The ameliorative effect of Moringa oleifera oil on tributyltin-induced brain toxicity in albino rats
Abstract
Tributyltin (TBT) is an organotin compound widely used as a biocide in antifouling paints. Moringa oleifera oil (MOO) has a promising antioxidant potential, which necessitates further exploration. This study was conducted to investigate the potential protective effect of MOO against TBT-induced brain toxicity. The 30 rats were grouped into five groups (six each), Group I negative control, Group II positive control (vehicle), Group III MOO (5 ml/kg body weight [b.wt.]), Group IV TBT (10 mg/kg b.wt.), and Group V TBT & MOO. All treatments were given orally for 28 days. Thereafter, brains were exposed to oxidative stress and neurological parameters analyses. Histopathological and immunohistochemical (caspase-3, Bax, Bcl-2) examinations were also carried out. In rats administered TBT, increased malondialdehyde level, decreased reduced glutathione, and low total antioxidant capacity levels were in support of oxidative stress mechanism. Neurotoxicity was indicated by high nitric oxide level and increased acetylcholinestrase activity. Along with the histopathological alterations, the dysregulated expression of caspase-3, Bax, and Bcl-2 were indicative of the apoptotic mechanism mediated by TBT. Co-administration of MOO with TBT ameliorated the aforementioned toxic effects. In conclusion, TBT causes brain toxicity via oxidative, nitrosative, and apoptotic mechanisms. MOO demonstrates protective effect against TBT-induced brain toxicity mostly via potent antioxidant and antiapoptotic properties.
1 INTRODUCTION
Tributyltin (TBT) is a major organometallic compound primarily used to prevent the adhesion of microorganisms to underwater structures. Over time, TBT becomes involved in many products like preservatives, polyvinyl chloride stabilizers, disinfectants, and insecticides.1 Despite being banned since 2008; TBT continues to be detected as a common contaminant in different ecosystems and biological tissues.2
Contamination of the marine environment is triggered by direct leakage of TBT from ships into the water.3 The extreme stability and resistance to natural degradation, make TBT one of the most toxic chemicals introduced to the marine environment.4 TBT concentration in sediment has been classified by Dowson et al.5 as follow: uncontaminated (below 3 ng/g), lightly contaminated (3–20 ng/g), moderately contaminated (20–100 ng/g), highly contaminated (100–500 ng/g), and grossly contaminated (above 500 ng/g). The Egyptian Mediterranean Coasts are highly to grossly contaminated by TBT.3
Human exposure to TBT takes place via water, sea food, and household products.6 World Health Organization has adopted 0.25 mg/kg body weight (b.wt.) as a tolerable daily intake of TBT in humans.7 TBT causes seizures, violent pain, and psychological disorders in humans. Thus, the European Commission8 has identified TBT as a “priority hazardous substance”.
TBT can disrupt blood–brain barrier and alter chemical neurotransmitters. Thus, brain is considered as a potential target for TBT toxicity.9 Oxidative stress and apoptosis have been perceived as two inseparable phenomena in TBT toxicity.7 Oxidative stress refers to the imbalance between reactive oxygen species (ROS) and antioxidant defense.10 Unfortunately, brain is highly vulnerable to oxidative stress because of high O2 consumption, fatty acid content, and defective neuronal glutathione.11 Under normal conditions, a state of steady balance is maintained between the pro-oxidants and antioxidants in brain. When this balance is disrupted, neuro-pathologies will follow.12
Oxidative stress has been implicated in the progression of many neurodegenerative disorders like Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis.13 ROS attack lipids causing lipid peroxidation, modify proteins causing dysfunction of enzymes (glutamine synthase, superoxide dismutase [SOD]), and interact with DNA causing mutations. ROS enhance excitotoxicity of glutamate receptors by excess calcium leading to apoptosis and neuronal cell death.14
The relation between pesticides and neurodegenerative disorders is well established. Organochlorines generate oxygen free radicals, disrupt the ubiquitin-proteasome system, and impair mitochondrial membrane potential. Paraquat inhibits mitochondrial complex I enzyme and activates N-methyl-D-aspartate receptors causing apoptosis or necrotic cell death.15 Fipronil impairs the mitochondrial oxidative phosphorylation of neuronal cells with ATP depletion, glycolysis, and lactate accumulation with subsequent activation of the apoptotic cascade.16
Apoptosis eliminates damaged or redundant cells in an ordered process of cellular disintegration.17 ROS increase cytosolic calcium by disrupting mitochondrial trans-membrane potential and respiratory chain.18 Excess calcium enhances the release of cytochrome c and initiates apoptosis cascade.14 Besides, oxidative stress increases expression of proapototic B-cell lymphoma-2 (Bcl-2) associated-X-protein (Bax) and decreases expression of the antiapoptotic Bcl-2 causing caspases activation.19 Caspase-3 is a major member of caspases which initiates apoptosis.17
Great attention is currently paid to natural antioxidants like herbs and food to block neurotoxicity. Phytochemicals have shown an ability to scavenge free radicals and to enhance neuronal survival and regeneration.20 Therefore, they have been used to ameliorate neurotoxicity of variable intoxicants such as pesticides.16 Likewise, phytochemicals have been recommended to protect against neurodegenerative disorders.21
Moringa oleifera (MO) (Family: Moringaceae) is a phytochemical plant cultivated in tropical and subtropical countries. Different parts of MO (seeds, roots, bark, leaves, fruit, flowers, and immature pods) have been used as cardiac stimulants, antitumor, antipyretics, antiinflammatory, antiulcer, antispasmodic, antihypertensive, and antidiabetic.22 Most of these effects have been attributed to the unique antioxidant nature of this plant.23 Extracts of different parts of MO have been proved to mitigate the redox homeostasis in different tissues. They have shown hepatoprotective effect against carbon tetrachloride (CCl4)24 and acetaminophen,25 nephroprotective effect against cobalt26 and lead acetate,27 and neuroprotective effect against TiO2-NPs-induced cerebral toxicity.28
Moringa oleifera oil (MOO) is the main constituent of the seed.29 MOO is enriched with phytosterols and tocopherols which potentiate its oxidative stability.29 Phenolic compounds in MOO act as terminators of free radicals and can retard oxidative degradation of lipids.30 Additionally, flavonoids carry out antioxidant potential via scavenging and chelating free radicals.31
The worldwide exposure to TBT has drawn much more attention to its neurological hazards.32 Therefore, this study was designed to investigate the sub-acute oral toxicity of TBT on brain of adult male albino rats and the potential protective effect of MOO against such toxicity. To the best of our knowledge, we report for the first time, the neuroprotective effect of MOO against TBT-induced brain toxicity.
2 MATERIAL AND METHODS
2.1 Chemicals
2.1.1 Tributyltin
TBT chloride (CAS Number: 1461-22-9) in the form of clear liquid, 96% purity, was purchased from Sigma–Aldrich Chemical Company, St. Louis, MO, USA.
2.1.2 Moringa oleifera oil
Was purchased from the Egyptian Scientific Society of Moringa, National Research Center, Dokki, Cairo, Egypt. MOO was obtained from MO seeds by cold pressure without any chemical treatment.
Gas chromatography–mass spectrometry analysis of fatty acids, methyl esters of MOO
Following the conversion of fatty acids in MOO into their methyl esters according to IUPAC,33 fatty acids were analyzed using a HP 6890 gas chromatograph with an HP 5973 Network mass selective detector and TR-FAME capillary column (MS, 30 m × 0.25 mm i.d., [70%-cyanopropyl-polysil phenylene], 0.25 μm film thickness), temperature of injector (250°C), and temperature of transfer line (240°C). The oven temperature was initially adjusted at 80°C for 2 min, and gradually increased by 3°C /min up to 220°C. Thereafter, temperature was held at 220°C for 20 min. The carrier gas was Helium and the flow rate was 1.5 ml/min. The sample injected was l μl (about 2 mg/ml) and the ionization energy was 70 eV. Fatty acids were identified by comparing the retention times of each peak with known fatty acid standard mixture. Peak areas were computed automatically by an integrator. The amount of each fatty acid was given as a percentage relative to the total fatty acid content.
Identification of unsaponifiable matter of MOO
Was analyzed using Hp 5890 gas chromatograph equipped with FID detector and DB-5 capillary column (30 m, 0.25 mm (5% phenyl)-95% methyl polysiloxane, 0.25 μm film thickness, temperature of the injector (280°C), and temperature of transfer line (300°C). The oven temperature was adjusted initially at 100°C for 2 min, increased by 10°C each min up to 300°C, and then held at 300°C for 20 min. N2 (2 ml/min) was the carrier gas. The identification of compounds was made by comparing relative retention times with their authentic reference compounds.
Determination of total phenolic content in MOO
The total phenolic (TP) content was determined using Folin–Ciocalteu reagent method according to Slinkard and Singleton.34 In test tube, 1 ml of oil sample was oxidized by the addition of 2.5 ml Folin–Ciocalteu reagent. Then, it was neutralized by 2 ml sodium carbonate (7.5%) and left to stand in the dark for 20 min at room temperature. Thereafter, absorbance was determined using spectrophotometer at 650 nm. The TP content was calculated as mean ± SD (n = 3) and expressed as μg of gallic acid equivalents (GAE) per g of sample.
Determination of total flavonoid content in MOO
Method of Chang et al.35 was used to determine total flavonoid (TF) content using the aluminum chloride colorimetric assay. The technique was based on the formation of a flavonoid-aluminum complex. Briefly, the reaction mixture (3 ml) formed of 1 ml sample (1 mg/ml), 1 ml methanol, 0.5 ml (1.2%) aluminum chloride, and 0.5 ml (120 mM) potassium acetate, was incubated for 30 min at room temperature. The absorbance of the reaction mixture was determined by spectrophotometer at 415 nm against a blank without aluminum chloride. The TF content was calculated as mean ± SD (n = 3) and expressed as μg of quercetin equivalents (QUE) per g of sample.
Determination of total tocopherol content in MOO
The total tocopherol (TOC) content of MOO was determined according to Wong et al.36 where 0.01 g of oil was added in volumetric flask (10 ml) with 5 ml of toluene, 3.5 ml of 2,2 bipyridine (0.07% [w/v] in 95% ethanol) and 0.5 ml FeCl3.6H2O (0.2% w/v in 95% ethanol). Then, the total volume of the mixture was brought to 10 ml solution with 95% ethanol. After that, absorbance of mixture was measured at 520 nm using spectrophotometer. The TOC was calculated as mean ± SD (n = 3) and expressed as mg/ 100 g oil.
2.2 Animals
Thirty adult male albino rats (each weighting 150–200 g, 12–16 weeks) were obtained from the animal house, faculty of medicine, Zagazig University, Egypt. Before experimentation, the animals were acclimatized for 2 weeks under standard laboratory conditions (temperature [23 ± 2°C], humidity [50 ± 5%], light–dark cycle of 12 h [dark at 8 pm, light at 8 am]). Animals were fed a standard diet and water ad libitum. The experiment was approved by the Institutional Animal Care and Use Committee of Zagazig University (ZU-IACUC/3/F/33/2020) and conducted in accordance with guidelines of the National Institute of Health (NIH).
Animals were randomly grouped into five equal groups (six rats each); Group I (negative control) left without any treatment for measurement of basic parameters, Group II (positive control group: vehicle group) received 1 ml corn oil as a vehicle of TBT, Group III (MOO group) received MOO at a dose of 5 ml per kg b.wt., Group IV (TBT-treated group) received TBT at a dose of 10 mg per kg b.wt. dissolved in 1 ml corn oil, and Group V (TBT & MOO group) received MOO (5 ml/kg b.wt.) 1 h prior to administration of TBT (10 mg/kg b.wt. in 1 ml corn oil). All treatments were administered orally by gavage on a daily bases for 28 days. Rats were weighed every week and the dose was adjusted according to the changes in body weight. The dose of TBT represented 1/10 of LD509 of TBT chloride, and was in accordance with previous study of Mitra et al.32 MOO dose was chosen according to Famurewa et al.11 and Edeogu et al.37
2.3 Specimen collection
At the end of the study, rats were anesthetized by intra-peritoneal injection with pentobarbital (50 mg/kg) and sacrificed by cervical dislocation. Brains of all rats were dissected and washed thoroughly by ice-cold saline to drain all red blood cells. Thereafter, brains were inspected for anomalies and the right hemispheres were homogenized with 10 times (W/V) ice-cold phosphate buffer (0.1 M, pH 7.4) and centrifuged at (10 000 rpm for 10 min at 4°C) to separate the supernatant for analysis of oxidative stress and neurological parameters. The supernatant was frozen at −80 °C in aliquots until analysis. The left hemisphere of each rat was immediately fixed in 4% paraformaldehyde for 20 h. Then, they were embedded in paraffin for hisopathological and immunohistochemical examinations.
2.4 Oxidative parameters
2.4.1 Malondialdehyde
Was determined according to Ohkawa et al.38 using kits obtained from Biodiagnostic (CAT. No. MD2529). After being homogenized, an aliquot of 0.2 ml tissue supernatant was mixed with trichloroacetic acid (1 ml) and thiobarbituric acid (2 ml) and heated for 1 h at 100°C. Thereafter, the mixture was allowed to cool and the supernatant was centrifuged again at 3000 g for 10 min. The absorbance of the organic layer was measured at 532 nm.
2.4.2 Reduced glutathione
Brain reduced glutathione (GSH) was measured following the method of Bump et al.39 using Myobiosource kits (USA, Cat. No. MBS265966). The reaction mixture contained 0.05 ml supernatant, 3 ml 0.05 M phosphate buffer (pH = 8), and 0.02 ml (5,5 - dithiobis-(2-nitro-benzoic acid). The reaction of GSH and 5,5 - dithiobis-(2-nitro-benzoic acid) resulted in formation of yellow complex. The absorbance was measured at 412 nm.
2.4.3 Total antioxidant capacity
Was determined according to the spectrophotometric method described by Koracevic et al.40 using kits of Biodiagnostic (Egypt, Cat. No. TA 2513). This method measures the ability of antioxidants in sample to scavenge free radicals generated from hydrogen peroxide (H2O2).
2.5 Neurological parameters
2.5.1 Nitric oxide
As nitric oxide (NO) is rapidly oxidized within few seconds to nitrite (NO-2) and nitrate (NO-3), so, both NO-2 and NO-3 can be used as indicators for NO production. NO level was determined following the Griess reagent method.41 Kits of Mybiosource (USA, Cat. No. MBS164965) were used. Samples (100 μl tissue suspension) were initially deproteinized by Somogyi reagent which precipitated proteins by adding 50 μl of 10% trichloroacetic acid. Nitrates were converted to nitrites by adding copperized cadmium granules. Total nitrites were measured by spectrophotometer at 545 nm.
2.5.2 Acetylcholinesterase
The activity of acetylcholinestrase (AChE) in the brain was assayed according to Ellman et al.42 by using kits obtained from Myobiosource (USA, Cat. No.MBS164965). The core of this method is to measure photometrically the yellow color product (2-nitro-5-mercaptobenzoate) resulting from the reaction between thiocholine and dithiobisnitrobenzoate ions at 450 nm. The rate of formation of 2-nitro-5-mercaptobenzoate is proportional to the AChE activity.
2.6 Histopathology
The Paraffin blocks were sectioned, prepared, and stained by hematoxylin and eosin (H&E) for 20 min and 10 min respectively.43 Slides were subjected to routine histopathological examination using light microscopy (OLYMPUS, Japan).
2.6.1 Semi-quantitative analysis (scoring) of cerebral lesions (gliosis and vacuolation)
Independently done by two investigators (one anatomical morphologists and one histologist) and scored according to Gibson-Corley et al.44 where, lesions in 10 non-overlapping fields were randomly chosen from each slide for each rat and averaged. The lesions were blindly scored as follow: 0 = normal, 1 = (≤ 25%), 2 = (26%–50%), 3 = (51%–75%), 4 = (76–100%).
2.7 Immunohistochemistrty (caspase-3, Bax, and Bcl-2)
Was done following the streptavidin-biotin immunoperoxidase technique. Paraffin sections (4 μm) were mounted, dewaxed, and rehydrated with phosphate buffered Saline (PBS) (pH 7.2). Heat-induced antigen retrival was done for 20 min in citrate buffer at PH 6. Sections were treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, and then washed in PBS. Slides were incubated over night with the primary antibodies; anticaspase-3 rabbit monoclonal antibody (1: 50 dilution in PBS, Cat. No. ab4051, ABclonal, USA), anti Bax rabbit antihuman, mouse, rat, polyclonal antibody (1:100 dilution in PBS, Cat. No. ab16152320, Abnova™ Lab Vision, USA), and anti Bcl-2 rabbit antihuman, mouse, rat, polyclonal, proteintech (Ready to use, Cat No. ab16896274, Abnova™, Lab Vision, USA). Thereafter, incubation with a secondary antibody and product visualization by chromagen, 3, 3′-diaminobenzidine tetrahydrochloride were done. Sections were counterstained with Mayer's hematoxylin, washed by distilled water and PBS, dehydrated, mounted and then examined microscopically.
2.7.1 Morphometric analysis
The area percentages of immune reaction to caspase-3, Bax, and Bcl-2 were measured within six fields for each rat at a magnification ×400 using g ImageJ IHC Profiler plugin ImageJ (Fiji Image J) software (National Institute of Health; NIH, Bethesda, MD, USA) at Histology Department, Faculty of Medicine, Zagazig University, Egypt. Thereafter, results were statistically represented as means ± SD.
2.8 Statistics
Data were analyzed using a commercially available statistics software package (SPSS for Windows version 19, Chicago, USA). One-way analysis of variance test was performed and multiple comparisons were done using the least significant difference. Results were presented as means ± SD where p values <.05 were considered as statistically significant.
3 RESULTS
3.1 Fatty acid composition of MOO
The chromatogram of MOO showed five peaks indicating the presence of five fatty acid compounds; oleic acid (C18:1 n-9, 39.88%), linoleic acid (C18:2, 37.73%), palmitic acid (C16:0, 15.18%), stearic acid (C18:0, 4.74%), and linolenic acid (C18:2 n-6, 2.47%) (Table 1, Figure 1).
| Peak | Retention time | Name | Formula | Area | Area sum% |
|---|---|---|---|---|---|
| 1 | 8.769 | Palmitic acid (C16:0) | C17H34O2 | 5609373.82 | 15.18 |
| 2 | 10.704 | Stearic acid (C18:0) | C19H38O2 | 1750404.78 | 4.74 |
| 3 | 10.973 | Oleic acid (C18:1 n-9) | C19H36O2 | 14740401.1 | 39.88 |
| 4 | 11.608 | Linoleic acid (C18:2) | C19H34O2 | 13945011.5 | 37.73 |
| 5 | 12.603 | Linolenic acid (C18:2n-6) | C19H32O2 | 912977.69 | 2.47 |
- Abbreviation: MOO, Moringa oleifera oil.
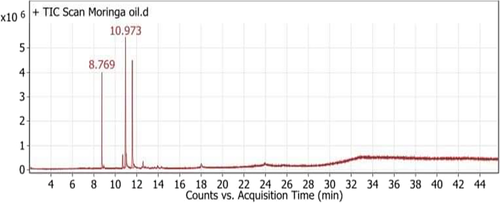
3.2 Unsaponifiable matter of MOO
The unsaponified matter of MOO showed 12 peaks. The major compounds found in oil were β-sitosterol (42.77%), stigmasterol (20.69%), campesterol (16.11%), and γ-sitosterol (8.92%). Furthermore, δ-tocopherol (2.04%) was also detected (Table 2, Figure 2).
| Peak | Retention time | Name | Formula | Area | Area Sum % |
|---|---|---|---|---|---|
| 1 | 5.794 | 1-Hexadecanol, 2-methyl- | C17H36O | 1215861.35 | 0.47 |
| 2 | 6.609 | trans-Geranylgeraniol | C20H34O | 2817297.4 | 1.08 |
| 3 | 6.965 | Heptacosane | C27H56 | 5046494.48 | 1.93 |
| 4 | 7.14 | δ-Tocopherol, TMS derivative | C30H54O2Si | 5067967.49 | 2.04 |
| 5 | 7.263 | 1-Heptatriacotanol | C37H76O | 2698321.53 | 1.03 |
| 6 | 10.698 | Campesterol, TMS derivative | C31H56OSi | 42051125.9 | 16.11 |
| 7 | 11.092 | Stigmasterol, TMS derivative | C32H56OSi | 54010793.1 | 20.69 |
| 8 | 12.024 | β-Sitosterol | C29H50O | 111 664 922 | 42.77 |
| 9 | 12.134 | γ-Sitosterol | C29H50O | 23294995.4 | 8.92 |
| 10 | 12.412 | Ergosta-5,22-dien-3-ol, acetate, (3.beta.,22E)- | C30H48O2 | 2669772.48 | 1.02 |
| 11 | 12.677 | 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, (3.beta.,5Z,7E)- | C27H44O3 | 6778034.04 | 2.6 |
| 12 | 12.897 | Stigmasta-5,22-dien-3-ol, acetate, (3.beta.)- | C31H50O2 | 3752144.49 | 1.34 |
- Abbreviation: MOO, Moringa oleifera oil; TMS derivative, trimethylsilyl ether.
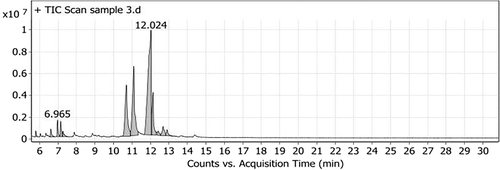
3.3 TP, TF, and TOC contents
MOO revealed the presence of TP, TF, and TOC with values of 81.51 ± 1.24 μg GAE /g oil, 44.09 ± 1.15 μg QUE/g oil and 23.12 ± 0.76 mg/100 g oil, respectively (Table 3).
| Content | MOO |
|---|---|
| TP (μg GAE/g oil) | 81.51 ± 1.24 |
| TF (μg QUE/g oil) | 44.09 ± 1.15 |
| TOC (mg/100 g oil) | 23.12 ± 0.76 |
- Abbreviations: GAE, gallic acid equivalents; MOO, Moringa oleifera oil; TP, total phenolic; TF, total flavonoids; TOC, tocopherol; QUE, quercetin equivalents.
- Note: Values denote mean ± SD for MOO analyzed individually in triplicate.
3.4 Oxidative stress parameters
For all oxidative stress parameters, no significant difference was detected among negative control, positive control, and MOO groups. Consequently, the negative control group was used for comparison with other groups.
A highly significant (p < .001) increase in malondialdehyde (MDA) levels was detected in TBT-treated group when compared to the negative control group. The combination of both TBT and MOO resulted in a highly significant (p < .001) decrease in MDA levels compared to the TBT-treated group. Contrary, TBT caused a highly significant (p < .001) decrease in GSH and total antioxidant capacity (TAC) levels compared to the negative control group. While, co-administration of TBT & MOO significantly (p < .001) increased GSH and TAC levels compared to TBT-treated group (Figure 3).
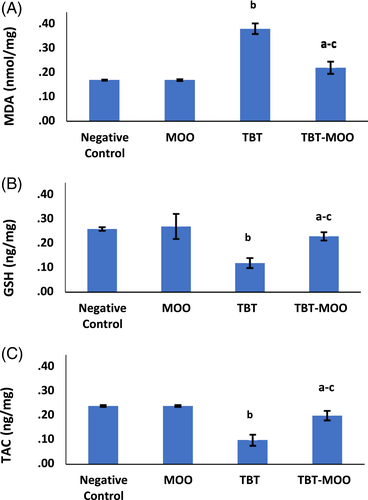
3.5 Neurological parameters
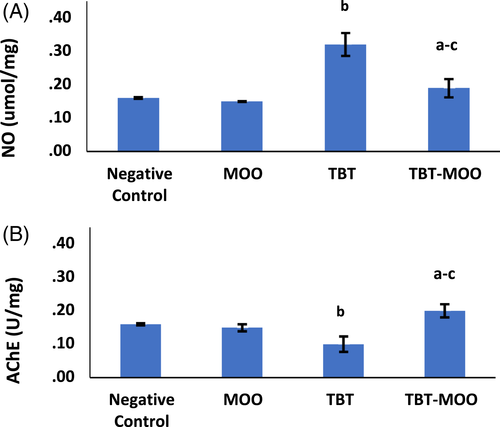
Results of NO and AChE parameters revealed no significant difference among negative control, positive control, and MOO groups Therefore, the negative control group was used for comparison with other groups. TBT resulted in a highly significant (p < .001) increase in NO level and AChE activity compared to the negative control. Treatment with a combination of both TBT and MOO significantly (p < .001) decreased both parameters (Figure 4).
3.6 Histopathology
Gross examination of brains of all groups revealed no gross anomalies. Microscopically, no structural abnormalities were detected in the negative control, positive control, and MOO groups. The cerebral cortex of negative control and MOO groups was covered by a delicate layer of pia mater and arranged into six layers: the outer molecular, external granular, external pyramidal, internal granular, internal pyramidal, and the multiform layer (Figure 5(A),(B)). The six layers showed neurons and glial cells scattered in homogenous pink neuropil. Cortical neurons were demonstrated as cells with large open face nuclei, prominent nucleoli, basophilic cytoplasm, and long processes. Glial cells were presented as small dark stained cells. Blood vessels with narrow perivascular spaces were also seen (Figure 6(A),(B)).
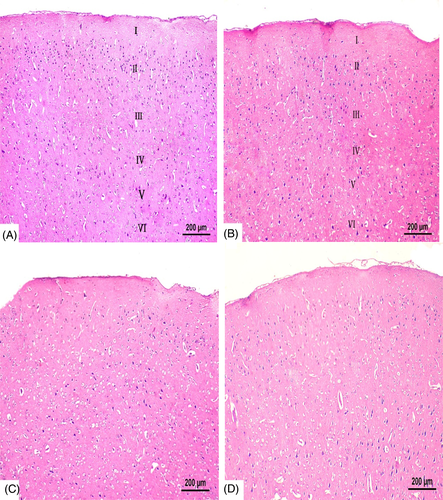
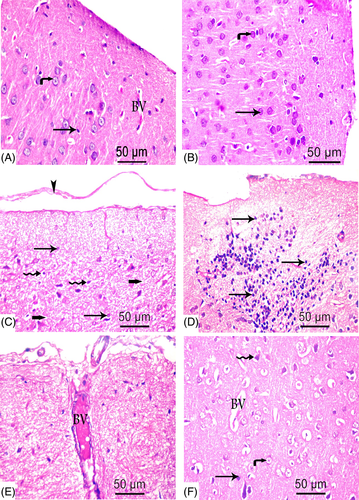
In TBT treated group, sections revealed marked disturbance in the normal structure of cerebral cortex with difficult identification of the six cortical layers (Figure 5(C)) and wide separation of Pia mater (Figure 6(C)). The cortical neurons were noted as dark stained cells surrounded by vacuolated cytoplasm and scattered in multiple vacuolated neuropil. Also, areas of gliosis and infiltration with small dark glial cells were shown. The blood vessels were dilated and congested with wide perivascular spaces (Figure 6(C)–(E)).
In the combined group, restoration of nearly normal cortical structure was evident (Figure 5(D)). Cortical sections demonstrated few dark stained cells scattered in nonvacuolated neurophil, few glial cells, minimally dilated blood vessels, and narrow perivascular spaces (Figure 6(F)).
Semi-quantitative analysis (scoring) of cerebral lesions (gliosis and vacuolation) was presented in (Table 4).
| Parameter | Treatment group (n = 6) | |||
|---|---|---|---|---|
| Group I negative control | Group III MOO | Group IV TBT | Group V TBT-MOO | |
| Gliosis | 0 | 0 | 2 | 1 |
| Vacuolation | 0 | 0 | 3 | 1 |
- Abbreviations: MOO, Moringa oleifera oil; TBT, tributyltin.
- Note: Score scale: 0 = normal; 1 = (≤25%); 2 = (26–50%); 3 = (51–75%); and 4 = (76–100%).
3.7 Immunohistochemistry
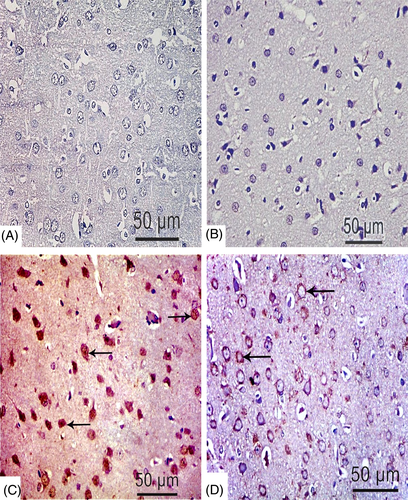
For further evaluation of apoptotic effect of TBT, immunohistochemical examinations of caspase-3, Bax, and Bcl-2 were undertaken. Neurons stained with anticaspase-3 showed dark brown staining (nuclear and cytoplasmic), whereas healthy neurons were blue with counter stain. The immunoreaction was nearly negative in the negative control and MOO groups (Figure 7(A),(B)), while a strong reaction was shown in TBT treated group (Figure 7(C)). In the combined group, moderate reaction was detected (Figure 7(D)).
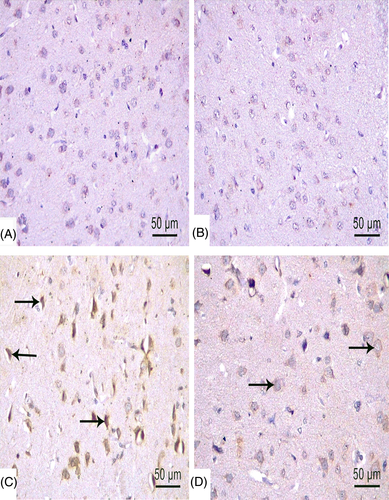
Administration of TBT increased Bax expression (Figure 8(C)) and decreased Bcl-2 expression (Figure 9(C)). On the contrary, decreased expression of Bax and increased Bcl-2 expression were observed in negative control and MOO groups (Figures 8 and 9(A),(B)). Moderate Bax and Bcl-2 expression was evident in rats administered both TBT & MOO (Figures 8(D), 9(D)).
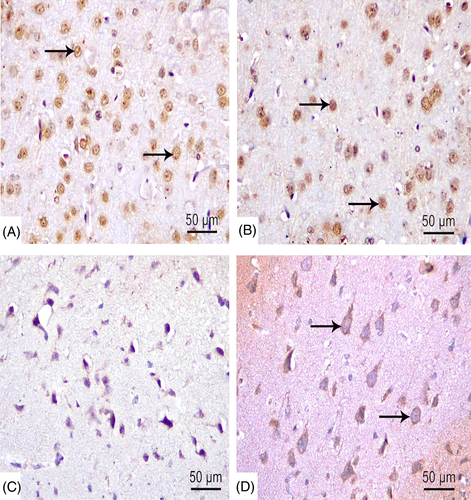
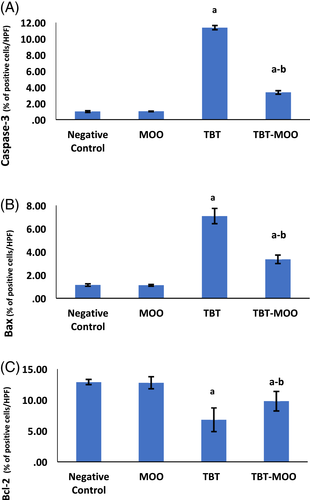
The morphometric analysis revealed no significant difference among negative control, positive control, and MOO groups. Accordingly, the negative control was used for the comparison with other groups. As to the TBT group, a highly significant (p < .001) increase in area % of immune reaction to caspase-3 and Bax was detected. The combination of TBT & MOO resulted in a highly significant (p < .001) reduction in area % of immunoreaction to caspase-3 and Bax. Contrary, the area % of immune reaction due to Bcl-2 was significantly (p < .001) higher in negative control and MOO groups than the TBT-treated group. Co-administration of MOO with TBT significantly (p < .001) increased the area % of Bcl-2 expression in comparison with the TBT-treated group (Figure 10).
4 DISCUSSION
Oleic acid, linoleic acid, and palmitic acid were the major fatty acids detected in MOO. These findings were supported by Aly et al.45 The high oleic acid content increases the nutritional value and stability of MOO during cooking and frying.46 Moreover, linoleic acid has shown beneficial effects on blood lipids, pressure and cholesterols.46 The antioxidant activity of oleic and α-linoleic acids has been reported in previous studies.27, 47
β-sitosterol, campsterol, and sigmasterol were the main phytosterols found in MOO. Our results are in line with Lalas and Tsaknis48 and Basuny and Al-Marzouq.46 Sterols exhibit cholesterol-lowering effect and potent antioxidant activity via formation of allylic free radical which is further isomerized into a more stable free radical.49
Phenolic compounds and tocopherols enhance the antioxidant potential of different vegetable oils. Therefore, determination of TP and TOC contents act as an indicator of the antioxidant capacity of the oil. 50 Compared to other methods of extraction, MOO obtained by cold pressure shows the highest content of δ-tocopherol. The δ-tocopherol possesses the highest antioxidant activity among all types of tocopherols.48 The presence of considerable amounts of TP, TOC in MOO has been reported in previous studies.45, 46 The TF content detected by this study is lower than TP content, which is contributed to the fact that flavonoids are subgroups of phenolics and contribute to the overall phenolic content.51
Administration of TBT resulted in a significant increase in MDA levels associated with a decrease in both GSH and TAC levels. These results support the oxidative stress mechanism of TBT. The presence of large amounts of polyunsaturated fatty acids as well as the neuronal sensitivity to ROS, increase brain's vulnerability to TBT-induced lipid peroxidation.52 MDA is the end product of polyunsaturated fatty acid degradation that can be used as a marker for lipid peroxidation.17
GSH is a nonenzymatic antioxidant naturally occurring in all types of cells. GSH provides a crucial defense against free radicals via the thiol group, which detoxifies H2O2 and lipid hydroperoxides. Moreover, GSH inhibits dopamine-induced oxidative damage in the brain.53 Accordingly, depletion of GSH impairs the brain's detoxification capacity and sensitizes cells to lipid peroxidation.11 The diminished GSH can be ascribed to its continuous consumption during the elimination of ROS generated by TBT.54
The TAC measures the susceptibility of cells to oxidative stress.55 The reduced antioxidant capacity is attributed to the strong direct affinity of TBT to free thiol group in GSH and its inhibitory effect on other antioxidant enzymes.54 Also, TBT diminishes zinc content in cortical tissues which reduces the brain's antioxidant capacity.9
TBT has been proved to enhance oxidative stress in various tissues including kidney,56 adrenals, and pituitary glands.57 TBT exposure has altered the redox homeostasis and depleted cortical antioxidants in rats treated by a single dose (30 mg/kg) of TBT.32 Also, in freshwater teleost, a significant elevation in brain MDA has been reported following exposure to sub lethal concentrations of TBT (75 ng/L, 0.75 μg/L, and 7.5 μg/L).52
As to the combined group, co-administration of MOO with TBT diminished MDA levels and elevated GSH and TAC levels in brain tissues. Parallel to our results, MOO (5 ml/kg) has successfully repleted cortical antioxidants (Catalase, SOD, and GSH) and diminished lipid peroxidation (MDA) in rats treated by methotrexate.11 Similarly, MOO at a dose of 1.798 mg/kg has ameliorated HgCl2-mediated oxidative stress in livers and kidneys of rats.58 Moreover, 2 ml/kg MOO has shown ability to reverse or even abolish the lipid peroxidation induced by CCl4 in testes59 and livers60 of treated rats. Likewise, Omotoso et al.23 demonstrated the ability of MOO (4 mg/kg) to restore the oxidative balance and to normalize the antioxidant enzymes in geniculate bodies of rats treated by cadmium for 3 weeks. Furthermore, Edeogu et al.37 declared the ability of MOO (5 ml/kg) to alleviate nephrotoxicity of gentamicin by lowering renal MDA and increasing renal SOD, catalase, and GSH levels.
MOO has potent antioxidants like tocopherols and sterols which are natural breakers of ROS and inhibitors of lipid peroxidation.37, 58 These antioxidants can scavenge DPPH radical and reduce Fe3+ to Fe2+ in a way comparable to the standard antioxidants.61 Tocopherols have been found to preserve the activity of SOD enzyme. Besides, sterols have inhibitory effect on generation of superoxide radicals and release of elastases from neutrophils.37 Additionally, under oxidative conditions, β-sitosterol has shown ameliorative effect on antioxidant enzymes (SOD and GSH peroxidase) in macrophages.62 Moreover, MOO contains phenolic compounds and flavonoids that are naturally occurring antioxidants with free radical scavenging activity.11, 58 These compounds are hydrogen atom donors, reducing agents, singlet and triplet oxygen quenchers, and peroxides decomposers.46 All these contents make the MOO one of the most promising natural antioxidants.
At physiological concentration, NO has a regulatory function in the brain and spinal cord. NO plays a crucial role in cell signaling, neurotransmission, and protection. Under pathological conditions, excess NO is highly toxic to cells and can initiate a cascade of nitrosative stress. NO interacts with ROS or superoxide anions resulting in formation of peroxynitrite anion (ONOO−) which causes nitrosylation of essential structures like proteins resulting in alteration of their functions.52
The present study revealed a highly significant elevation in NO level in the TBT-treated group. This result indicates the neurotoxic effect of TBT and the close association between nitrosative and oxidative stress mechanisms.11 Similar alteration has been reported in brains of female Sebastiscus marmoratus exposed to TBT (1, 10, and 100 ng) in a dose dependent manner.63 Combination of TBT and MOO could significantly reduce NO level compared to TBT-treated group. Consistent with our result, MOO has reduced NO levels in the brains of rats treated with methotrexate.11 Additionally, antioxidant assays of MO has demonstrated its obvious ability to scavenge NO and reduce ROS.29, 37
At cholinergic synapses, AChE plays a critical role in regulating acetylcholine action, neuronal excitability, synaptic transmission, synaptic plasticity, and neuronal firing. Dysfunction of AChE has been implicated in a wide range of neuropsychiatric disorders.52 In current study; TBT-treated group demonstrated a highly significant increase in AChE activity compared to the control group. Similarly, Ciona juveniles exposed to TBT has revealed a significant increase in AChE activity in nerve cells in a way comparable to those of AD and PD.64 Mansueto et al.64 has suggested a possible relationship between AChE activity and stress conditions where amplification of AChE gene might be a compensatory defense mechanism.
In combined group, AChE activity was significantly reduced when compared to TBT group. In rat models of AD, MO has demonstrated an inhibitory effect on AChE activity and stimulatory effect on cholinergic pathways and antioxidant mechanisms.65 Pathogenesis of AD is complex involving both cholinergic signaling deficit and oxidative stress.65 Furthermore, as per the study conducted by Rahmath et al.66 MO has revealed protective effect against scopolamine mediated neurotoxicity via mitigating oxidative stress and enhancing cholinergic activity. Interestingly, in the previous study,66 Co-administration of MO has reduced AChE activity even lower than that in the control group.
TBT finds its access to the brain directly via sensory organs like the retina and axonal transport or indirectly after its metabolism.67 Fortier et al.68 has observed the highest levels of TBT in the cerebral cortex, and the lowest levels in the cerebellum. Therefore, cerebral cortex is representing the target area of structural alterations caused by TBT in brain.
The histological findings of this study revealed apoptotic changes in the form of dark stained cortical neurons surrounded by vacuolated cytoplasm and scattered in vacuolated neuropil. Also, there were areas of gliosis and infiltration with small dark glial cells. The blood vessels were dilated and congested with wide perivascular spaces. Apoptotic changes can be attributed to the ROS mediated cytotoxicity.69 Our findings are in line with Mitra et al.32 where a marked increase in cortical apoptosis has been detected in rats 7 days after exposure to a single dose of TBT. Other studies have also reported similar apoptotic changes in renal cortex56 and pancreatic β-cells.70
Caspase-3 is the main effector caspase involved in apoptosis.18 Results of the current study revealed strong immunoreactivity to caspase-3 in TBT treated group and moderate reaction in the combined group. These finding were further supported by earlier studies56, 70, 71 where activation of caspase-3 has been detected in renal tubular cells, pancreatic β-cell, and hepatocytes respectively.
TBT promotes apoptosis via mitochondrial and death receptors pathways. TBT enhances the release of calcium from mitochondria with subsequent release of cytochrome c, followed by activation of caspase-9, and then caspase-3. On the other hand, the death receptors pathway can be activated by TBT via enhancing the transformation of Fas-associated death domain into death-inducing signaling complex at cell membranes. Thereafter, caspase-8 and caspase-3 are activated subsequently.71
The Bcl-2 family of proteins is another factor controlling apoptosis. Bax enhances opening of mitochondrial permeability transition pores, leading to cytochrome c release and activation of caspase-3.72 On the other hand, Bcl-2 prevents apoptosis via decreasing release of mitochondrial apoptogenic factors or segregating proforms of death-driving cysteine proteases.73 TBT group showed up regulation of Bax and down regulation of Bcl-2 immunoexpression. Similar results have been demonstrated by Zhang et al.74 and Mitra et al.32 in mouse hepatocytes and rat cerebral cortex respectively.
The combination of TBT and MOO has partially alleviated the structural alterations and apoptotic changes. In harmony, Edeogu et al.37 has reported structural alleviation in kidneys of rats treated with gentamicin and MOO. Likewise, Omotoso et al.23 has demonstrated the ability of MOO to reverse the toxic alterations induced by cadmium in the geniculate body. Mousa et al.75 have revealed the antiapoptotic effect of MO via down regulating caspase-3 activity in the liver of thioacetamide treated rats. Also, Soliman et al.19 demonstrated the ability of MO leaf extract to restore or reverse the hepato-renal altered Bax and Bcl-2 expression in mice caused by methotrexate.
5 CONCLUSION
Sub-acute exposure to TBT resulted in detrimental functional and structural alterations in brains of albino rats. The increased lipid peroxidation, depleted antioxidants, elevated NO levels, strong immunoreactivity to caspase-3, as well as the altered expression of Bax and Bcl-2 were all indicative of the oxidative, nitrosative, and apoptotic mechanisms mediated by TBT. MOO demonstrated a noticeable ameliorative effect on TBT-induced brain toxicity, mostly via potent antioxidant and antiapoptotic properties.
CONFLICT OF INTEREST
Authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




