Imbalanced inflammatory response in subchronic arsenic-induced liver injury and the protective effects of Ginkgo biloba extract in rats: Potential role of cytokines mediated cell–cell interactions
Ling Dong and Yonglian Liu contributed equally to this study.
Funding information: Natural Science Foundations of China, Grant/Award Numbers: U1812403, 81430077
Abstract
Arsenic is a well-known environmental toxicant and carcinogen, which has been epidemiologically proved related to the increased hepatic disorders. Researches have shown that aseptic inflammation and abnormal immune response are associated with arsenic-induced liver injury. However, the immunotoxic effects of liver have not been extensively characterized. Ginkgo biloba extract (GBE), a natural products of G. biloba leaves with proven anti-inflammatory and potential immunoregulatory activities, was used as intervention agent to explore its protective effects on arsenic-induced hepatotoxicity. Thus, the underlying mechanism of the immunotoxic effects on arsenic-induced liver injury were investigated in 2.5, 5.0, and 10.0 mg/kg NaAsO2 of Wistar rats for 16 weeks. Subsequently, GBE was used as intervention agent in 50 mg/kg for 6 weeks after cessation of arsenic exposure. The ratio of Th17 to Treg cells in peripheral blood as well as the secretion of inflammatory cytokines IL-17A, IL-6, TGF-β1, and IL-10 in serum and liver were detected. Meanwhile, the notable activation of aseptic inflammation-related molecule TLR4 and its downstream targets MyD88 and NF-κB in the liver were observed. In this work, we confirmed that subchronic exposed to arsenic triggered the infiltration of inflammatory cells in rat liver, coupled with obvious histopathological changes and aberrant hepatic serum biochemical parameters. Meanwhile, imbalanced immune response was verified by the notable abnormal ratio of Th17 to Treg cells in peripheral blood as well as the secretion of inflammatory cytokines IL-17A, IL-6, TGF-β1, and IL-10 in serum and liver of arsenic exposed rats. Further, the level of TLR4, MyD88, and NF-κB in liver both transcription and translation activity were raised. Subsequently, GBE markedly mitigated arsenic-induced liver injury, most impressively, post treatment with GBE prominently suppressed the overactivated inflammatory-related TLR4-MyD88-NF-κB pathway and evidently decreased the secretion of inflammation cytokines. Meanwhile, the disturbance of pro- and anti-inflammatory response was reversed. We concluded that the disruption of pro- and anti-inflammatory T-cells balance caused by cytokines mediated cell–cell interactions may be one of the mechanisms underlying arsenic-induced liver injury and that GBE intervention exerts an evidence protective effects, which might be closely associated with the suppression of inflammatory-related TLR4 pathway.
1 INTRODUCTION
Arsenic (As), as a well-known naturally occurring environmental toxicant and carcinogen, is ubiquitously distributed in the environment in both inorganic and organic forms.1 Epidemiological studies have proved that long-term exposure to As via contaminated drinking water, diet, or air can cause a variety of adverse health effects in humans. Apart from typical dermal changes,2 multiorgan system damages, such as respiratory,3digestive,4 urinary,5 cardiovascular,6 immune systems7 and many other harmful effects also have been demonstrated. Among them, the liver, as the primarily metabolic and detoxifying organ, is already confirmed as a susceptible toxicity target to arsenic.8 Recent studies have indicated that chronic arsenic exposure through environmental media with high levels of arsenic could result in increased risks of various hepatic diseases, such as hepatomegaly, fibrosis, cirrhosis, and even liver cancer.9, 10 Though the severity and types of liver injury caused by arsenic are different, the pathological process with abnormal inflammatory response is common. Nevertheless, the mechanisms of arsenic-induced inflammatory in liver have not been extensively investigated.
It has been widely recognized that the liver is not only a vital substance metabolism organ but also function as an important immune-regulate organ and the unique anatomical and immunological site make it important for the host defenses.11 Recent years, increasing attention is being towards pathological immune responses in the pathogenesis of many different types of liver injuries, in which the role of Th17 and Treg cells that has been revealed gradually. Th17 cells, the recently identified pro-inflammatory subset of CD4+ T helper cells has been proved important for the host against extracellular pathogenic substance, while, some literatures claimed that the overactivation of Th17 cells is pathogenic and closely related to the pathological processes of many liver disease.12, 13 Opposite to Th17 cells, another subset of CD4+ T cells that named regulatory T (Treg) cells are viewed as a critical immune-suppressor in excessive inflammation responses, especially in autoimmune and inflammatory diseases.14 Existing studies indicated that Th17 and Treg cells represent two aspects of immune response and the subtle balance of Th17 and Treg cells is vital in the maintenance of liver immune homeostasis.12 Our previous research found that the skewed balance of Th17 and Treg cells was closely associated with the pathogenesis of arsenicosis,15 and investigation in arsenic poisoning rats further revealed that arsenic-induced imbalanced immune responses was involved in arsenic-induced liver damage.16, 17 Although the correlations between arsenic induced immunotoxicity and liver damage have been explored to some extent, the molecular mechanism underlying remains unclear.
Increasing lines of studies demonstrated that, toll like receptor 4 (TLR4), one of the most representative pattern recognition receptors, is reported function as a “bridge” to link the innate immune and adaptive immune system work together to response pathogenic factors through via myeloid differentiation primary response gene 88-(MyD88-) independent or MyD88-dependent pathways.18, 19 In case of infection or injury, TLR4 is able to recognize specific molecular structures of pathogenic microorganisms or damage-associated molecular patterns and then initiates inflammatory cascade, with the pathological factors sustained, aberrant stimulation of TLR4 finally elicits excessive inflammatory response.20, 21 Moreover, additional evidence showed that TLR4 is also involved in the regulation of Th17 and Treg cells differentiation.22 Literatures reported that the activation of TLR4-MyD88-NF-κB pathway and the mediated inflammatory responses play an important role in the pathological process of liver diseases.23 However, the role of TLR4 in arsenic-induced liver injury have not been extensively investigated in vivo.
Ginkgo biloba extract (GBE), a defined complex mixture that contains active compounds of flavonoids and ginkgolides, which is used as a therapeutic agent for some cardiovascular and neurological disorders.24, 25 Several mechanisms have been proposed for the beneficial effects of GBE, including anti-oxidation,26 anti-inflammatory,27 and immunoregulatory.28 In a recent study, we present a scientific evidence indicated that GBE treatment can ameliorate arsenicosis patients immunological dysfunction by regulating the balance of inflammatory response.15 Furthermore, some studies have found that GBE intervention showed a protective effect on inflammatory-related liver damage.29-31 Nevertheless, the role and mechanisms of GBE on arsenic-induced liver immunotoxicity are poorly understood.
Comprehensively, the present study was focus on the changes of the inflammatory molecules and immune-related TLR4-MyD88-NF-κB pathway in the liver of arsenic exposed rats and to investigate the protection and probable mechanisms of GBE treatment in arsenic-induced liver injury.
2 MATERIALS AND METHODS
2.1 Drugs, reagents, and instruments
GBE was produced by the Yangtze River Pharmaceutical Group Co. under approval number Zhunzi Z20028023, China. Sodium arsenite (NaAsO2, ≥99.0%) was purchased from Sigma Chemical Co. (St. Louis, MO). RPMI1640 medium (HyClone, South Logan, UT), fotal bovine serum (Gibco-Invitrogen, Carlsbad, CA), and phosphate-buffered saline (PBS) were obtained from Gibco-Invitrogen (Carlsbad, CA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-10, TGF-β1, IL-17A, and IL-6 determining were purchased from Elabscience Biotechnology Co., Ltd, (Wuhan, China). FITC-labeled mouse anti-rat CD4 was purchased from BD Biosciences (BD Biosciences, San Jose, CA), APC-labeled mouse anti-rat -CD25, PE-cyanine7- labeled mouse anti-rat -IL-17, and PE-labeled mouse anti-rat -Foxp3, corresponding isotype control of IgG2a κ isotype control PE, mouse IgG1 κ isotype control APC, rat IgG2a κ isotype control PE-cyanine7, as well as the transcription factor staining buffer set, fixation/permeabilization buffer set and leukocyte-activation cocktail were purchased from eBioscience (San Diego, CA). Serum alanine aminotransferase (ALT), albumin (ALB), albumin/globulin (A/G), and the cholinesterase (CHE) were measured by colorimetry using a commercially assay kits from Jiancheng Bioengineering Institute (Nanjing, China). Real-time polymerase chain reaction (RT-PCR) kits were purchased from Takara Co (Japan). Primary antibodies of western blotting including anti-TLR4 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX). Anti-MyD88, anti-NF-κB p65, and anti-p-NF-κB p65 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-NF-κB p50 and anti-p-NF-κB p50 antibodies were purchased from Abcam (Cambridge, MA). Anti-beta-actin and secondary antibodies were purchased from Proteintech (WUHAN SANYING, Wuhan, China). Primary antibody dilution buffer (P0023A) and secondary antibody dilution buffer (P0023D) were purchased from Beyotime Biotechnology (Beyotime Institute of Biotechnology, Shanghai, China). Antibodies for immunohistochemistry analysis including anti-TLR4 (Abcam, Cambridge, MA), anti-MyD88, anti-NF-κB p65 and anti-NF-κB p50 were purchased from proteintech (WUHAN SANYING, Wuhan, China). All of the chemicals used were of analytical grade.
2.2 Animals and experimental administration
A total of 56 specific pathogen-free (SPF) Wistar rats (weighing 120–130 g, half female half male) were obtained from Liaoning Changsheng Biotechnology Co., Ltd (Liaoning, China) with a National Animal Use License number of SCXK-LN2015-0003. Animal used has been approved by the Animal Experimental Ethical Committee of Guizhou Medical University with an approval number 1403059.
During the experiment, experimental rats were group-housed under standard laboratory conditions (maintained temperature 22 to 24°C, relative humidity of 60%–70%) with 12 h light–dark cycle. After 1 week of acclimatization under laboratory conditions, those rats were randomly divided into seven groups according to their body weight. This study includes two parts: arsenic poisoning model established and GBE treatment. The doses of NaAsO2 were selected on the basis of our preliminary experiments as well as previously published research.32 Arsenic poisoning model groups included a control (A) group (received only deionized water) and three arsenic treatment groups were exposed to 2.5, 5.0, and 10.0 mg/kg NaAsO2 intragastrically for 16 weeks, respectively. The treatment group including control (B), high arsenic (10.0 mg/kgNaAsO2), recovery, and GBE-treated group. GBE was administered at 50 mg/kg by gavage for 6 weeks, and the doses were designed based on the clinical application of GBE. The specific animal groups and administration methods are given in Table 1.
| Groups | Administration and time | |
|---|---|---|
| NaAsO2 (mg/kg)—16 weeks | GBE (mg/kg)—6 weeks | |
| Model groups | ||
| Control (A) | 0 | —— |
| Low | 2.5 | —— |
| Middle | 5.0 | —— |
| High | 10.0 | —— |
| GBE-treated groups | ||
| Control (B) | 0 | 0 |
| Natural recovery | 10.0 | 0 |
| GBE-treated | 10.0 | 50 |
- Abbreviation: GBE, Ginkgo biloba extract.
To validate whether the model of liver damage caused by arsenic poisoning was successful established, the rats poisoning symptoms and changes of body weight were observed daily and weekly, respectively. What's more, at each end point of the experiment, the urine production was collected for each rat for 24 h using a metabolic cage, and a 10% nitric acid-treated polyethylene plastic tube was used to collect the urine. After centrifugal precipitation, 1 ml of urine was acidified with hydrochloric acid to a final pH of less than 2 for the urinary arsenic (UAs) assay. All the urinary samples were stored at −80°C until analysis. Secondly, the rats were anesthetized by ether, approximately 2 ml of blood from the heart was collected in both heparin-containing and serum tubes for T lymphocyte subsets, cytokines and liver function analysis. Finally, all the rats were sacrificed by anesthesia and the livers were collected for histopathological examination, liver arsenic (LAs) content and immune related molecular detection.
2.3 Calculation of the liver index
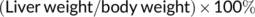
2.4 Determination of total arsenic levels in urinary and liver tissue
The total As content in urine and liver tissue were detected according to GB/T 5009.11–2014.33 Before the determination of L-As, approximate 0.3 g liver tissue of experimental rats was digested with 6 ml concentration HNO3 and 2 ml hydrogen peroxide using a microwave digestion instrument (Anton Paar, Multiwave GO, Sweden) for 1 h. For the determination of the urine arsenic concentration, 1 ml acidified urine was firstly diluted to 10 ml with 2% nitric acid. Then, inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Fisher, XSeries2) was used to analyze the total arsenic content in urine and liver tissue samples. UAs were standardized to the concentration of urinary creatinine (UCr). The limits of detection (LOD), limits of quantitation (LOQ), intra-and inter-day precision, and recovery rate of ICP-MS for the total arsenic analysis are given in Table 2.
| Instrumentation | ICP-MS |
|---|---|
| LOD (μg/L) | 0.16 |
| LOQ (μg/L) | 1.63 |
| Intra-day precision (RSD, %) | 0.35 ~ 1.37 |
| Inter-day precision (RSD, %) | 1.87 ~ 5.46 |
| Recovery rate (%) | 91.90 ~ 109.42 |
- Abbreviations: ICP-MS, inductively coupled plasma mass spectrometry; LOD, limits of detection; LOQ, limits of quantitation; RSD, relative standard deviation.
2.5 Analysis of liver biochemistry parameters levels in serum
To determine the serum biomarkers of liver damage, the blood samples of all rats were collected from each rat in every group and centrifuged at 3000 rpm for 15 min. Serum samples were separated and stored at −80°C until determination. The activities of ALT, AST, CHE, and the ratio of A/G in serum were detected using an automatic biochemical analyzer (Olympus AU400, Tokyo, Japan).
2.6 Histopathological evaluation
To validate the arsenic-induced liver damage model was successfully established, liver histology was observed. For histopathology examination, the liver tissues were fixed in 10% phosphate-buffered formalin for 48 h, embedded in paraffin blocks, and sectioned at 5 μm by a microtome (EM UC7, Leica, Germany), After hematoxylin and eosin (H&E) staining, the histopathological changes were visualized with a microscope (Olympus, Tokyo, Japan), and the typical histopathological changes were presented in the results.
2.7 Cell isolation and flow cytometric analysis
To evaluate the effect of arsenic exposure on the balance of immune response, the frequencies of Th17 and Treg cells in peripheral blood of rats were assessed. For the frequencies of Th17 and Treg cells analysis, the peripheral blood mononuclear cells (PBMCs) were isolated from the fresh heparinized blood by Ficoll–Hypaque (Tian Jin Hao Yang Biological Manufacture Co., Tianjin, China) gradient centrifugation at 2500 rpm for 20 min. And the isolated PBMCs were washed and re-suspended in complete medium, and then distributed in 24-well round bottom plates at a density of 2 × 106 cells/ml. For flow cytometry, Treg (CD4+ CD25hi Foxp3+) lymphocytes were evaluated using Foxp3 staining kit (eBioscience, San Diego, CA), while for the analysis of Th17(CD4+ IL-17A+) subsets, the isolated PBMCs were cultured in vitro at 37°C with 5% CO2 for 4–6 h and the Leukocyte Activation Cocktail, with BD Golgi Plug™ (BD Biosciences, San Jose, CA) was added to stimulate Th17 signature cytokine IL-17A production. After harvesting, the cells (2 × 106 cells/ml) were suspended in FACS solution and incubated with cell surface antibodies (FITC-labeled mouse anti-rat CD4, APC-labeled mouse anti-rat CD25) at 4°C for 30 min in the dark. For the intracellular staining, fixation& permeabilization solution was used to permeabilize the membrane of cell cytoplasm and nucleus at 4°C for 60 min, and then incubated with cell intracellular and nuclear antibodies (PE-labeled mouse anti-rat Foxp3, PE-cyanine7- labeled mouse anti-rat IL17A) at 4°C for 30 min in the dark. The isotype controls were treated with the same procedures and then washed with FACS solution. Subsequently, the cells were analyzed using BD FACS Canto II (BD Biosciences, San Jose, CA).
2.8 ELISA assays
To determine the inflammatory response in the liver of experimental rats, liver tissues were homogenized on ice with 500 μl ice-cold PBS and 0.05 g tissues to create a 10% tissue homogenate, and then centrifuged at 12 000 rpm for 15 min at 4 °C to remove precipitates, and the supernatants were collected and stored at −80°C until determined. For the measurement of systemic inflammatory levels, serum samples were obtained from thenon-anticoagulated whole blood. Then, the levels of inflammatory related cytokines IL-10, TGF-β1, IL-17A, and IL-6 in the serum and liver tissues homogenate were determined using commercially available ELISA kits according to the manufacturer's instructions (Elabscience, Wuhan, China). The absorbance was recorded at 450 nm in a microplate reader (Thermo Fisher). All of the tests were repeated two times.
2.9 Real-time PCR analysis
All of the primers were synthesized by Shanghai Jierui Biological Engineering Co., Ltd. (Shanghai, China). Primer sequences for real-time PCR are given in Table 3. Real-time PCR reactions were performed in 10 μl, with the final volume containing 0.5 μl of each primer, 1 μl of cDNA, 3 μl of RNase/DNase-free water, and 5 μl of SYBR Premix Ex Taq II (RR820) (TaKaRa, Japan). PCR amplification conditions consisted of initial denaturation for 5 min at 95°C, followed by 40 cycles of denaturation for 35 s at 95°C, annealing for 30 s at 56°C and extension for 30 s at 72°C. The mRNA levels were normalized against the mRNA levels of the reference gene GAPDH. Relative quantification was determined using the 2-△△Ct method, which resulted in ratios of the target genes to the housekeeping reference gene.
| Genes | Primers |
|---|---|
| TLR4 | Forward primer: 5′-ATCAGAGGAAGAACAAGAAGCAA-3′ |
| Reverse primer: 5′-AGAAACCCAGATGAACTGTAGCA-3′ | |
| MyD88 | Forward primer:5′-CTTCATCTGCTACTGCCCCAAT-3′ |
| Reverse primer: 5′-CACCATGCGACGACACCTTT-3′ | |
| NF-κB p65 | Forward primer: 5′-AGACCTGGAGCAAGCCATTAGC-3′ |
| Reverse primer: 5′-CGGACCGCATTCAAGTCATAGT-3′ | |
| NF-κB p50 | Forward primer: 5′-CTATGACAGCAAAGCCCCCAA-3′ |
| Reverse primer: 5′-CAAATCCTTCCCAAACTCCACC-3′ | |
| GAPDH | Forward primer: 5′-ACAGCAACAGGGTGGTGGAC-3′ |
| Reverse primer: 5′-TTTGAGGGTGCAGCGAACTT-3′ |
- Abbreviations: GAPDH, glyceraldehyde- 3-phosphate dehydrogenase; MyD88, myeloid differentiation factor; NF-κB, nuclear factor-κB; TLR4, toll-like receptors 4.
2.10 Western blotting analysis
After extracted, the proteins were separated by electrophoresis with 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). Then, the proteins were transferred to polyvinylidene difluoride membranes (PVDF, Millipore, Billerica, MA). The blots membranes were blocked and incubated with primary antibodies, including mouse monoclonal anti-TLR4 antibody (1:1000 dilution), MyD88 rabbit mAb (1:1000 dilution), NF-κB p65 XP rabbit mAb (1:1000 dilution), anti-NF-κB p105/p50 (1:3000 dilution), phospho-NF-κB p65 (Ser536) rabbit mAb (1:1000 dilution; 93H1, Cell Signaling Technology, MA), anti-NF-κB p50 (phospho S337) (1:1000 dilution), and beta-actin mouse antibody (1:10 000) at 4°C overnight, respectively. On the second day, membranes were washed and then incubated with corresponding HRP-conjugated secondary antibodies (anti-mouse IgG; 1:10 000 dilution; anti-rabbit IgG; 1:10 000 dilution) for 2 h at room temperature. Blots were visualized via ECL (Millipore[Millipore, Billerica, MA]), and analyzed by Image Lab system (Image Lab, BIO-RAD) after washed.
2.11 Immunohistochemistry analysis
The sectioned livers were paraffin-embedded, deparaffinized, dehydrated, antigen-repaired, and endogenous peroxidase-and goat serum-blocked. Then, the sectioned samples were incubated with primary antibodies, including mouse monoclonal anti-TLR4 antibody (1:100 dilution; ab22048, abcam), MyD88 Rabbit Polyclonal Antibody (1:50 dilution; 23 230-1-AP, proteintech), NF-κB p65 Rabbit Polyclonal Antibody (1:50 dilution; 10 745-1-AP, proteintech), and NF-κB p50 Rabbit Polyclonal Antibody (1:100 dilution; 14 220-1-AP, proteintech) at 4°C overnight. On the second day, secondary antibodies (Goat Anti-Mouse/Rabbit Antibody, Zhongshan Biotechnology Co., Ltd, Beijing, China) for 30 min at 37°C. The negative controls consisted of sections exposed to PBS instead of primary antibody. Diaminobenzidine (DAB) assay kit was used to observe the reaction of Antigen–antibody, and then counterstained with hematoxylin (K135925C, Zhongshan Biotechnology Co., Ltd). The results were assessed based on the evaluations of three independent observers. All of the slides were scanned using BA200 Digital Triple camera microscopic camera system (McAudi Industrial Group Co., Ltd) and analyzed with Image-Pro Plus version 6 software.
2.12 Statistical analysis
Data analysis in the present study were performed using SPSS 19.0 software (SPSS, Inc., Chicago, IL). All graph plotting was performed with GraphPad Prism software, version 7.0 (GraphPad Software). Data were presented as mean ± standard deviations (SD). Statistical significance was detected via one-way analysis of variance (ANOVA) followed by LSD (when the variance was equal) or Dunnett's T3 test (when the variance was uneven). Pearson's correlation was performed. Results were considered statistically significant at p < .05.
3 RESULTS
3.1 Arsenic exposure induced liver injury in rats
In the present study, rats in the arsenic poisoning group were treated with 0, 2.5, 5.0, and 10 mg/kg of NaAsO2 by intragastric administration. To validate the liver damage by arsenic exposed, the poisoning symptoms, changes of body weight, arsenic content in the urine and liver tissue, liver index, and liver histopathology were characterized. After 16 weeks of arsenic treatment, the arsenic-exposed rats exhibited varying degrees of poisoning symptoms, such as fluffy hair, slowed response, and decreased activity were observed, and these symptoms were exacerbated in the 10.0 mg/kg NaAsO2 treatment group. From the beginning of the twelfth week, the body weight of the arsenic-exposed rats tended to decrease compared with the control (A) group (Figure 1(A)). The levels of UAs and LAs were dose-dependently increased in different experimental groups (Figure 1(B)). It was quite clear that NaAsO2 could accumulated in liver. In addition, the liver index was calculated in each experimental group, while we failed to find any significant difference (Figure 1(C)). By observing the subsequent changes in liver function, we found that arsenic exposure decreased the levels of ALB, A/G, CHE (Figure 1(E–G)) and elevated the activity of ALT in serum (Figure 1(H)). Square frame indicates aberrant histopathologic structure, as well as the obvious infiltration of inflammatory cells (red arrow) and proliferation of fibroblast (green arrow) further confirming the arsenic-induced liver injury (Figure 1(D)).
3.2 Arsenic exposure disrupted the balance of Th17 and Treg cell in PBMCs of rats
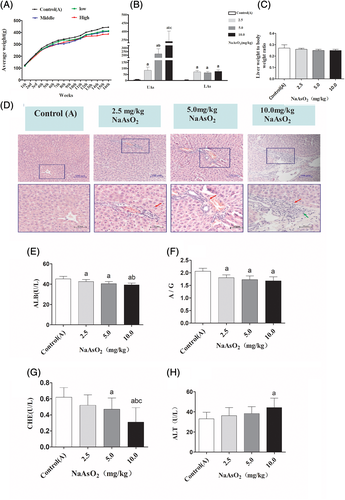
To evaluate the effects of arsenic exposure on the balance of Th17 and Treg cells, the percentages of Th17 cells and Treg cells in the PBMCs were detected according to their specific surface markers. The Th17 cells were defined as CD4+ IL-17+T cells, and the Treg cells were defined as CD4+CD25+Foxp3+Tcells. In the present study, we found that compared with control(A) group, the frequency of Th17 cells strikingly increased by 131%, 146%, and 196% in the PBMCs of rats exposed to 2.5, 5.0, and 10 mg/kg NaAsO2. On the other hand, the percentage of Treg cells was moderately raised by 135%, 130%, and 119%, respectively. While compared with the 2.5 mg/kg NaAsO2 treatment group, the percentage of Treg cells evidently decreased in the 10 mg/kg NaAsO2 group (Figure 2(D)). Remarkably, the ratio of Th17 to Treg cells were not altered significantly in 2.5 and 5.0 mg/kg NaAsO2 treatment group compared with control(A) group, respectively. However, the ratio was remarkably increased by 164% in the 10 mg/kg NaAsO2 group (Figure 2(F)). Totally, these results suggested that sustained arsenic exposure promoted Th17 cells differentiation and significantly disturbed the balance of Th17 and Treg cells.
3.3 Imbalanced inflammatory response was associated with arsenic-induced liver dysfunction
Our previous studies revealed that the oral administration of 2.5, 5.0, and 10.0 mg/kg of NaAsO2 significantly increased the levels of Th17 and Treg cells in the liver, as well as the concentration of Th17 and Treg cell related cytokines IL-10, TGF-β1, IL-17A, and IL-6 in serum and liver.16, 34 To further explore the role of immunological factors in arsenic-induced liver dysfunction, Pearson's correlation analysis was used to assay the indexes of liver injury and immunological changes in both peripheral blood and liver tissues of rats. As given in Table 4, there was a clear correlation between the imbalanced immune response and liver dysfunction.
| Variables | ALB | A/G | ALT | CHE | |||||
|---|---|---|---|---|---|---|---|---|---|
| R-coefficient (95%CI) | p-value | R-coefficient (95%CI) | p-value | R-coefficient (95%CI) | p-value | R-coefficient (95%CI) | p-value | ||
| Lymphocytes | Treg (%) | −0.37 (−0.64 to −0.02) | .040 | −0.67 (−0.47to 0.21) | .418 | 0.01 (−0.34 to 0.35) | .97 | −0.07 (−0.42 to 0.28) | .067 |
| Th17 (%) | −0.59 (−0.78 to −0.30) | <.001 | −0.15 (−0.83 to −0.43) | <.001 | 0.22 (−0.14 to 0.52) | .24 | −0.40 (−0.66 to −0.059) | .023 | |
| Serum | IL-10 | −0.45 (−0.69 to −0.12) | .010 | −0.55 (−0.75 to −0.25) | .001 | 0.22 (−0.14 to 0.53) | .223 | −0.25 (−0.55 to 0.11) | .165 |
| TGF-β1 | −0.70 (−0.84 to −0.45) | <.001 | −0.65 (−0.82 to −0.40) | <.001 | 0.32 (−0.03 to 0.60) | .074 | −0.62 (−0.80 to −0.34) | .000 | |
| IL-17 | −0.55 (−0.75 to −0.25) | .001 | −0.64 (−0.81 to −0.38) | <.001 | 0.41 (0.07 to 0.66) | .020 | −0.49 (−0.72 to −0.17) | .004 | |
| IL-6 | −0.50 (−0.72 to −0.18) | .004 | −0.29 (−0.58 to 0.07) | .113 | 0.13 (−0.23 to 0.46) | .475 | −0.36 (−0.63 to −0.01) | .045 | |
| Liver homogenate | IL-10 | −0.45 (−0.69 to −0.12) | .001 | −0.43 (−0.67 to −0.09) | .015 | 0.15 (−0.21 to 0.47) | .15 | −0.36 (−0.63 to −0.011) | .044 |
| TGF-β1 | −0.56 (−0.76 to −0.26) | .001 | −0.61 (−0.79 to −0.33) | <.001 | 0.27 (−0.09 to 0.56) | .14 | −0.49 (−0.72 to −0.17) | .004 | |
| IL-17 | −0.53 (−0.74 to −0.22) | .002 | −0.42 (−0.67 to −0.09) | .016 | 0.04 (−0.31 to 0.38) | .83 | −0.26 (−0.56 to 0.10) | .152 | |
| IL-6 | −0.56 (−0.76 to −0.26) | .001 | −0.39 (−0.65 to −0.05) | .026 | 0.13 (−0.23 to 0.46) | .48 | −0.41 (−0.66 to −0.07) | .021 | |
- Abbreviations: A/G, albumin/globulin; ALB, albumin; ALT, alanine aminotransferase; CHE, cholinesterase.
3.4 Arsenic exposure activated TLR4-MyD88-NF-κB pathway in rat liver
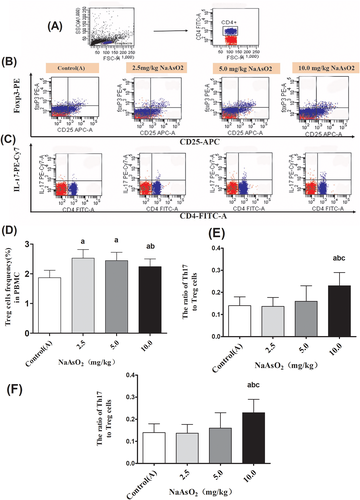
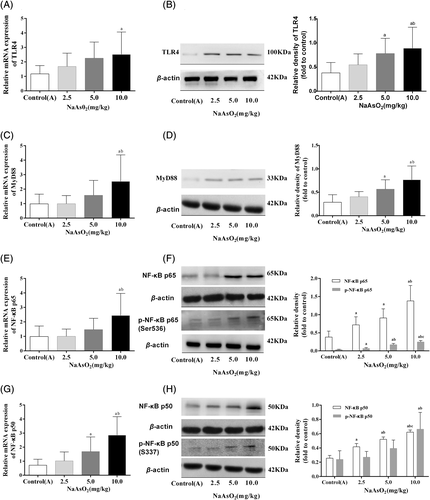
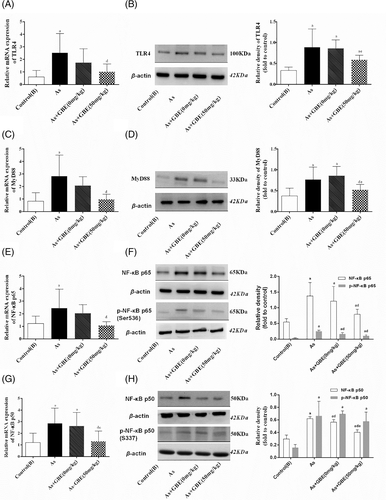
To explore the possible mechanisms underlying arsenic-induced immunotoxicity in liver, the expressions of inflammation-related molecule TLR4 and its downstream targets MyD88 and NF-κB in the liver were performed. The immunohistochemical staining showed that the expression of TLR4 and its down-stream pathway were observed only in a few hepatocytes and other cells of liver in the control(A) group. While, the higher expression of TLR4 was observed in the cytoplasm and membranes of large amounts of endothelial cells, macrophages and hepatocytes in the arsenic-exposed groups, especially in 10 mg/kg NaAsO2 group. Similar patterns expression of MyD88 (Figure 3), NF-κB p65 and NF-κB p50 (Figure 4) were observed in cytoplasm. Consistent with the results of immunohistochemical staining, RT-PCR and Western Blotting showed that the transcription activity and translation levels of TLR4 (Figure 5(A,B)), MyD88 (Figure 5(C,D)), NF-κB p65 (Figure 5(E,F)), and NF-κB p50 (Figure 5(G,H)) were significantly up-regulated by different doses of NaAsO2. Moreover, the phosphorylated forms of NF-κB p65 and NF-κB p50 were strongly induced (Figure 5(F,H)). Combine with the abnormal inflammatory response, our data showed that sub-chronic arsenic exposure activated the inflammatory related TLR4-MyD88-NF-κB pathway, which might be involved in arsenic-induced liver damage caused by excessive immune inflammatory response.
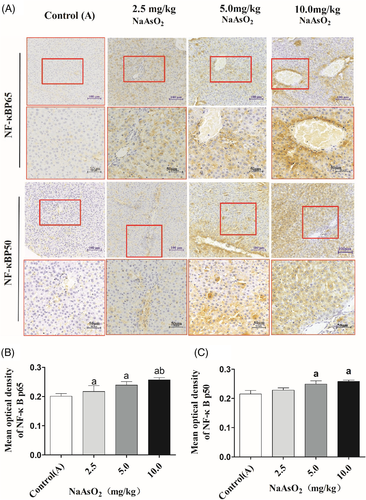
3.5 GBE treatment alleviated arsenic-induced hepatic dysfunction
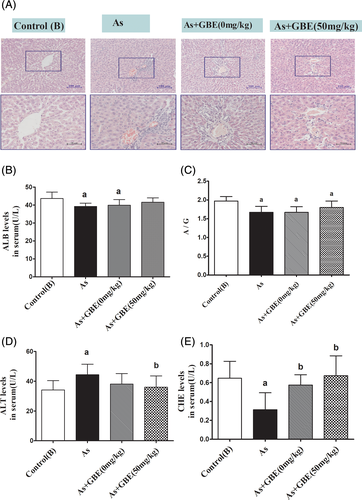
In the arsenic-treated rats, activities of ALT significantly increased, while the activity of ALB, CHE, as well as the ratio of A/G decreased compared with the control (B) group. Compared with the arsenic-exposed group, intervention of GBE markedly decreased the level of ALT and obviously increased the levels of ALB, CHE, and the ratio of A/G (Figure 6(B–E)). Furthermore, as shown in H&E staining sections in Figure 6(A), there was a clear aberrant histopathologic structure in the liver of arsenic-exposed rats. However, the pathological changes in the GBE treatment group were less than that in the arsenic-exposed and recovery group in Figure 6(A). These results suggested that GBE treatment could significantly alleviate the rat's liver injuries caused by arsenic exposure.
3.6 GBE treatment restored the balance of Th17/Treg in the PBMCs of arsenic-exposed rats
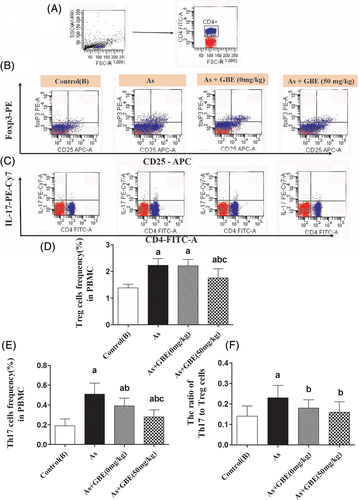
To investigate the potential regulative effects of GBE on the differentiation of Th17 and Treg cells, PBMCs were isolated and flow cytometric analysis was performed to determinate the frequency of Th17 and Treg cells. As shown in Figure 7, compared with the arsenic exposure and recovery group, the percentage of Th17 the ratio of Th17 to Treg cells were remarkably decrease in the GBE treatment group. What's more, the results here also observed a slight decrease of Treg cells. Whereas the percentage of Treg and Th17 cells in the recovery group significantly higher than that in the control(B) group. These experiment data showed that GBE could partially restore the balance between Th17 and Treg in PBMC of arsenic-exposed rats.
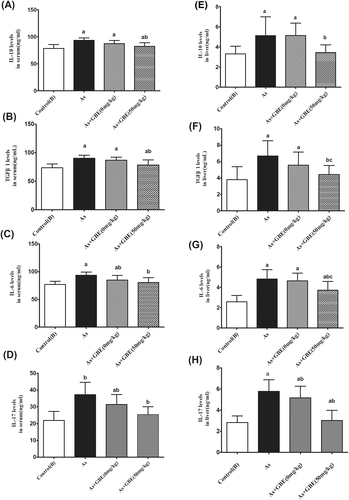
3.7 GBE treatment decreased inflammatory responses in the serum and liver of rats caused by arsenic exposure
To study the role of GBE on the arsenic-induced inflammatory response, we further focused on the levels of the corresponding signature cytokines of Th17 cell (IL-17A) and Treg cells (IL-10), as well as the inflammatory response mediator IL-6 and TGF-β1 in the rats' serum and liver tissue were detected. As shown in Figure 8, compared with the control (B) group, arsenic significantly increased the levels of IL-17, IL-10, IL-6, and TGF-β1 by 170%, 119%, 122%, and 121% in serum, respectively. Moreover, the above cytokines in liver tissue were remarkably raised by 205%, 155%, 186%, and 176%. In contrast, intervention of GBE drastically suppressed the arsenic-induced inflammatory cytokines secretion both in the serum and liver tissues. The experimental data indicated that GBE-treatment ameliorated the liver injury was accomplished by suppressing the production of inflammatory cytokines in liver tissue and regulating the secretion of corresponding signature cytokines from Th17 cell and Treg cell.
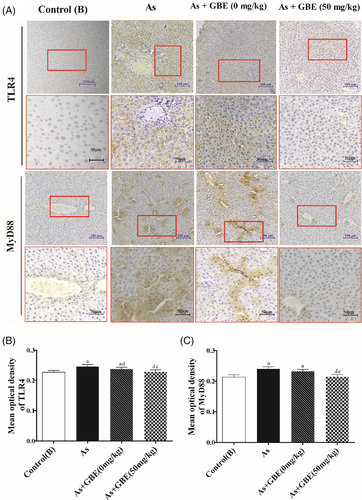
3.8 GBE-mediated protective effects may involve TLR4-MyD88-NF-κB suppression
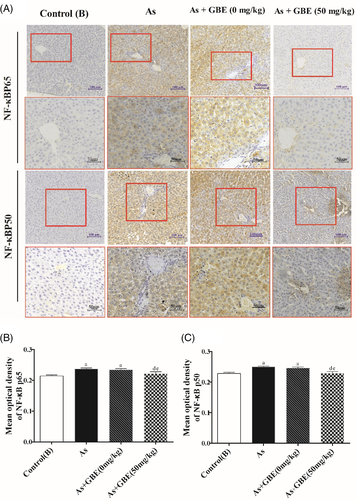
To explore whether the TLR4-MyD88-NF-κB pathway was involved in the protective effects of GBE, immunohistochemistry was used to detect the location and relative protein expressions of TLR4 and its downstream MyD88 and NF-κB in the arsenic-exposed and GBE-treated rats. As shown in Figure 9, TLR4-positive cell was significantly higher in the arsenic exposed and recovery group and lower expression in GBE treatment group than those in the control(B) group. The similar expression pattern of MyD88 NF-κBp65 and NF-κBp50 were observed in the cytoplasm (Figures 9 and 10). Simultaneously, the transcription activity and translation levels analysis showed that TLR4 and its downstream target MyD88, NF-κB mRNA, and protein levels in the liver tissues of arsenic exposure group were strongly increased compared with those in the control(B) group. Additionally, GBE treatment significantly suppressed those elevations in arsenic-exposed rats. The results suggested that GBE-mediated protective effects may be involved in the TLR4- MyD88-NF-κB pathway (Figure 11).
4 DISCUSSION
Increasing epidemiological studies have demonstrated the relationship between chronic As-contaminated environmental media exposure and hepatic disorders even hepatocellular carcinoma.35-38 However, the pathogenesis is still unelucidated and there is no specific treatment. Recent years, several mechanisms of As-induced hepatic damage have been proposed, such as DNA damage, oxidative stress, promotion of apoptosis and autophagy.39-41 According to the recent researches, the liver is not only a metabolic and detoxifying organ, but also an important immunological organ with numerous innate and adaptive immune cells.11 Moreover, increasing evidence indicated that immunological factors play an important role in the pathological process of various liver disease.42GBE has performance many physiological benefits and pharmacological effects, such as antioxidant, anti-inflammatory, and immunomodulatory.15, 30, 43 Thus, in this paper, GBE was used as an immunomodulatory agent to investigated its protective effects on As-induced liver injury.
Since the level of As in urine and organ tissue can directly reflect the absorption and accumulation of As in the body, it is widely to use the As concentration as biomarkers of exposure for arsenic poisoning. UAs is regard as an routine indicator of As exposure, whereas LAs may reflect the accumulation of arsenic in the liver.44 The present data showed the levels of UAs and LAs in As-exposed groups were higher than that in the control(A) group. Moreover, As treatment dramatically decreased serum levels of ALB, CHE, A/G and significantly increased the level of ALT. Histopathological examination showed that As-exposed for 16 weeks dose- dependently triggered the infiltration of inflammatory cells in rat's liver. Moreover, increased inflammatory related cytokines, as well as the imbalanced differentiation of Th17 and Treg cells and overactivation of inflammatory-related TLR4-MyD88-NF-κB pathway were also observed upon arsenic exposure. Together with the aberrant alteration of liver histopathology further confirmed the As-induced liver injury rat model was successful established. Furthermore, combine with the positive correlation between liver injury indexes and the immunological changes further indicated the hepatic immunotoxicity of arsenic in vivo.
Inflammatory response is viewed as an important mechanism for the body defend pathogenic invasion and thought to be triggered by the immune cells, and then expand by the adaptive immune system. Appropriate inflammatory response is benefit for foreign matter's clearance and even self-healing to some extent. Nevertheless, if the response is out of control, overactivated immune reaction that lacks suppressive regulation finally leading to serious results.45 Singh NJ et al46reported that, arsenic exposure increased the levels of inflammatory cytokines in mice, such as TNF-α, IL-1β, and IL-6. Furthermore, overexpression pro-inflammatory mediators of TNF-α and IL-6 was also observed in chronic arsenic exposure individuals.47 Our previous study also found that pro-inflammatory cytokines IL-6 and IL-17A were higher in patients with arsenicosis.15 In accordance with these observations, the present study showed that the concentration of pro-inflammatory mediators IL-6 and IL-17 in sub-chronic arsenic-exposed rat's serum and liver were consistently increased compared with the control group. Together with the verified increased infiltration of immune cells in the liver tissue of rats by biopsy further indicated that sub-chronic arsenic exposure resulting in obvious inflammatory changes and histopathological damage.
Treg cells start differentiation and suppress the excessive activation of effector immune cells to avoid inflammatory injury by releasing negative regulator cytokines or expressing immunosuppressive receptor molecule on the surface of cell membranes. IL-10 and TGF-β, which secreted by multiple cells, such as Treg cells, macrophages and DC cells were proved possessing powerful immunosuppressive properties and anti-inflammatory activity in protecting cells and tissues from injury caused by excessive inflammatory response,48, 49 the present data showed that the levels of IL-10 and TGF-β1were also significantly increased in the sub-chronic exposed rat's serum and liver. These results were consistent with the report by Li J et al,50 that sub-chronic As exposure moderate enhanced the expression of anti-inflammatory cytokines. While other research reported that individuals exposed to As via As-contaminated drinking water significantly decreased the anti- inflammatory cytokines.51The inconsistency may be related to the different types of exposure, time, dose and experimental systems. In the present study we found that, with the sustains of inflammatory response caused by arsenic, the levels of anti-inflammatory cytokines in serum and liver tissue were also significantly increased. However, the increased values of cytokines anti-inflammatory were lower than the values of pro-inflammatory cytokines. Together, these results implied that sub-chronic arsenic exposed weaken the body's immune regulatory function, resulting in imbalanced immune response.
Recent years, studies on the mechanism of imbalanced immune response further indicated that the interaction of appropriate innate and adaptive immune system is important for the body to exhibit a highly efficient immune response in the recognition and clearance of pathogens. It is demonstrated that the Th17 and Treg cells represent two aspects of the immune response, and their unique plasticity allows for shifting between pro-inflammatory and anti-inflammatory states. Duan X et al52 indicated that acute arsenic exposed significant disturbed the differentiation of Th17 and Treg cells both in spleen and thymus, as demonstrated by up-regulated the mRNA expression of Treg associated transcription factors foxp3 and cytokines IL-10. While, lessened the specific transcription factors Ror-γt and IL-17 mRNA of Th17 cells in mice.
Our findings indicated that sub-chronic arsenic exposure dose-dependently increased the frequency of Th17 cells. Meanwhile, with the increasing of Th17 numbers, the Treg cells that act as a negative regulator in immune responses also increased. While, the ratio of Th17 to Treg cells in the 10 mg/kg NaAsO2 was significantly increased by 164% compared to the baseline (Figure 2(D–F)). Combine with our previous study15 that As exposure significantly disrupted the balance of Th17 and Treg cells as well as the corresponding cytokines IL-17, IL-6, and IL-10 suggested that prolonged arsenic exposure might exhaust the immunoregulatory capacity of Treg subpopulations, ultimately resulting in Th17 predominant effects and disrupting the balance of pro-inflammatory and anti-inflammatory. Recent literature has indicated that the imbalance of Th17 and Treg cells is involved in the liver disease.12 In lines with those reports, a significantly positive correlation between the alteration of liver function index and the imbalance of Th17 and Treg cells was observed. Moreover, our previous study further revealed sub-chronic arsenic exposure induced the infiltration of Th17 and Treg cells in rats liver.17 Together, these results implied that the imbalanced of Th17 and Treg cells was involved in arsenic induced liver injury. However, the regulatory mechanism underlying is unknown.
As one of the first TLRs found in the body, TLR4 plays an important role both in the natural and adaptive immune responses, which is capable of recognizing signature motifs of exogenous pathogens, and also been activated by endogenous damage-related molecules that released from stressed or damaged cells.20, 53 Moreover, TLR4 plays a critical role in cellular stress and inflammation responses by activating the NF-κB.54 MyD88, is considered as a critical downstream ligand of TLR4 pathway and also served as an important adapter protein of NF-κB pathway. Jaya Chilakapati et al55demonstrated that TLR4 was up-regulated by various forms of arsenic, including iAs, trivalent and pentavalent methylated arsenicals at minimally cytotoxic concentration. In addition, elevated NF-κB was also observed in arsenic consumption individuals peripheral blood.56 Consistent with those reports, we found that both transcription and translation levels of TLR4, MyD88, NF-κB genes were markedly increased in the liver tissues of sub-chronic arsenic-exposed rats. Meanwhile, as immunohistochemical staining results showed, the expression of TLR4was enhancement in the cytoplasm and membranes of Kupffer cells, liver sinusoidal endothelial cells and few hepatocytes compared to the control group. The similar expression pattern of MyD88 was observed in the cytoplasm. Consistently, elevated expression of NF-κBp65 and NF-κBp50 were observed in arsenic exposure group. It has been demonstrated that the resident immune cells work as the first defend barrier of liver tissue. Furthermore, as a multifunctional organ, the liver has been found possess immune-regulating ability.42, 57 In addition, researchers have indicated that many hepatic diseases initiate with abnormal inflammation, and the progress of the immune response depends on cooperation between the innate and adaptive immune cells.11 Additional evidence further revealed TLR4-MyD88-NF-κB signaling pathway was closely related to the differentiation of Th17 and Treg cells, and the imbalanced polarization of Th17 and Treg cells can also be shifted through this pathway by regulating their specific transcription factors ROR-γt and Foxp3, respectively. Together with the elevated pro-inflammatory cytokines IL-6 in the liver, as well as the imbalanced of Th17 and Treg cells and their corresponding cytokines IL-17, IL-10, and TGF-β1 in sub-chronic arsenic exposure rats, we hypothesized that arsenic-induced liver immunotoxicity injury might be associated with the activation of the TLR4-MyD88-NF-κB pathway (Figure 12).
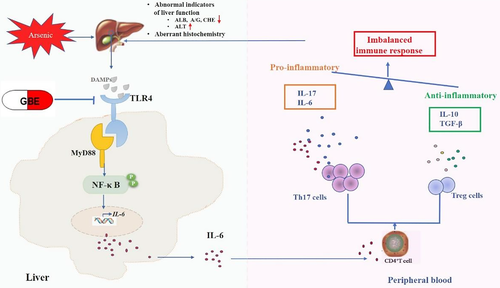
Recent years, GBE, a known bioactive extract from G. biloba leaves is receiving more and more attention and have been widely explored for its remarkable anti-inflammatory and antioxidant activity, especially in inflammatory related diseases, such as diabetes, colitis, cardiovascular and neurological disorders.27, 58 It has indicated that GBE intervention significantly decreased the levels of pro-inflammatory cytokines TNF-α and IL-6 in TNBS-induced colit.28 We found that GBE intervention showed a beneficial effect on the immune homeostasis of arsenicosis patients by reducing the RORγt and IL-17 of Th17 cells and enhancing Foxp3 and IL-10 of Treg cells in our previous study.15 The protective effects of GBE on arsenic-induced liver injury were investigated further. GBE treatment significantly decreased the percentage of Th17 subsets and remarkably adjusted the balance of Th17 and Treg cells. Furthermore, imbalanced inflammatory responses were relieved after GBE intervention, as results showed that the levels of pro-inflammatory cytokines IL-6 and IL-17 were dramatically decreased both in the liver and serum, whereas anti-inflammatory cytokines IL-10 and TGF-β were reduction slightly compared with the baseline. In addition, GBE dramatically inhibited the expression of TLR4, MyD88 and NF-κB. However, those changes mentioned above in the recovery group was higher than that in the control group, this result indicated that the self-healing ability for arsenic exposure induced liver injury is poorer. Combined with the histopathology determination results, arsenic-induced chronic inflammation infiltration and aberrant histopathologic structures of liver in the GBE treatment group was alleviated to some extent compared with the arsenic-exposed group and recovery group. Our results suggested that GBE treatment antagonized imbalanced inflammatory response by shifted the balance of Th17 and Treg cells in arsenic-induced liver injury via suppressing the overactivation of the TLR4-MyD88-NF-κB pathway.
5 CONCLUSIONS
In summary, this study demonstrated that sub-chronic exposed to arsenic triggers liver imbalanced immune responses by disrupting Th17 and Treg cells differentiation, which may be involved in the activation of inflammatory-related TLR4-MyD88-NF-κB pathway. In addition, the present work first demonstrated GBE intervention showed a beneficial effect on protecting rats against arsenic-induced liver inflammatory injury by restoring the balance of pro- and anti-inflammatory. Nonetheless, being limited to descriptive-based investigation data, more specific validation studies should be conducted to elucidate the mechanism of cytokines mediated cell–cell interactions in arsenic-induced liver damage and the hepato-protective effects of GBE in vivo and in vitro in our follow-up study.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundations of China (81430077 and U1812403).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study which titled with “Imbalanced inflammatory response in subchronic arsenic exposure induced liver injury and the protective effects of Ginkgo biloba extract in rats: potential role of cytokines mediated cell-cell interactions” are available on request from the corresponding author.




