhizopogon roseolus, and Tricholoma fracticum). Members of Agaricales can be found in almost every habitat, from woodlands and grasslands to deserts and sand dunes, on all continents. Agaricales is rich in edible macrofungus species [19-21]. Growing up to 15 cm, Macrolepiota mastoidea is an edible macrofungus with diverse bioactivities [21]. Fresh specimens of Bovista aestivalis, which grow individually or in groups in meadows, paths, roadsides, vacant lots, pastures, and thickets in summer, winter, and fall, are edible. Bovista aestivalis is among alternative foods due to its nutraceutical, antioxidant, and antimicrobial properties [2, 22]. Rhizopogon roseolus is an ectomycorrhizal fungus that grows under pine trees. Rhizopogon roseolus is considered a delicacy in East Asia and Japan and is used as a soil inoculant in agriculture and horticulture. Rhizopogon roseolus is noted for its anticancer and antioxidant properties [23-25]. Although it has a bitter taste, Tricholoma fracticum, which is consumed by people in Anatolia, has an antioxidant effect [3]. This species, which usually grows under coniferous trees, is common in temperate regions, including Anatolia, Europe, and North America [26].
Mushroom cultivation and traditional mushroom procurement are very common in Türkiye and worldwide. Therefore, risk assessment of wild edible mushrooms is important for the extent of the risk of elemental impurity exposure in mushroom consumption. In recent years, the accumulation of heavy metals in mushrooms and the associated health risks have become a significant area of research, particularly in evaluating the impact of environmental pollution on food safety. Toxic elements such as Cd, Pb, As, and Hg, which originate from soil and the atmosphere, can accumulate biologically during the growth process of mushrooms, posing a risk of chronic toxicity to consumers. This risk is particularly pronounced for wild mushrooms collected in areas close to industrial zones, areas with intensive mining activities, or areas where agricultural pesticides are frequently used. In a recent study, four boletes of the genus Leccinum collected from 16 forested areas in Slovakia were analyzed for total mercury (Hg) content in both soil and fruiting bodies. The study employed contamination factor (Cif), bioaccumulation factor (BCF), translocation factor (Qc/s), percentage of provisional tolerable weekly intake (%PTWI), and target hazard quotient (THQ) to assess mercury uptake, translocation, and potential health risks. While none of the sites exceeded PTWI values, three locations presented THQ values indicating possible health risks [5]. Similarly, another study evaluated the antioxidant properties of three wild Agaricus species alongside their toxic element content (Cd, Pb, As, and Hg) and performed both non-carcinogenic (hazard index, HI) and carcinogenic (cancer risk, CR) risk assessments. Their findings revealed that arsenic and cadmium levels in particular species posed health concerns, with HI values exceeding 1 and CR values approaching 1 × 10−4. Despite their antioxidant potential, these results suggest that wild edible mushrooms may pose significant health risks due to the presence of elemental impurities [10]. Although studies on this topic are increasing, comprehensive evaluations of naturally occurring mushrooms from different geographical regions are still needed. In this context, our study aims to investigate both the elemental impurity level and potential health risks associated with mushrooms collected from various locations. The main objective of this study was to determine the concentrations of four elemental impurities (Cd, Pb, As, and Hg) in edible mushrooms in Çanakkale and Van regions of Türkiye and to investigate their potential health risks.
2 Materials and Methods
2.1 Chemicals
Nitric acid (HNO3; 60%) and hydrogen peroxide (H2O2; ≥ 30%) for elemental impurity analysis were purchased from Sigma Aldrich (USA). Ultrapure water was used for sample dilutions. Ultrapure water (18 M−cm) was produced through a Milli-Q water purification system (Millipore, Bedford, Massachusetts, USA).
2.2 Collection and Identification of Mushroom Samples
The mushrooms collected in their natural habitat were identified with the help of the relevant kinds of literature [3, 21, 22, 27], considering their macro and micromorphology. The study materials were collected from Çanakkale and Van provinces (Türkiye) between 2022 and 2023 (Figure 1). The specimens collected from the field were placed on blotting paper. Mushroom samples were cleaned with a brush and a scalpel. It is dried at room temperature, away from sunlight, and turned into fungarium material. The dried mushrooms were ground for homogenization and turned into powder before analysis. During sampling, the caps and stems of the mushrooms were processed together without being separated.
2.3 Analysis of Cd, Pb, As and Hg in Mushrooms
For elemental impurity analyses, 0.3 g of dried mushroom samples were weighed into polytetrafluoroethylene (PTFE) tubes, and then 8 mL of HNO3 (60%) and 2 mL of H2O2 were added. Before closing the tubes, the samples were left in a fume hood for approximately 20 min to allow for controlled release of the gas. The tubes were then closed and digested in a microwave digestion system (Ethos Easy, Milestone Srl., IT) for 30 min [10]. After microwave digestion, the sample solutions were diluted to 30 mL with ultrapure water. The prepared sample solutions were analyzed by Inductively coupled plasma-mass spectrometry (ICP-MS) For this, elemental impurity analyses were carried out separately in two different laboratories. ICP-MS (7800, Agilent Technologies Inc., USA) was used to analyze Cd, Pb, As, and Hg in mushroom samples at İzmir Katip Çelebi University Central Research Laboratories (IKÇÜ-MERLAB). The instrument parameters are given in Table 1. In addition, mushroom samples were analyzed by Thermo iCAP RQ ICP-MS (Thermo Fisher Scientific, USA) at Eskişehir Osmangazi University Central Research Laboratory Application and Research Center (ESOGU ARUM).
| Parameter | Value |
|---|---|
| RF power | 1500 W |
| RF voltage | 1,80 V |
| S/C temperature | 2°C |
| Sample depth | 10 mm |
| Nebulizer gas | 1,00 L/min |
| Nebulizer pump | 0,10 rps |
| Internal standards | 6Li, 45Sc, 72Ge, 89Y, 115In, 159Tb, 209Bi |
| Tune solution | 7Li, 89Y, 205Tl |
2.4 Risk Assessment
The carcinogenic and non-carcinogenic human health risk models developed by the US Environmental Protection Agency (USEPA) have been successful and accepted worldwide. This study used calculations and threshold values to assess the potential human health risks posed by elemental impurity pollution [1, 4]. The risk calculation was performed for two different scenarios. The first scenario was for adult exposure, and the second scenario was for child exposure.
2.4.1 Estimated Daily Intake (EDI)
EDI represents estimated daily intake; C is the elemental impurity concentration in edible mushrooms (mg/kg); IR is the intake rate (6.6 × 10−3 kg/person/day); EF is the exposure frequency (365 days/year); ED corresponds to the exposure duration (adult 70 years; child 7 years); BW is the body weight (adult 70 kg; child 25.6 kg); AT (EF × ED) [1, 4, 28, 29].
2.5 Non-Carcinogenic Risks Assessment
2.5.1 Target Hazard Quotient (THQ)
RfD is the risk element's oral reference dose in mg/kg/day. The RfD values for As, Cd, Hg, and Pb are 3 × 10−4, 1 × 10−3, 3 × 10−4, and 3.5 × 10−3 mg/kg/day, respectively [28].
2.6 Carcinogenic Risk (CR) Assessment
Carcinogenic risk is used to assess the potential risk of lifetime exposure of consumers to carcinogenic substances [31-33]. Equation (4) can be used for the CR.
Risks exceeding 1 × 10−4 are considered potentially carcinogenic, whereas a CR value below 1 × 10−6 is generally regarded as posing no significant health risk. CR values in the range of 1 × 10−6 to 1 × 10−4 are typically considered to represent a tolerable level of risk [4].
2.7 Probabilistic Risk Assessment
In the literature, probabilistic risk assessment was performed using the Monte Carlo simulation method to evaluate the potential risk distribution and uncertainties created by elemental impurity exposure levels [9, 34]. Considering the literature methods, the Monte Carlo simulation method was used in this study to evaluate health risks due to elemental impurity contamination. The simulation was implemented using Python 3.9.13 and the following libraries: NumPy 1.24.4, Pandas 1.5.3, and Matplotlib 3.7.1. The simulation model was created using the parameters specified in Table 2 for the values included in the risk analysis. The simulation was run with 10 000 iterations, and total HI for non-carcinogenic health risk and total CR for carcinogenic health risk were calculated. Initially, the data set was loaded. Then, elemental impurity concentration was randomly sampled from a log-normal distribution, and body weight was randomly sampled from a normal distribution. With the fixed parameters determined, the EDI was calculated. Using this value, the total THQ (TTHQ) and total CR were calculated. Analyses were performed at the mean and 95% percentile of the results and visualized with histograms to show the risk levels.
| Parameters | Unit | Symbol | Distribution | Value | References |
|---|---|---|---|---|---|
| Elemental impurity concentration | μg/kg | MC | Lognormal | [9, 28] | |
| Mushroom intake rate |
Kg/day |
IR |
Uniform |
0.0033–0.03 | [4, 6, 33] |
|
Exposure frequency |
Days/year |
EF |
Uniform |
365 |
[1] |
|
Exposure duration |
Year |
ED |
Uniform |
Child = 7 Adult = 70 |
[1, 4] |
|
Body weight |
Kg |
BW |
Normal |
Child = 15 g–49 Adult = 50–90 |
[1, 4, 6, 33] |
|
Average time |
Days |
AT |
Uniform |
EF*ED |
[1] |
2.8 Statistical Evaluation
In this study, the levels of four elemental impurities measured in five different mushroom species were compared, and statistical analyses were performed using the IBM SPSS Statistics 23 program. Prior to the analysis, the Shapiro–Wilk test was applied for each group to determine whether the data showed a normal distribution. The test results yielded p > 0.05 values, indicating that the data were normally distributed and that parametric tests were appropriate for use. Therefore, one-way analysis of variance was preferred for intergroup comparisons. One-Way ANOVA is a commonly used parametric test for comparing mean differences among three or more independent groups. For elements where significant differences were detected between groups in the ANOVA results, the Tukey HSD test was applied post hoc to determine which groups the differences occurred between.
3 Results and Discussion
This study included an elemental impurity risk assessment of mushroom species (Figure 2), which can easily grow in the natural environment and are widely consumed by local people. The specimens collected in systematic order were as follows: Helvella sp., (Figure 2A): Van, Centennial University Campus, near the Green Area Directorate, under Populus sp. trees, 38° 34¢10′′N, 43° 17¢07′′E, 1666 m, 16.05.2022, collector no: Acar 1196; Macrolepiota mastoidea (Fr.) Singer (Figure 2B): Çanakkale, Lâpseki, near Kocabaşlar village, under Pinus brutia trees, 40° 18¢12′′N, 26° 48¢15′′E, 415 m, 10.12.2023, collector no: Acar 1407; Tricholoma fracticum (Britzelm.) Kreisel (Figure 2C): Çanakkale, Onsekiz University, Near Safiye girls' dormitory, under Pinus brutia trees, 40° 06¢41′′N, 26° 25¢54′′E, 218 m, 20.12.2023, collector no: Acar 1563; Bovista aestivalis (Bonord.) Demoulin (Figure 2D): Çanakkale, Ezine, Yeniköy exit, in meadow, 39° 55¢40′′N, 26° 09¢51′′E, 54 m, 21.11.2023, collector no: Acar 1292; Rhizopogon roseolus (Corda) Th. Fr. (Figure 2E): Çanakkale, Onsekiz Mart University, next to Science Faculty B/Block, parking area, under Pinus brutia trees, 40° 06¢33′′N, 26° 25¢04′′E, 87 m, 29.11.2023, collector no: Acar 1324. Some of the collected specimens were kept as voucher specimens in VANF Furgarium, Department of Biology, Faculty of Science, Van Yüzüncü Yıl University. The study specimens were properly cleaned from all kinds of dust and contaminants before pulverization.

The literature has reported that elemental impurities released into the ecological system due to industrial and anthropogenic activities can penetrate the structure of food sources grown in natural environments and pose a risk to human health through food consumption [1]. There are several guidelines for risk assessment for metal toxicity in foods [31, 32, 35]. These guidelines guide the risk assessment of elemental impurity to ensure food safety and protect consumers' health. For example, the USFDA provides specific guidelines (USP <232>, USP <233>) for assessing elemental impurities [36]. These USFDA guidelines establish the allowable amounts of toxic elements in foods and provide food manufacturers with appropriate testing methods and monitoring strategies. Furthermore, EFSA sets guidelines and standards for assessing metal content in foods [37] These EFSA guidelines are based on scientific data to identify metal toxicity risks in foods and provide food manufacturers with appropriate test methods and monitoring strategies. These guidelines are the main resources used to assess metal toxicity in food. Risk assessments conducted in accordance with these guidelines are important to ensure that consumers consume safe and healthy foods. Elemental impurities in foods are usually categorized into classes according to their toxic effects and potential risks to human health. These classifications are usually based on the type of element, its toxicity, and routes of exposure to the human body. Organizations such as the USFDA and EFSA generally use three classifications when setting maximum permissible levels of metal elements in food. Class I: This class generally includes elements with the highest toxicity and poses the greatest risk to human health. Cd, Pb, Hg, and As are included in this category. Class II: This class includes elements that pose a lower risk to human health. These elements have lower toxicity or pose a risk to human health at lower exposure levels. Class III: This class includes elements generally posing a low risk to human health. These elements typically have no apparent toxic effects on human health at foodborne exposure levels or have very low toxicity.
The Cd, Pb, As, and Hg levels found in the five mushroom species examined in our study are shown in Table 3. The amounts of risk elements in mushrooms are listed in order from the highest to the lowest: Cd, As, Pb, and Hg in Macrolepiota mastoidea; As, Pb, Hg, and Cd in Bovista aestivalis; Cd, As, Pb, and Hg in Tricholoma fracticum; As, Cd, Pb, and Hg were detected in Helvella sp.; As, Pb, Hg, and Cd were detected in Rhizopogon roseolus.
| Mushroom | Element | MC (μg/kg) mean ± std.dev | EDI (mg/kg/day) | THQ | HI | CR |
|---|---|---|---|---|---|---|
| Adult | ||||||
| Macrolepiota mastoidea | Cd | 4183.51 ± 98.22 | 3.94 × 10−4 | 0.394 | 1.498 | 2.48 × 10−3 |
| Pb | 495.43 ± 11.82 | 0.47 × 10−4 | 0.013 | 3.97 × 10−7 | ||
| As | 3267.52 ± 100.23 | 3.08 × 10−4 | 1.026 | 4.62 × 10−4 | ||
| Hg | 200.50 ± 8.34 | 0.19 × 10−4 | 0.063 | |||
| Bovista aestivalis | Cd | 152.22 ± 7.51 | 0.14 × 10−4 | 0.014 | 2.996 | 9.04 × 10−5 |
| Pb | 2836.81 ± 89.27 | 2.67 × 10−4 | 0.076 | 2.27 × 10−6 | ||
| As | 7648.02 ± 102.31 | 7.21 × 10−4 | 2.404 | 1.08 × 10−3 | ||
| Hg | 1594.50 ± 75.32 | 1.50 × 10−4 | 0.501 | |||
| Tricholoma fracticum | Cd | 4838.71 ± 105.34 | 4.56 × 10−4 | 0.456 | 1.972 | 2.87 × 10−3 |
| Pb | 1782.10 ± 77.36 | 1.68 × 10−4 | 0.048 | 1.43 × 10−6 | ||
| As | 4601.85 ± 83.73 | 4.33 × 10−4 | 1.446 | 6.51 × 10−4 | ||
| Hg | 67.67 ± 13.51 | 0.06 × 10−4 | 0.021 | |||
| Helvella sp. | Cd | 2776.55 ± 102.32 | 2.62 × 10−4 | 0.261 | 2.325 | 1.65 × 10−3 |
| Pb | 1134.86 ± 53.53 | 1.07 × 10−4 | 0.031 | 9.09 × 10−7 | ||
| As | 6467.61 ± 62.47 | 6.10 × 10−4 | 2.032 | 9.14 × 10−4 | ||
| Hg | ND | NC | NC | |||
| Rhizopogon roseolus | Cd | 11.19 ± 3.54 | 0.01 × 10−4 | 0.001 | 8.171 | 6.65 × 10−6 |
| Pb | 828.67 ± 23.71 | 0.78 × 10−4 | 0.022 | 6.64 × 10−7 | ||
| As | 25823.07 ± 108.74 | 24.35 × 10−4 | 8.116 | 3.65 × 10−4 | ||
| Hg | 100.78 ± 11.72 | 0.09 × 10−4 | 0.032 | |||
| Child | ||||||
| Macrolepiota mastoidea | Cd | 4183.51 ± 98.22 | 10.79 × 10−4 | 1.079 | 4.095 | 6.79 × 10−3 |
| Pb | 495.43 ± 11.82 | 1.28 × 10−4 | 0.036 | 1.09 × 10−6 | ||
| As | 3267.52 ± 100.23 | 8.42 × 10−4 | 2.808 | 1.26 × 10−3 | ||
| Hg | 200.50 ± 8.34 | 0.52 × 10−4 | 0.172 | |||
| Bovista aestivalis | Cd | 152.22 ± 7.51 | 0.39 × 10−4 | 0.0392 | 8.191 | 2.47 × 10−4 |
| Pb | 2836.81 ± 89.27 | 7.31 × 10−4 | 0.209 | 6.22 × 10−6 | ||
| As | 7648.02 ± 102.31 | 19.72 × 10−4 | 6.572 | 2.96 × 10−3 | ||
| Hg | 1594.50 ± 75.32 | 4.11 × 10−4 | 1.370 | |||
| Tricholoma fracticum | Cd | 4838.71 ± 105.34 | 12.47 × 10−4 | 1.247 | 5.392 | 7.86 × 10−3 |
| Pb | 1782.10 ± 77.36 | 4.59 × 10−4 | 0.131 | 3.91 × 10−6 | ||
| As | 4601.85 ± 83.73 | 11.86 | 3.955 | 1.78 × 10−3 | ||
| Hg | 67.67 ± 13.51 | 0.17 × 10−4 | 0.058 | |||
| Helvella sp. | Cd | 2776.55 ± 102.32 | 7.16 × 10−4 | 0.716 | 6.358 | 4.51 × 10−3 |
| Pb | 1134.86 ± 53.53 | 2.92 × 10−4 | 0.084 | 2.49 × 10−6 | ||
| As | 6467.61 ± 62.47 | 16.67 × 10−4 | 5.558 | 2.50 × 10−3 | ||
| Hg | ND | NC | NC | |||
| Rhizopogon roseolus | Cd | 11.19 ± 3.54 | 0.03 × 10−4 | 0.003 | 22.342 | 1.82 × 10−5 |
| Pb | 828.67 ± 23.71 | 2.13 × 10−4 | 0.061 | 1.82 × 10−6 | ||
| As | 25823.07 ± 108.74 | 66.57 × 10−4 | 22.192 | 9.99 × 10−3 | ||
| Hg | 100.78 ± 11.72 | 0.26 × 10−4 | 0.087 | |||
- Abbreviations: CR, carcinogenic risk; EDI, the estimated daily intake; HI, the hazard index; MC, elemental impurity concentration; NC, not calculated; ND, not detected; THQ, the target hazard quotient.
When Figure 3 is examined, Cd, Pb, As, and Hg levels showed different bioaccumulation in the mushrooms. Cd levels of Bovista aestivalis and Rhizopogon roseolus were not significantly different, while Cd levels were significantly different among all other mushrooms (p < 0.05). Pb and As levels differed significantly (p < 0.05) among investigated mushrooms. Hg has not been detected in Helvella sp. When Hg levels in other mushrooms were analyzed, it was found that the Hg level in Rhizopogon roseolus was similar to that in Macrolepiota mastoidea and Tricholoma fracticum. Hg levels were significantly different between M. mastoidea, Bovista aestivalis, and Helvella sp. (p < 0.05).
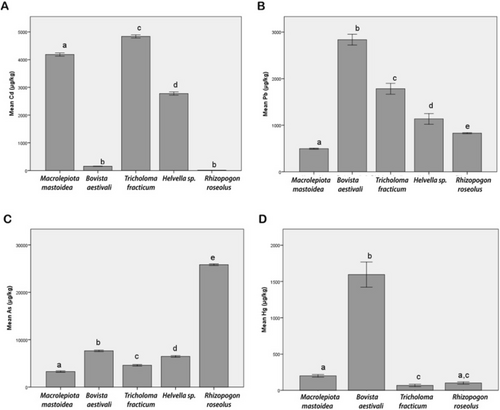
In the five mushroom species considered in the study, Cd level in Class I was found in the range of 11.19–4838.71 μg/kg. In a review study, 69 analyses of heavy metal levels in mushrooms reported that Cd levels ranged from 541.20 to 2246.00 μg/kg [1]. One recent study has reported that the Cd maximum level in mushrooms was 26350.00 μg/kg [28]. It is seen that Cd levels in the mushrooms in this study are comparable to those in other mushrooms analyzed in the literature (Table 3). However, although the Cd level THQ value for adults was less than 1 in all mushrooms examined in this study, the HI value of each mushroom was greater than 1. Nevertheless, in children, the THQ value for Cd was greater than 1 in some mushrooms. Therefore, it is thought that the Cd level contributed to the high HI value and that a noncarcinogenic risk may arise (Table 3). In this study, Rhizopogon roseolus had the lowest Cd level, while Tricholoma fracticum had the highest Cd level. Cd mainly accumulates in the human body's spleen, liver, and kidneys and may pose a serious health risk [4].
Pb levels in mushrooms were found in the range of 495.43–2836.81 μg/kg (Table 3). When previous studies were reviewed, it was reported that Pb levels ranged from 823.60 to 4145.80 μg/kg in 63 studies related to mushrooms [1]. Another study reported that the maximum Pb level in mushrooms was 5757.00 μg/kg [28]. Another study reported that the Pb level in black mushrooms ranged from 18.00 to 1300.00 μg/kg [34]. Pb levels detected in the mushrooms analyzed in this study were found to be within the range reported in previous literature. However, when the contribution of Pb to non-carcinogenic risk was assessed for all mushroom samples, the THQ value was found to be below 1 (Table 3). The mushroom species with the lowest Pb level was Macrolepiota mastoidea, while the mushroom species with the highest Pb level was Bovista aestivalis. Pb mainly causes many symptoms in the human body, such as anemia, kidney damage, and neurotoxic effects [4].
As levels ranged from 3267.52–25823.07 μg/kg (Table 3). In the literature, the range of As levels in 18 wild edible mushroom species was reported to be in the range of 160.00–34500.00 μg/kg [4]. Although the As levels in the mushrooms in this study were comparable to the literature, As levels were close to the upper limit detected previously in the literature. In addition, when the effect of As level on non-carcinogenic risk in all mushrooms considered in the study was examined, the THQ value was greater than 1 (Table 3). The non-carcinogenic risk potential of As level was observed in all mushrooms. This value also affected that of HI in mushrooms; the HI value in all mushrooms was greater than 1. In this study, the mushroom species with the lowest As level was Macrolepiota mastoidea, while the mushroom species with the highest level was Rhizopogon roseolus. Although As is generally classified as a heavy metal, it exhibits both metallic and non-metallic properties in terms of its chemical characteristics and is therefore classified as a metalloid in the periodic table. For this reason, it is important to evaluate As in our study by taking into account both its toxicological effects and its chemical classification. High arsenic intake can cause serious damage to the peripheral vascular system, as well as the central nervous system and cardiovascular system [4]. It is clearly seen that the consumption of the mushroom species considered in the study may pose a serious health risk due to the toxic effects of As on human health. M. mastoidea, with the lowest As value, was collected from the forest area far from the city. R. roseolus, which had the highest As value, was collected from around the Faculty of Science parking lot. In this case, it is thought that mushrooms exposed to exhaust gas will carry more elemental impurities in their bodies. Hg is one of the toxic elements especially for humans and animals, and it is found among the top 10 chemicals or groups of chemicals of major public health concern. Large anthropogenic Hg emissions and its increased accumulation occurred with intensive industrial activities after the Industrial Revolution [7]. Hg is a global pollutant that has raised great concerns worldwide. Unlike other risk metals, Hg can remain in the atmosphere for a long period and migrate long distances. Eventually, about 93.7% of Hg enters the land and water ecosystem through dry and wet deposition. The usual contents of mercury in wild-growing mushrooms range between < 0.5 and 5.0 mg/kg (DW) and some species have been reported to be Hg bioaccumulators [5]. A recent study reported that the Hg level was 1.00–2613.00 μg/kg in the study of mushroom heavy metal levels [28]. Similarly, another study investigated and compared Hg concentrations in caps and stipes of Neoboletus luridiformis collected from 38 different areas across Slovakia. The study reported that Hg accumulation in the fruiting bodies of the mushroom was directly correlated with the Hg content of the soil/substrate. The median Hg concentration in the soil exceeded 5.00 mg/kg (DW) in only two localities, while in the remaining 36 areas, it ranged from 0.05 to 0.50 mg/kg (DW). These findings highlight the significant impact of environmental contamination on mercury accumulation in wild mushrooms, emphasizing their sensitivity to local pollution levels [7]. The Hg levels detected in this study were within the range of Hg levels in the literature. Hg levels in the five mushrooms ranged from 0.00 to 1594.50 μg/kg (Table 3). The mushroom species with the highest Hg level was Bovista aestivalis, while Helvella sp. was undetected. When the effect of Hg level on non-carcinogenic risk was analyzed, the THQ value was less than 1 in all mushrooms (Table 3). Hg has numerous acute and chronic adverse effects on humans. Acute exposure can lead to nervous system and lung damage. Chronic poisoning is characterized by psychological and neurological symptoms [4].
When Table 3 is examined, the non-carcinogenic and carcinogenic risk values for adults and children clearly reveal a health risk. The HI value, effective in determining the non-carcinogenic health risk in all five mushroom species, is found in the range of 1.498–8.171 and is greater than 1. While all mushroom species pose non-carcinogenic risk, Rhizopogon roseolus has the highest non-carcinogenic risk potential in both adults and children. Carcinogenic risks may occur due to exposure to Cd, Pb, and As heavy metals through food consumption. Consumption of food with a CR value greater than 1 × 10−4 poses a potential carcinogenic hazard, whereas consumption with a CR value less than 1 × 10−6 is not considered a health hazard as there are tolerable risks. A CR value between 1 × 10−6 and 1 × 10−4 generally indicates an acceptable level of exposure and does not pose a health hazard [28]. The CR values of Cd, Pb, and As, heavy metals with carcinogenic risk potential, in mushrooms were analyzed separately for each metal in our study. Mushrooms with a CR value of Cd exposure greater than 1 × 10−4 and which may pose a potential carcinogenic hazard due to mushroom consumption in adults and children are Macrolepiota mastoidea and Tricholoma fracticum. Mushrooms with a CR between 1 × 10−4 and 1 × 10−6, that have an acceptable level of exposure from mushroom consumption in adults and children, are Bovista aestivalis and Rhizopogon roseolus.
Mushrooms with a Pb exposure CR of less than 1 × 10−6 that do not pose a health risk to adults are Macrolepiota mastoidea, Helvella sp., and Rhizopogon roseolus. Mushrooms with CR values around 1 × 10−6 that cause acceptable exposure in adults are Bovista aestivalis and Tricholoma fracticum. The CR value for Pb exposure in children was approximately 1 × 10−6. In contrast, the CR value associated with As exposure from consuming all mushrooms in the study was found to be at or above 1 × 10−4, with values exceeding 1 × 10−4 observed specifically in children. Since As was detected in all mushrooms in this study and the CR value of this metal was found to be at a level that may pose a potential carcinogenic hazard in all mushrooms, it is thought that the consumption of these mushrooms may cause serious health problems. The mushroom samples evaluated in the study were collected from university campuses located in the provinces of Çanakkale and Van, as well as from forested areas surrounding these campuses. Although these areas appear to be natural, their proximity to city centers means that they are potentially exposed to human activity, either directly or indirectly. Especially in regions with a geologically rich structure in terms of elemental impurity accumulation, such as Van province, anthropogenic sources such as past or present mining activities, proximity to transportation routes, agricultural pesticide residues, construction materials, and fossil fuel use can lead to elemental impurity accumulation in the soil. Similarly, sampling points in Çanakkale Province are located at certain distances from the region's dense industrial and transportation networks, which may increase the risk of environmental contamination. Considering the direct relationship between mushrooms and soil and their bioaccumulation properties, it is likely that these environmental factors have elevated elemental impurity levels and, consequently, risk assessment values.
Considering the variability of the parameters (MC, BW, and IR) that may affect the formation of risk in determining carcinogenic and non-carcinogenic health risks, the probabilistic risk dimension of elemental impurity exposure in children and adults was also evaluated by simulation methods. When Figure 4 is examined, it is seen that the health risks arising from metal accumulation in mushrooms may increase significantly in children and adults, and preventive measures should be taken.
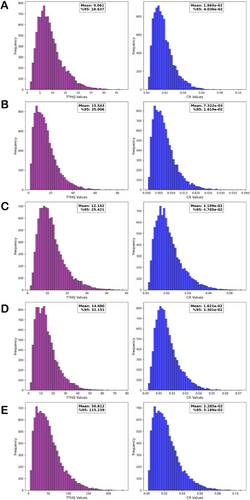
When the Monte Carlo simulation method was used to evaluate the probabilistic health risks of elemental impurities in mushroom species, Figures 4 and 5 showed that carcinogenic and non-carcinogenic health risks reached high levels in children and adults. When Figure 4 is examined, the mean TTHQ values in the non-carcinogenic risk assessment for children were found to be greater than 1, indicating significant health risks, especially for children. TTHQ values were also found to be higher in the 95th percentile. When the carcinogenic risk assessment in children is analyzed, the mean and 95% CR values were greater than 1 × 10−4, indicating that the carcinogenic risk in children will be high.
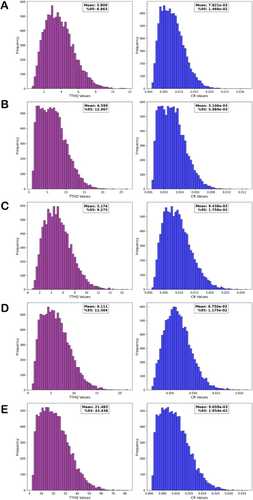
Figure 5 presents the distribution of health risk values calculated for adults. The mean TTHQ values for adults were greater than 1. When the 95% percentile is considered, total HI values were greater than 1, and the health risk increased. When the carcinogenic risk assessment in adults was examined, the mean and 95% CR values were greater than 1 × 10−4, indicating that the carcinogenic risk in adults may be high.
These results indicate that elemental impurity accumulation in mushrooms can cause serious health problems for children and adults. Considering the body weight of children in particular, exposure to elemental impurities can cause more serious health problems than in adults. Therefore, protective measures should be taken for the entire population, especially children. The high levels of carcinogenic toxic elements in the mushrooms investigated indicate that long-term consumption may increase the risk of cancer. In addition, the presence of non-carcinogenic substances that may have harmful effects through bioaccumulation should also be considered. These results suggest that the mushrooms in this study may pose a significant threat to public health, and their consumption should be carefully evaluated.
4 Conclusion
The findings of this study reveal that mushrooms growing in areas close to residential areas can accumulate high levels of elemental impurities due to environmental pollution. Elemental impurity contamination originating from factories, transportation routes, industrial activities, and past mining operations not only affects soil and water resources but also directly impacts natural products such as mushrooms that grow in these environments. Considering the strong bioaccumulation properties of mushrooms, it is understood that such natural products may contain high levels of risk elements even in forest ecosystems. This situation poses a significant risk in terms of environmental health, food safety, and public health, emphasizing the need for increased regulatory oversight and consumer awareness.
In conclusion, this study provides important evidence regarding the potential effects of environmental elemental impurity pollution on the food chain and establishes a scientific basis for the development of preventive policies to protect public health. Our findings highlight the need for expanded environmental and epidemiological research, including time-dependent monitoring studies that comparatively evaluate the bioaccumulation profiles of edible natural products grown in different ecosystems. Such advanced research will contribute to the development of food safety policies and a better understanding of the environmental circulation of pollutants.
Author Contributions
Conceptualization: İ.A. and F.C. Methodology: İ.A., F.C., and F.A. Software: İ.A., F.C., and F.A. Validation: F.C. and F.A. Formal analysis: F.C. and F.A. Investigation: İ.A., F.C., and F.A. Resources: İ.A. and F.C. Data curation: F.C. and F.A. Writing ‑ original draft preparation: İ.A., F.C., and F.A. Writing ‑ review and editing: İ.A., F.C., and F.A. Visualization: İ.A., F.C., and F.A. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank the institutions from which we outsourced the elemental impurity analysis for their contributions.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




