Luteolin-7-O-glucoside inhibits cell proliferation and modulates apoptosis through the AKT signaling pathway in human nasopharyngeal carcinoma
Hsin-Yu Ho and Ping-Ju Chen contributed equally to this work.
Funding information: Changhua Christian Hospital, Grant/Award Number: 109-CCH-IRP-014; National Science Council, Taiwan, Grant/Award Number: MOST 109-2314-B-371-005
Abstract
Nasopharyngeal carcinoma (NPC) is an unnoticeable malignant tumor with a high potential of lymphatic metastasis, and its prevalence is high in Asia. Ionizing radiation is the mainstay of treatment for patients with NPC without metastasis. However, patients with metastatic lesions require advanced treatments such as chemotherapy. The present study investigated the apoptotic effect of luteolin-7-O-glucoside on NPC cells and elucidated its underlying signaling mechanisms. The results revealed that luteolin-7-O-glucoside significantly reduced the proliferation of NPC cell lines (NPC-039 and NPC-BM). Flow cytometry and morphological analysis results demonstrated that luteolin-7-O-glucoside treatment induced S and G2/M cell cycle arrest, chromatin condensation, and apoptosis. In addition, mitochondrial membrane potential was observed to be depolarized with an increasing concentration of luteolin-7-O-glucoside. Proteins involved in the extrinsic and intrinsic pathways of apoptosis, such as death receptor, caspase-3, caspase-8, caspase-9, and Bcl-2 family proteins (Bax, t-Bid, Bcl-2, and Bcl-xL), were downregulated and upregulated after treatment with luteolin-7-O-glucoside, respectively. Moreover, the addition of a PI3K/AKT inhibitor enhanced the activation of poly-ADP-ribose-polymerase (PARP) and attenuated cell viability, indicating that luteolin-7-O-glucoside induced apoptosis in NPC cells through the AKT signaling pathway. These results indicated that the apoptosis of NPC cells modulated by luteolin-7-O-glucoside may be preceded by mitochondrial depolarization, cell cycle arrest, extrinsic and intrinsic apoptosis pathway activation, and AKT signaling modulation. Thus, luteolin-7-O-glucoside can be a promising anticancer agent against human NPC.
1 INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a malignant tumor located in the nasopharynx. According to the International Agency for Research on Cancer, NPC accounted for approximately 0.7% of all newly diagnosed cancer cases in 2018.1 Compared with other tumor types, NPC is relatively infrequent. However, the global distribution of patients with NPC is extremely unbalanced. Over 80% and 9% of patients newly diagnosed as having NPC were from Asia and Africa, respectively.2 Risk factors for NPC include genetic susceptibility, Epstein–Barr virus infection, and environmental factors.3 Although NPC is potentially curable in the early stage because of locoregional control, NPC is often diagnosed in advanced stages, leading to poor prognosis.4 Moreover, approximately 6–15% of patients with NPC exhibit metastatic lesions at the time of initial diagnosis without any prior treatment, and the common metastatic sites tend to be the bones, lungs, and liver.5, 6 In patients with early-stage NPC without metastasis, ionizing radiation is the mainstay of treatment because of its high sensitivity. In addition, locoregionally advanced disease requires radiotherapy as well as chemotherapy combined with radiotherapy, such as concurrent chemoradiotherapy7 and adjuvant chemotherapy.8
Apoptosis, which is programmed cell death, plays a crucial role in biological development and homeostasis in mammals.9 Apoptosis is activated through two pathways: the death receptor–activated (extrinsic) pathway and cellular stress–activated (intrinsic) pathway. Cysteine aspartyl-specific proteases (caspases) execute the apoptosis process through cleaved target proteins. In addition, the B-cell lymphoma-2 (Bcl-2) protein family, including proapoptotic and antiapoptotic proteins, is particularly involved in the intrinsic pathway of apoptosis.10 These processes remove unnecessary or unwanted cells when DNA damage or uncontrolled cell proliferation occurs. However, apoptosis was found to be inhibited in various cancers. Dysfunction in apoptosis controls cell repair and prolongs cancer cell survival, thus leading to increased mutations, angiogenesis, and tumor metastasis and interfering with cell differentiation.11 Cancer cells evade apoptosis through caspase inactivation and apoptotic protein dysregulation, which result in tumor cells resisting apoptotic stimuli or some anticancer drugs such as cisplatin.12 Hence, targeting apoptosis is regarded as the most effective nonsurgical cancer therapy. The inhibition of the X-linked inhibitor of apoptosis protein (XIAP), an antiapoptosis protein, could induce tumor apoptosis in gastric cancer,13 advanced prostate cancer,14 and pancreatic cancer.15 Moreover, Bcl-2 inhibitors, such as venetoclax, combined with obinutuzumab were used as the first-line therapeutic agent in patients with chronic lymphocytic leukemia.16
In recent years, natural products derived from medicinal herbs have been reported to possess anticancer properties, including curcumin,17 resveratrol,18 and paclitaxel.19 Flavonoids, a large group of polyphenolic secondary metabolites commonly found in vegetables and fruits, have demonstrated their potential in the prevention and treatment of various diseases.20-22 Luteolin-7-O-glucoside, also known as cynaroside, is a glycosyloxyflavone derived from luteolin. Because of its antioxidative property, luteolin-7-O-glucoside has been reported to scavenge 2,2-diphenyl-1-picrylhydrazyl radicals in lipopolysaccharide (LPS)-stimulated RAW264.7 cells23 and free radicals in colon cancer cells.24 In addition, luteolin-7-O-glucoside could improve cisplatin-induced side effects by reducing cisplatin-induced nephrotoxicity.25 The anticancer property of luteolin-7-O-glucoside has been primarily associated with the inhibition of metastasis26 and cell proliferation27 and the induction of apoptosis.28 Although studies have reported the antiproliferative and chemopreventive effects of luteolin-7-O-glucoside, its anticancer effect on NPC has not yet been studied. Hence, the present study investigated the apoptotic effect of luteolin-7-O-glucoside on NPC and elucidated the underlying molecular mechanisms.
2 MATERIALS AND METHODS
2.1 Chemicals
Luteolin-7-O-glucoside was purchased from Sigma-Aldrich (St. Louis, MO, USA). The preparation of stock was dissolved in dimethyl sulfoxide (DMSO), while the final concentration of DMSO for all treatments were contained less than 0.1%. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and 4′,6-diamidino-2-phenylindole (DAPI, DNA stain dye) were purchased from Sigma Aldrich. Z-VAD-FMK, pan-caspase inhibitor, was purchase from InvivoGen (San Diego, CA, USA). Primary antibodies including cdc2 (cat. No. 9116), phospho-cdc2 (Tyr15) (cat. No. 4539), CDK 2 (cat. No. 2546), CDK 4 (cat. No. 12790), CDK 6 (cat. No. 3136), cyclin A2 (cat. No. 4656), cyclin B1 (cat. No. 12231), p21 (cat. No. 2947), cleaved caspase-3 (cat. No. 9664), cleaved caspase-8 (cat. No. 9496), cleaved caspase-9 (cat. No. 9505), cleaved PARP (cat. No. 9542), t-Bid (cat. No. 2002), Bcl-2 (cat. No. 2872), Bcl-xL (cat. No. 2764), Bax (cat. No. 2772), DR5 (cat. No. 8074), Fas (cat. No. 4233), TNF-R1 (cat. No. 3736), RIP (cat. No. 3493), phospho-AKT (cat. No. 4060), AKT (cat. No.4298), phospho-p38 (cat. No. 9211), p38 (cat. No. 9212), phospho-ERK1/2 (cat. No. 4370), ERK1/2(cat. No. 4695), phospho-JNK1/2 (cat. no. 4668), and JNK1/2 (cat. no. 9258) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). β-actin were purchased from Novus Biologicals (Littleton, CO). Specific inhibitors for ERK (U0126), p38 (SB203580), JNK (SP600125) and PI3K/AKT (LY294002) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2 Cell culture
Human NPC cell lines NPC-039 and NPC-BM cells were the gifts from Dr. Jen-Tsun Lin, Hematology & Oncology, Changhua Christian Hospital. NPC-039 and NPC-BM cells were cultured in RPMI 1640 Medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1 mM glutamine, 1.5 g/L sodium bicarbonate, and 1 mM sodium pyruvate (Sigma). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
2.3 Cell cytotoxicity assay
NPC-039 and NPC-BM were plated in 96-well plates and treated with various concentrations (0, 20, 40, and 80 μM) of luteolin-7-O-glucoside for 24, 48 or 72 hours. Then, cells were incubated with 0.5 mg/mL MTT reagent at 37°C in 5% CO2 incubator for 4 hours. After removing the supernatant, DMSO was added to dissolve the formed blue formazan crystals. The absorbance of the converted dye was measured at 595 nm using microplate reader (BioTek, Winooski, VT).
2.4 Colony-formation assay
The colony formation assay was described previously.29 Cell lines were seeded at a concentration of 5 × 103 cells/mL in 6-well cell culture plates. Then cells were incubated with media contained luteolin-7-O-glucoside at indicated concentrations. Incubation medium changed every 3 days. After two weeks, colonies were fixed with 4% paraformaldehyde, then stained with 0.5% crystal violet and counted by a stereomicroscope. Colonies of greater than 50 cells were counted.
2.5 Cell cycle analysis
Cells were plated into the 6-well plate, then treated with indicated concentrations of luteolin-7-O-glucoside for 24 hours. After drug treatments, cells were collected by centrifugation and fixed in 70% ethanol for 12 h at −20°C. After fixation, ethanol was removed and the cells were suspended into PI buffer (BD Biosciences, San Jose, CA) for 30 min at room temperature in dark. Cell cycle analysis was evaluated by BD Accuri C6 Plus flow cytometry (BD Biosciences), while data were analyzed by BD CSampler Plus software (BD Biosciences).
2.6 DAPI staining
Cells were plated in a 6-well plate for 18 h, then treated with indicated concentrations of luteolin-7-O-glucoside for 24 h. Cells were fixed with 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.1% (v/v) Triton X-100. After washed twice in PBS, cells were stained with DAPI (50 μg/mL) for 15 min at room temperature. Morphological changes were then photographed using fluorescence microscopy (Lecia, Bensheim, Germany). Percentage of cells was scored on at least 500 cells.
2.7 Annexin V/PI double staining assay
Cells were plated into 6-well plates for 18 h, then treated with indicated concentrations of luteolin-7-O-glucoside for 24 h. Cells were harvested and suspended in PBS contained 2% BSA, and then incubated with Muse Annexin V & Dead Cell reagent (EMD Millipore) for 20 min at room temperature and avoid light. Collected cells were analyzed by Muse Cell Analyzer flow cytometry (EMD Millipore) and by MUSE 1.4 Analysis software (EMD Millipore).
2.8 Mitochondrial membrane potential (ΔΨm) measurement
Cells were plated in 6-well plates and incubated with luteolin-7-O-glucoside for 24 h. The cells were harvested, washed and suspended in Muse MitoPotential working solution at 37°C for 20 min. After that, cells were incubated with 7-ADD at room temperature. Samples were monitored by Muse Cell Analyzer flow cytometry (EMD Millipore) and data were assessed using Muse 1.4 Analysis Software (EMD Millipore).
2.9 Western blotting analysis
Cells were lysed with RIPA buffer containing protease inhibitor cocktail and phosphatase inhibitor cocktail (EMD Millipore). Appropriated amounts of protein were separated on a 10% or 12.5% polyacrylamide gel and transferred onto PVDF membranes (EMD Millipore). The membranes were incubated with 5% skim milk for 1 h at room temperature, and probed with the primary antibodies (All primary antibodies were diluted in 1:1000) at 4°C overnight. After that, membranes were washed in TBS-T three times, and then incubated with an appropriate peroxidase-conjugated secondary antibody at room temperature for 1 h. The immunoreactivity signal was assessed using an enhanced chemiluminescence detection system (EMD Millipore), and the relative density was quantitated with gel documentation and analysis (AlphaImager 2000; Alpha Innotech Corporation, San Leandro, CA). The protein levels were normalized by a comparison with the β-actin levels.
2.10 Statistical analysis
Statistical analyses were performed using GraphPad Prism Software Version 5.0 (GraphPad Software Inc., La Jolla, CA). All the experiments were repeated at least three times, and quantitative data are expressed as the mean ± SD. One-tailed Student's t test was used to compare the differences between two groups (control and drug concentration). P-values of <.05 were considered to represent statistically significant changes.
3 RESULTS
3.1 Cytotoxic effect of luteolin-7-O-glucoside on human nasopharyngeal carcinoma cell lines
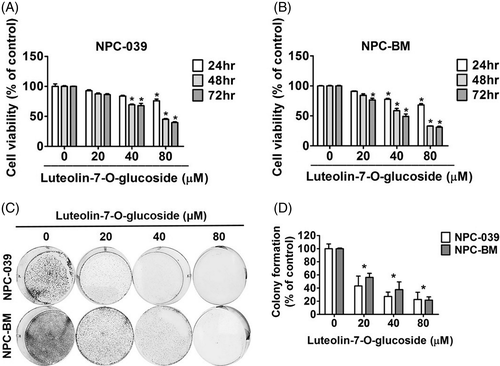
To determine the antitumor effects of luteolin-7-O-glucoside on NPC, the cell viabilities of two NPC cell lines, NPC-039 (Figure 1(A)) and NPC-BM (Figure 1(B)), were analyzed by performing an MTT assay. The two NPC cell lines were treated with 0, 20, 40, and 80 μM of luteolin-7-O-glucoside for 24, 48, and 72 hours, respectively. Both NPC-039 and NPC-BM exhibited a steady decline in cell viability in a dose- and time-dependent manner. The results of a colony formation assay showed that the number of cell colonies were significantly decreased to 20% in cells treated with a high dose of luteolin-7-O-glucoside compared with control cells (Figure 1(C) and (D)). These findings indicate that luteolin-7-O-glucoside could reduce cell proliferation in NPC cell lines.
3.2 Luteolin-7-O-glucoside causes apoptosis in NPC-039 and NPC-BM cell lines
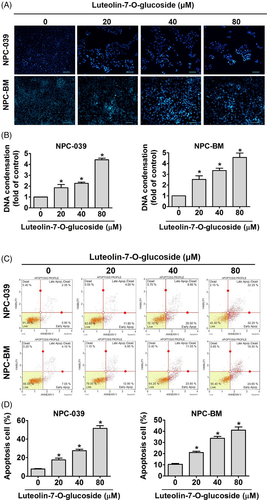
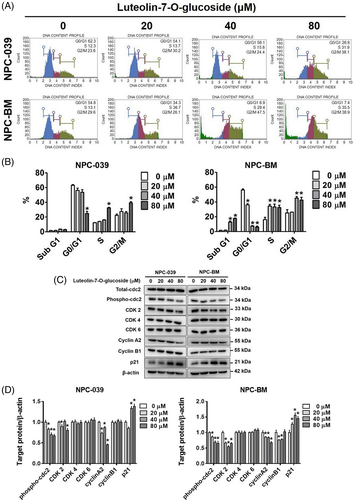
To explore the apoptotic effects of luteolin-7-O-glucoside on NPC cell lines, we examined the cell cycle, annexin V/PI ratio, and chromatin condensation through flow cytometry and DAPI staining. The numbers of both NPC-039 and NPC-BM cells were increased in the S phase and G2/M phases but decreased in the G0/G1 phases after treatment with luteolin-7-O-glucoside (Figure 2(A)). In addition, the distribution of NPC-BM cells was increased in the sub-G1 phase (Figure 2(B)). Cell cycle related proteins were performed by Western blot analysis to further clarify the effect of luteolin-7-O-glucoside which involves in regulating cell cycle arrest. As shown in Figure 2(C) and (D), we observe that the protein expression levels of phosphorylated cdc-2 (also known as cyclin-dependent kinase 1, CDK 1), CDK 2 and cyclin A2 were decreased, while p21 was increased after luteolin-7-O-glucoside treatment. Besides, the protein expression levels of luteolin-7-O-glucoside treated cyclin B1, CDK 4, and CDK 6 were remain unchanged compared with those of untreated cells. Chromatin condensation is a morphological hallmark of apoptosis; chromatin changes from a heterogeneous to a highly condensed form during apoptosis.30 Figure 3(A) and (B) shows that after treatment with different concentrations of luteolin-7-O-glucoside, DNA condensation increased by two- and five-fold in NPC-039 and NPC-BM cell lines, respectively. In addition, the population of annexin V-positive NPC-039 and NPC-BM cells, including early and late apoptotic cells, increased to 54.45% and 43.95% after treatment with a high dose of luteolin-7-O-glucoside, respectively (Figure 3(C) and (D)). These findings indicate that luteolin-7-O-glucoside could activate apoptotic processes in NPC cell lines.
3.3 Luteolin-7-O-glucoside induces cell apoptosis through mitochondrial membrane potential regulation and the death receptor pathway
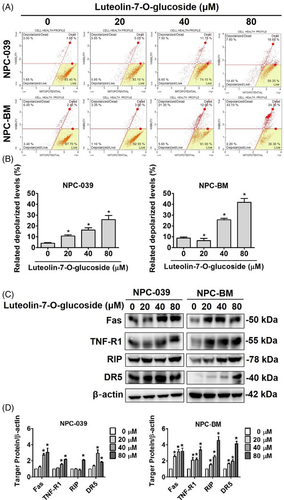
Loss of mitochondrial membrane potential is regarded as an apoptosis index because it is linked to the initiation and activation of apoptosis.31 To confirm whether luteolin-7-O-glucoside affected mitochondrial membrane potential in NPC cell lines, the Muse MitoPotential Kit and Muse Cell Analyzer were used in this study. As shown in Figure 4(A), increased cell depolarization was observed in both cell lines. After treatment with different concentrations of luteolin-7-O-glucoside, the percentage of depolarized NPC-039 and NPC-BM cells increased to approximately 25% and 40%, respectively (Figure 4(B)). To elucidate the mechanism underlying the activation of apoptosis, we examined the death receptor pathways, including the death receptor 5 (DR5) pathway; the first active signal (Fas) pathway; and the tumor necrosis factor (TNF) pathway. After treatment with luteolin-7-O-glucoside, a significant increase was observed in the protein levels of TNF receptor-1 (TNF-R1) and DR5 (Figure 4(C)). In addition, the protein levels of Fas and RIP were upregulated after treatment with luteolin-7-O-glucoside. The results indicated that luteolin-7-O-glucoside-induced apoptosis may be regulated through the death receptor pathway. In addition, when the expression of death receptor proteins was compared between two NPC cell lines, NPC-BM cells were found to be more sensitive to luteolin-7-O-glucoside treatment compared with NPC-039 cells (Figure 4(D)).
3.4 Luteolin-7-O-glucoside treatment activates the intrinsic pathway in NPC cell lines
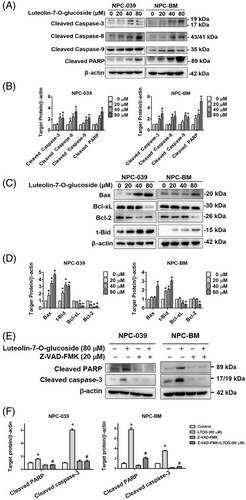
Because luteolin-7-O-glucoside induced the death receptor pathway of apoptosis, the activation of the intrinsic pathway of apoptosis was also examined. The cleaved forms of caspase-3, caspase-8, caspase-9, and PARP were significantly increased by more than 2-fold relative to the control in both NPC-039 and NPC-BM cell lines after treatment with luteolin-7-O-glucoside (Figure 5(A) and (B)). The expressions of proapoptotic and antiapoptotic proteins were also affected by luteolin-7-O-glucoside (Figure 5(C)). Treatment with luteolin-7-O-glucoside upregulated proapoptotic proteins, namely Bax and truncated-Bid (t-Bid), and downregulated antiapoptotic proteins, namely Bcl-2 and Bcl-xL, in a dose-dependent manner (Figure 5(D)). To further confirm whether luteolin-7-O-glucoside regulated apoptosis mechanism in NPC cells, the pan-caspase inhibitor (Z-VAD-FMK) which directly regulates caspases activation was used for subsequent examination. As shown in Figure 5(E) and (F), the suppression of cleaved PARP and cleaved casepase-3 exhibited that luteolin-7-O-glucoside treatment group significantly induced cleaved PARP and cleaved caspase-3 while Z-VAD-FMK/luteolin-7-O-glucoside co-treatment group showed restoration of these pro-apoptotic proteins. The results revealed that luteolin-7-O-glucoside induced apoptosis through caspase activation in NPC cells.
3.5 Luteolin-7-O-glucoside treatment regulates the AKT pathway in NPC cell lines
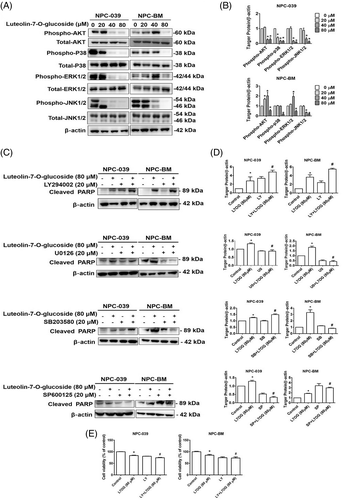
The mitogen-activated protein kinase (MAPK) pathway and AKT pathway are signaling cascades that regulate extracellular and intracellular signaling. To examine the molecular mechanism of luteolin-7-O-glucoside in NPC cell lines, the protein levels of AKT, p38, ERK1/2, and JNK1/2 were measured through Western blot (Figure 6(A)). As shown in Figure 6(B), treatment with luteolin-7-O-glucoside significantly reduced the protein levels of the phosphorylated form of p38, ERK1/2, JNK1/2, and AKT in a dose-dependent manner. To further determine the main signaling cascade that leads to luteolin-7-O-glucoside-induced apoptosis, inhibitors of each protein were co-treated with luteolin-7-O-glucoside in two NPC cell lines, respectively. As shown in Figure 6(C) and (D), cleaved PARP was upregulated after treatment with the PI3K/AKT inhibitor LY294002 alone while the co-treatment with LY294002 and luteolin-7-O-glucoside induced the protein expression of cleaved PARP in both NPC-039 and NPC-BM cell lines. Moreover, cell viability demonstrated the attenuation in LY294002/ luteolin-7-O-glucoside co-treatment group compared with luteolin-7-O-glucoside treatment group (Figure 6(E)). These results indicate that luteolin-7-O-glucoside regulated apoptosis in NPC cell lines through the AKT pathway.
4 DISCUSSION
Although many cancer therapeutic agents have been discovered in the past few decades, numerous unknown therapeutic agents have yet to be explored. In the present study, we demonstrated the apoptotic effect of luteolin-7-O-glucoside, a flavonoid, on NPC cell lines. The present study investigated the effects of different concentrations of luteolin-7-O-glucoside (20, 40, 80 μM) on NPC cell lines and compared the results with those of untreated cells. The MTT and colony formation assays were first performed to examine the cytotoxic effect of luteolin-7-O-glucoside (Figure 1). The results indicated that luteolin-7-O-glucoside reduced the proliferation of NPC cells; this finding is in agreement with those of previous studies reporting its antiproliferative effect on cancer cells.28, 32 A study reported that luteolin-7-O-glucoside reduced cell proliferation through apoptotic processes in cervical cancer.33
We then examined whether luteolin-7-O-glucoside reduced cell proliferation by regulating the cell cycle and inducing apoptosis in NPC cell lines. We examined the cell cycle, chromatin condensation, and apoptosis in NPC cells lines after treatment with luteolin-7-O-glucoside. The results demonstrated that treatment with luteolin-7-O-glucoside induced S and G2/M phase cell cycle arrest in both NPC-039 and NPC-BM cell lines (Figure 2(A) and (B)). This observation is consistent with a previous study which reported that luteolin-7-O-glucoside induced cytotoxicity through G2/M phase cell cycle arrest in human liver cancer.32 Studies have indicated that S phase progression is controlled by cyclin A/CDK 2, whereas G2/M phase progression is controlled by cyclin B/cdc2 complex.34, 35 Our study reveals that luteolin-7-O-glucoside-induced S phase and G2/M phase arrest might control by cell cycle regulating proteins (p-cdc, CDK2 and cyclin A2) (Figure 2(C) and (D)). Also, cyclin-CDK inhibitor p21, the major inhibitor that suppresses the binding of cyclin and CDK complexes, was found increasing in luteolin-7-O-glucoside treated NPC cells. Moreover, the present study is the first to report the presence of sub-G1 cell cycle arrest in NPC-BM cells after treatment with luteolin-7-O-glucoside. Interestingly, some studies have reported that luteolin, a precursor compound of luteolin-7-O-glucoside, regulated sub-G1 cell cycle arrest and apoptosis in breast, colon, and cervical cancer cells.36-38 These findings are similar with our observation that luteolin-7-O-glucoside induced sub-G1 cell cycle arrest in NPC-BM cells. Chromatin condensation and apoptotic cells were found to be increased after treatment with luteolin-7-O-glucoside (Figure 3). In addition, the finding that luteolin-7-O-glucoside increased apoptotic cells is in line with that reported by Shao et al.33
Mitochondria plays a crucial role in the apoptotic process. The key events of mitochondria-controlled apoptosis include changes in mitochondrial transmembrane potential and mitochondrial membrane permeabilization that result in the release of apoptotic proteins, such as caspase activators and pro- and anti-apoptotic Bcl-2 family proteins.39 We examined changes in mitochondrial membrane potential in our study. Treatment with luteolin-7-O-glucoside induced depolarization of the mitochondrial membrane potential in a dose-dependent manner in NPC cell lines (Figure 4(A) and (B)). To further confirm the apoptotic effect of luteolin-7-O-glucoside, the apoptosis pathways were investigated. The extrinsic and intrinsic pathways of apoptosis both lead to cell death by activating cell signaling cascades. The extrinsic pathway is initiated by proapoptotic ligands that interact with cell surface death receptors, such as the TNF receptor, Fas receptor, death receptor 4, and DR5.40 These ligand–receptor interactions can independently form the death-inducing signaling complex (DISC) by recruiting RIP, the TNF-R1-associated death domain, and the Fas-associated death domain, thus resulting in caspase-8 activation.41, 42 Our results demonstrated that luteolin-7-O-glucoside significantly induced the protein expressions of Fas, TNF-R1, RIP, and DR5 (Figure 4(C) and (D)). After the cleavage of the cytosolic proapoptotic protein Bid into t-Bid by activated caspase-8, t-Bid translocated into mitochondria, thus transducing apoptotic signals from the cytoplasm to mitochondria.43 Apoptotic stimulation leads to the activation of Bax and Bak, and these activated proteins are then translocated from the cytosol to mitochondria.44 A previous study reported that aspirin-induced apoptosis occurred through the activation of caspase-8/Bid and Bax in gastric cancer.45 The expression of cleaved caspase-8, t-Bid, and Bax was upregulated in luteolin-7-O-glucoside–treated cell lines, indicating that luteolin-7-O-glucoside regulated apoptosis through the extrinsic pathway (Figure 5). In the intrinsic pathway, Bcl-2 family members, including proapoptotic and antiapoptotic proteins, play crucial roles in regulating the activation of the apoptotic process. Our results revealed that luteolin-7-O-glucoside reduced the expression level of antiapoptotic proteins, namely Bcl-xL and Bcl-2 (Figure 5(C) and (D)). A previous study demonstrated that luteolin-7-O-glucoside treatment downregulated Bcl-2 in cervical cancer cells.33 The cascade of caspase activation in both extrinsic and intrinsic pathways leads to the cleavage of PARP,46 which is consistent with our observation that the expressions of caspase-3, caspase-8, caspase-9, and cleaved PARP increased after luteolin-7-O-glucoside treatment in NPC cells (Figure 5(A) and (B)). In additionally, our study provides evidence that luteolin-7-O-glucoside regulated apoptotic process through caspase activation in NPC cells by caspase inhibitor, namely Z-VAD-FMK, which function has been referred to several type of apoptotic induction studies.47, 48 (Figure 5(E) and (F)).
Luteolin-7-O-glucoside may affect the regulation of the MAPK signaling pathway in different cancer types. Zhou et al. reported that luteolin-7-O-glucoside induced autophagy through the AKT/mTOR/p70S6K pathway in lung cancer cells.49 A previous study revealed that luteolin-7-O-glucoside modulated NFκB/AP-1/PI3K-AKT signaling cascades in LPS-induced RAW264.7 cells.50 In the present study, we found that luteolin-7-O-glucoside reduced the activation of AKT, JNK1/2, ERK1/2, and p38 (Figure 6(A) and (B)). To examine direct anticancer signaling in luteolin-7-O-glucoside– treated NPC cells, we used signaling inhibitors (LY294002, SP600125, SB203580, and U0126). As shown in Figure 6(C) and (D), the induction of cleaved PARP demonstrated that luteolin-7-O-glucoside regulated apoptosis through the AKT signaling pathway. Moreover, the cotreatment of LY294002/luteolin-7-O-glucoside further attenuated luteolin-7-O-glucoside caused cytotoxicity, which reveals that luteolin-7-O-glucoside regulated cell viability via AKT pathway in NPC cells (Figure 6(E)). In cervical cancer cells, luteolin-7-O-glucoside mediated apoptosis through the p38 and JNK1/2 signaling pathways33; this finding differs from our results. Thus, we inferred that luteolin-7-O-glucoside regulates different MAPK signaling in different types of cancer cells. However, additional studies are required to verify this inference.
In conclusion, the results of the present study indicated that luteolin-7-O-glucoside regulated the cell cycle and the extrinsic and intrinsic pathways of apoptosis in NPC cells. In addition, luteolin-7-O-glucoside-induced apoptosis in NPC cells was modulated by the AKT signaling pathway. To the best of our knowledge, ours is the first study to elucidate the molecular mechanism underlying luteolin-7-O-glucoside-induced apoptosis in NPC. These findings indicate that luteolin-7-O-glucoside can be a promising anticancer agent.
ACKNOWLEDGMENTS
This study was supported by grants from National Science Council, Taiwan (MOST 109-2314-B-371-005) and Changhua Christian Hospital (109-CCH-IRP-014). The authors of the manuscript do not have a direct financial relation with the commercial identity mentioned in this article.
AUTHOR CONTRIBUTIONS
Conceptualization: Ming-Ju Hsieh and Mu-Kuan Chen. Methodology: Hsin-Yu Ho, Yu-Sheng Lo, Chia-Chieh Lin, Yi-Ching Chuang. Software: Yu-Sheng Lo, Chia-Chieh Lin, Yi-Ching Chuang. Writing—original draft preparation: Hsin-Yu Ho and Ping-Ju Chen. Writing—review and editing: Ming-Ju Hsieh and Mu-Kuan Chen.
CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




