Effects of low-dose melamine exposure during pregnancy on maternal and fetal kidneys in rats
Funding information: Studies Related to Melamine Incident from Food and Health Bureau, Hong Kong Special Administration Region, Grant/Award Number: MI-BS-06.
Abstract
Despite the previous reports on melamine contamination in high concentrations some years ago, there were not many studies on low-level exposure in daily life, particularly in pregnancy. We investigated the effect of low-dose melamine on the kidneys of the pregnant rats and their developing embryos/fetuses during various gestational stages namely implantation, gastrulation, organogenesis, maturation and whole pregnancy. Our results showed that the repeated low level of melamine (12.5, 25, and 50 mg/kg bw/d) during pregnancy did not cause obstruction of renal tubules although more precipitating crystals were found in the early gestational periods. Simple hyperplasia in the maternal tubules and pelvic epithelium were more prominent after exposed to melamine during the whole gestational period. Neonatal kidneys significantly suffered more from congestion in glomeruli and interstitium, dilated tubules and interstitial edema after melamine administration to the mother in the late and the whole gestational periods. A trend of advance of glomerular development in fetuses was also observed. We conclude that in utero exposure of low-level melamine could post a risk on the kidneys of the pregnant mother as well as the developing fetuses, which may further increase the possibility of other health problems later in life.
Abbreviations
-
- ANOVA
-
- Analysis of variance
-
- Bwt
-
- body weight
-
- CAS
-
- Chemical Abstracts Service
-
- FDA
-
- Food & Drug Administration (US)
-
- OECD
-
- Organization for Economic Co-operation and Development
-
- TDI
-
- Tolerable Daily Intake
-
- WHO
-
- World Health Organization
1 INTRODUCTION
The first study of the melamine toxicity was carried out in the 1980s, which revealed solely its metabolic fate. The results showed that most of the ingested melamine was excreted in urine per se.1 Although subchronic and chronic studies reported that melamine can cause carcinomas in urinary bladder, but the doses studied were very high.2 Not until the melamine incidences in 2007, little toxicology data were available. Even though more discussion has been raised since then, they focused mostly on the nephrotoxicity, in both human and animals.3-9 More recent studies also include the neurotoxicity,10-12, reproductive toxicity,13, 14 and genotoxicity15, 16 of melamine. In spite of the victims were mostly infants, adult subjects were always chosen in the experiments.17-21 Few studies in pregnant animal models have been reported.22-25
Melamine-adulterated dairy products were the major sources of human contact in the milk scandal early in this millennium.8, 9, 26 They can be the infant formulae that serve as the only food source of some of the infants, as well as the dietary supplements for some of the pregnant mothers and school children. Nonetheless, melamine indeed exists in many forms in our household and is commonly found in food-contact materials, most importantly the “melaware”, from which leakage of melamine was detected in many studies.27-32 Although the melamine contamination in high dose has ceased, the increased risk of damages to the mothers and fetuses and its developmental adverse outcomes during pregnancy at an intake level lower than the WHO recommendation should be studied.
We have previously demonstrated the transfer of low-dose melamine through placenta and mammary gland in a rat model,33, 34 characterized the toxicokinetics of melamine in the pregnant rats, the fetuses and the neonates,35 and have also proven the reproductive effects in utero.36 The other published studies of reproductive and developmental effects of melamine in rats have longer administration period, ranging from 8 to 21 days22-25 but few have focused on the nephrotoxicity effect of the contaminant. Nevertheless, deciphering its effects on the individual developmental window is still lacking. In this study, we would like to further investigate the nephrotoxic effects of melamine during different gestational and developmental periods on maternal and fetal kidneys under the repeated maternal intake of low-dose melamine.
2 MATERIALS AND METHODS
2.1 Chemicals
Melamine (CAS Number 108-78-1, Cat No. M2659) was purchased from Sigma-Aldrich (St Louis, MO).
2.2 Animals
Sprague-Dawley rats were used in this study. Pregnant rats were obtained from the Laboratory Animal Services Centre, the Chinese University of Hong Kong. Ethics approval for the study was obtained from the Animal Experimentation Ethics Committee of the University (AEEC numbers: 08/059/MIS and 08/072/MIS). Animals were housed at 25°C ± 2°C with a 12-hour light-dark cycle, with water and food ad libitum.
2.3 Dosage
Three different doses, with reference to the dose in the previous experiment as 12.5, 25, and 50 mg/kg body weight/d,35 plus 1 vehicle control were used in this study. Our doses used are 20%, 40%, and 80% of the NOAEL for rats, that is, 63 mg/kg.37
2.4 Grouping and administration
This study was a repeated administration study. The length of melamine administration is determined by the length of the developmental windows investigated. The pregnancy duration of the rat is usually 22.5 days. Five developmental windows were chosen in this study, namely: (1) implantation (gestational day 5.5–8.5, E5.5-E8.5), (2) gastrulation (gestational day 8.5–10.5, E8.5-E10.5), (3) organogenesis (gestational day 10.5–16.5, E10.5-E16.5), (4) maturation (gestational day 16.5-delivery, E16.5-delivery), and (5) whole gestational period (gestational day 0.5-delivery, E0.5-delivery).
Previous data showed the gestational stage-specific toxicokinetics of melamine in maternal serum and its distribution in fetal and neonatal organs during pregnancy.35 The combination of these developmental windows cover the examination periods for the reproductive and developmental effects of melamine on early pregnancy, embryo-fetal development, prenatal growth, and the whole gestation.
The animals were randomly assigned to the 3 dose groups or to the control group. Gavage was carried out using a gastric tube, with daily weight-adjusted volume, from the beginning of the experiment to the day before sacrifice. Water consumption was also recorded daily. Loss of weight and appetite, mortality, any signs of morbidity and abortion, pertinent behavioral changes and overt toxicity were noted. Animals showing signs of abortion or premature delivery would be euthanized despite the scheduled day of sacrifice. The dams of the maturation and the whole gestation groups were sacrifice on the day of delivery to record any effect of melamine during pregnancy. Nonpregnant rats were excluded.
2.5 Sample collection
After delivery, the dams were euthanized and examined for gross morphological changes. Maternal kidneys were collected; vertical and horizontal dimensions were measured and weighed. Neonates were weighed, sacrificed by decapitation, and examined for gross morphological changes. Neonatal kidneys were collected; their dimensions were measured and weighed. Kidneys were fixed, embedded in paraffin and sectioned for histological examination.
2.6 Histological examination of maternal and neonatal kidneys
Maternal and neonatal kidneys were removed bilaterally, dissected transversely into equal halves, fixed and sectioned for histological examinations. Melamine crystals in the sections were screened by the birefringence property of melamine precipitations under polarizing light microscope. Pathological changes of melamine renal toxicity, including dilated tubules, crowding of nuclei and hyperplasia38 were examined by routine hematoxylin and eosin staining under optical microscope. Melamine crystal and the pathological features were presented as percentage of the number of sections with positive finding over the total number of sections examined in the dose group. For neonatal kidneys, medullary rays were identified and a pair of neighboring medullary rays that were closest to the middle of the section and extended to the medullar was selected. The differentiating glomeruli, including primitive (appearance of the renal vesicle), S-shaped (twisting nephron), transitional (mature nephron, without capillary loops), and mature (thinning of the epithelium and presence of capillary loops), confined by the selected pair of medullary rays were counted in each section.39 Percentages of different stages of glomeruli over the total number of glomeruli were compared. Other pathological changes were also recorded.
2.7 Statistical analysis
Sample size was calculated based on the anticipated difference in decreased fetal growth parameters as 0.04 and the anticipated standard deviations as 0.01 for at least 4 per treatment group with type I error 0.01 and power 90%. Homogeneity of variances was tested by Bartlett's examination. Homogenous data analyzed using 1-way ANOVA (parametric) or Kruskal-Wallis tests with post hoc Bonferroni correction amongst group comparison. Significant difference between each test group and control group was analyzed using Student's independent t test. Nonhomogenous data and the counting of the histological changes were analyzed using Chi-square test. Analysis of the control against all the treatment groups was done first. If significance was detected in the Pearson's test, comparison by Chi-square between the control group and the individual group was done separately.
3 RESULTS
3.1 General conditions
Maternal weight was measured every day for the calculation of melamine gavage. Water intake, appetite, mortality and morbidity, and so on were observed before the daily gavage. Signs of those clinical observation was not noticed, except a drop of maternal weight (30 g, around 13%) of half of the 50 mg/kg body weight/d rats of the E0.5-delivery group from the third day concurrent with a drop of water intake (data not shown). The increase in weight gain caught up quickly within 3 days without other noticeable morbidity. No adverse effects or maternal mortality were recorded in any dose group.
3.2 Maternal kidneys
Gestational effects of melamine on maternal kidney in various gestational periods are summarized in Table 1. Size and weight of the maternal kidneys in any dose groups were not much significantly different from the control group, except during maturation period that the kidney weight was significantly increased in the 50 mg/kg bwt/d group. No obvious gross morphological changes were found.
| Control group | Study group | |||
|---|---|---|---|---|
| 0 mg/kg/d | 12.5 mg/kg/d | 25 mg/kg/d | 50 mg/kg/d | |
| Implantation period: E5.5–8.5 | N = 9 | N = 8 | N = 8 | N = 8 |
| Litter size (mean ± SD) | 13.33 ± 1.73 | 13.63 ± 0.92 | 13.0 ± 1.69 | 12.88 ± 1.13 |
| Kidney length, vertical (mm; mean ± SD) | 17.40 ± 0.87 | 17.55 ± 1.0 | 17.42 ± 1.02 | 17.27 ± 0.95 |
| Kidney length, horizontal (mm; mean ± SD) | 10.84 ± 0.85 | 10.81 ± 0.79 | 10.79 ± 1.21 | 10.26 ± 1.23 |
| Kidney weight (g; mean ± SD) | 0.99 ± 0.08 | 0.97 ± 0.12 | 0.98 ± 0.06 | 0.95 ± 0.14 |
| Kidney-to-body weight (%; mean ± SD) | 0.39 ± 0.04 | 0.39 ± 0.04 | 0.40 ± 0.04 | 0.39 ± 0.05 |
| No of kidney sections for examination | n = 12 | n = 12 | n = 12 | n = 12 |
| Crystal in cortex (%) | 0 (0%) | 1 (8.3%) | 4 (33.3%)‡ | 6 (50%)‡‡‡ |
| Crystal in medulla (%) | 0 (0%) | 0 (0%) | 6 (50%)‡‡‡ | 6 (50%)‡‡‡ |
| Dilated tubules (%) | 0 (0%) | 1 (8.3%) | 0 (0%) | 0 (0%) |
| Crowding of nuclei, tubular (%) | 0 (0%) | 4 (33.3%)‡ | 5 (41.7%)‡ | 3 (25%) |
| Crowding of nuclei, inter-tubular (%) | 0 (0%) | 2 (16.7%) | 4 (33.3%) | 1 (8.3%) |
| Hyperplasia, tubular (%) | 0 (0%) | 3 (25%) | 6 (50%)‡‡‡ | 2 (16.7%) |
| Hyperplasia, pelvic epithelium (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gastrulation period: E8.5–10.5 | N = 8 | N = 8 | N = 8 | N = 8 |
| Litter size (mean ± SD) | 13.75 ± 3.06 | 12.63 ± 1.30 | 13.13 ± 2.03 | 12.25 ± 1.49 |
| Kidney length, vertical (mm; mean ± SD) | 17.87 ± 0.97 | 18.0 ± 1.22 | 17.67 ± 1.16 | 17.80 ± 0.91 |
| Kidney length, horizontal (mm; mean ± SD) | 10.93 ± 1.03 | 10.92 ± 0.86 | 10.92 ± 0.86 | 10.57 ± 0.51 |
| Kidney weight (g; mean ± SD) | 0.98 ± 0.09 | 1.04 ± 0.12 | 0.99 ± 0.07 | 1.02 ± 0.07 |
| Kidney-to-body weight (%; mean ± SD) | 0.37 ± 0.02 | 0.39 ± 0.04 | 0.39 ± 0.02 | 0.40 ± 0.04 |
| No of kidney sections for examination | n = 12 | n = 12 | n = 12 | n = 12 |
| Crystal in cortex (%) | 0 (0%) | 7 (58.3%)‡‡‡ | 9 (75%)‡‡‡ | 6 (50%)‡‡ |
| Crystal in medulla (%) | 1 (8.3%) | 3 (25%) | 5 (41.7%) | 5 (41.7%) |
| Dilated tubules (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Crowding of nuclei, tubular (%) | 1 (8.3%) | 2 (16.7%) | 4 (33.3%) | 4 (33.3%) |
| Crowding of nuclei, inter-tubular (%) | 0 (0%) | 2 (16.7%) | 4 (33.3%) | 2 (16.7%) |
| Hyperplasia, tubular (%) | 1 (8.3%) | 1 (8.3%) | 5 (41.7%) | 3 (25%) |
| Hyperplasia, pelvic epithelium (%) | 0 (0%) | 0 (0%) | 2 (16.7%) | 2 (16.7%) |
| Organogenesis period: E10.5-E16.5 | N = 8 | N = 8 | N = 8 | N = 8 |
| Litter size (mean ± SEM) | 12.38 ± 4.0 | 14.25 ± 1.58 | 12.88 ± 1.46 | 13.75 ± 1.04 |
| Kidney length, vertical (mm; mean ± SD) | 17.27 ± 0.83 | 17.13 ± 0.68 | 17.80 ± 1.18 | 17.37 ± 0.73 |
| Kidney length, horizontal (mm; mean ± SD) | 10.88 ± 0.78 | 10.82 ± 1.07 | 11.24 ± 0.92 | 11.13 ± 0.84 |
| Kidney weight (g; mean ± SD) | 1.02 ± 0.09 | 0.96 ± 0.06 | 1.09 ± 0.16 | 1.03 ± 0.10 |
| Kidney-to-body weight (%; mean ± SD) | 0.34 ± 0.30 | 0.33 ± 0.03 | 0.38 ± 0.05 | 0.34 ± 0.03 |
| No of kidney sections for examination | n = 12 | n = 12 | n = 12 | n = 12 |
| Crystal in cortex (%) | 0 (0%) | 3 (25%) | 3 (25%) | 0 (0%) |
| Crystal in medulla (%) | 0 (0%) | 1 (8.3%) | 5 (41.7%) | 1 (8.3%) |
| Dilated tubules (%) | 0 (0%) | 1 (8.3%) | 1 (8.3%) | 0 (0%) |
| Crowding of nuclei, tubular (%) | 1 (8.3%) | 3 (25%) | 5 (41.7%) | 1 (8.3%) |
| Crowding of nuclei, inter-tubular (%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 1 (8.3%) |
| Hyperplasia, tubular (%) | 1 (8.3%) | 2 (16.7%) | 3 (25%) | 0 (0%) |
| Hyperplasia, pelvic epithelium (%) | 0 (0%) | 2 (16.7%) | 1 (8.3%) | 2 (16.7%) |
| Maturation period: E16.5-delivery | N = 14 | N = 9 | N = 12 | N = 9 |
| Litter size (mean ± SD) | 11.79 ± 2.63 | 12.33 ± 1.94 | 11.33 ± 2.31 | 13.33 ± 2.29 |
| Kidney length, vertical (mm; mean ± SD) | 16.0 ± 1.11 | 16.62 ± 0.80 | 16.07 ± 0.93 | 16.46 ± 0.82 |
| Kidney length, horizontal (mm; mean ± SD) | 9.75 ± 0.87 | 10.12 ± 0.64 | 9.74 ± 1.14 | 10.48 ± 0.61 |
| Kidney weight (g; mean ± SD) | 0.82 ± 0.11 | 0.90 ± 0.06 | 0.82 ± 0.12 | 0.93 ± 0.11* |
| Kidney-to-body weight (%; mean ± SD) | 0.34 ± 0.03 | 0.38 ± 0.05 | 0.33 ± 0.04 | 0.39 ± 0.08* |
| No of kidney sections for examination | n = 24 | n = 16 | n = 21 | n = 16 |
| Crystal in cortex (%) | 1 (4.2%) | 1 (6.3%) | 2 (16.7%) | 4 (25%) |
| Crystal in medulla (%) | 0 (0%) | 2 (12.5%) | 0 (0%) | 4 (25%) |
| Dilated tubules (%) | 2 (8.3%) | 0 (0%) | 8 (38%) | 0 (0%) |
| Crowding of nuclei, tubular (%) | 2 (8.3%) | 3 (18.8%) | 3 (14.3%) | 4 (25%) |
| Crowding of nuclei, inter-tubular (%) | 2 (8.3%) | 3 (18.8%) | 2 (16.7%) | 1 (6.3%) |
| Hyperplasia, tubular (%) | 2(8.3%) | 2 (12.5%) | 4 (19.0%) | 3 (18.8%) |
| Hyperplasia, pelvic epithelium (%) | 1 (4.2%) | 2 (12.5%) | 2 (16.7%) | 1 (6.3%) |
| Whole gestational period: E0.5-delivery | N = 16 | N = 8 | N = 14 | N = 9 |
| Litter size (mean ± SD) | 10.8 ± 2.30 | 12.13 ± 2.30 | 9.29 ± 3.20 | 11.33 ± 1.66 |
| Kidney length, vertical (mm; mean ± SD) | 16.37 ± 1.17 | 16.74 ± 0.82 | 16.32 ± 0.83 | 16.78 ± 0.65 |
| Kidney length, horizontal (mm; mean ± SD) | 9.60 ± 0.99 | 10.52 ± 0.76* | 9.66 ± 0.89 | 10.50 ± 0.47* |
| Kidney weight (g; mean ± SD) | 0.83 ± 0.12 | 0.94 ± 0.22 | 0.84 ± 0.11 | 0.92 ± 0.56 |
| Kidney-to-body weight (%; mean ± SD) | 0.35 ± 0.04 | 0.40 ± 0.07* | 0.33 ± 0.03 | 0.40 ± 0.05* |
| No of kidney sections for examination | n = 28 | n = 16 | n = 27 | n = 16 |
| Crystal in cortex (%) | 0 (0%) | 3 (18.8%) | 2 (7.4%) | 0 (0%) |
| Crystal in medulla (%) | 0 (0%) | 1 (6.25%) | 2 (7.4%) | 3 (18.8%) |
| Dilated tubules (%) | 2 (7.1%) | 0 (0%) | 4 (14.8%) | 1 (6.3%) |
| Crowding of nuclei, tubular (%) | 2 (7.1%) | 4 (25%) | 3 (11.1%) | 3 (18.8%) |
| Crowding of nuclei, inter-tubular (%) | 1 (3.6%) | 2 (12.5%) | 1 (3.7%) | 3 (18.8%) |
| Hyperplasia, tubular (%) | 1 (3.6%) | 6 (37.5%)‡‡‡ | 4 (14.8%) | 4 (25%)‡ |
| Hyperplasia, pelvic epithelium (%) | 0 (0%) | 0 (0%) | 4 (14.8%)‡ | 5 (31.3%)‡‡‡ |
- One-way ANOVA with post hoc Student t test: *P < .05, compared with 0 mg/kg bwt/d with Bonferroni correction; Chi-Square test: ‡P < .05, ‡‡P < .01, ‡‡‡P < .005, compared with 0 mg/kg.
Crystals were more common in the renal cortex than in the renal medulla, and significantly increased by the melamine administration in the implantation and the gastrulation periods. The crystals found in the study was tiny that did not obstruct the renal tubules as shown in other studies using higher concentration of melamine or with combination with cyanuric acid.3, 40-42 No colocalization of crystals and other histological changes was seen.
Focally dilated tubules, mainly in the cortex, were more commonly found in the kidney sections with longer melamine administration, but they were not significantly different from the control group. No atypical hyperplasia, but simple hyperplasia, was found. Some of the transitional cell hyperplasia in the pelvic epithelium appeared to be of the papillary type, instead of the multiple-layered type (Figure 1, upper panel). The incidence of the simple hyperplasia significantly increased in renal cortex during the implantation period and the whole pregnancy period; and in pelvic epithelium during the whole pregnancy period only. Some of the hyperplastic tubules were also dilated. There is a significant increase in tubular hyperplasia of the 25 mg/kg bwt/d of the implantation group as well as the pelvic epithelial hyperplasia in the 12.5 mg/kg bwt/d of the whole gestation group. An extensive basophilic simple hyperplasia with tubular dilation spanning from the outer cortex to the inner medulla was identified in the 25 mg/kg bwt/d of the maturation period group (Figure 1, lower panel).
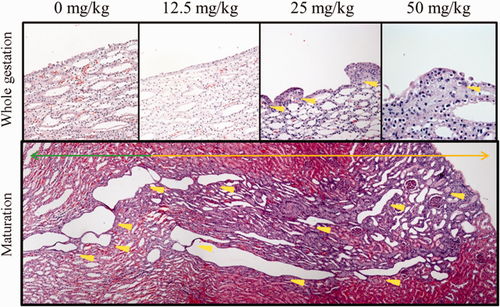
Histological change of maternal melamine nephrotoxicity. Upper panel: simple pelvis epithelium hyperplasia in maternal kidneys in the whole gestation group; yellow arrow heads denote epithelium hyperplasia. Lower panel: an extensive tubular hyperplasia in a maternal kidney of maturation group, 25 mg/kg dose; orange arrow denotes simple hyperplasia in cortex, green arrow simple hyperplasia in medulla, yellow arrow heads dilated tubules. Magnification: 20× [Color figure can be viewed at wileyonlinelibrary.com]
Intertubular crowding of nuclei denotes recruitment of inflammatory cells. There was a general increase in the incidence of the treatment group but not statistically significant. Other pathological changes included crescentic glomeruli (E0.5-delivery, 12.5 mg/kg bwt/d), parenchymal detachment, that is, shedding of the epithelial cells of the apical membrane or the necrostic debris of the tubules into the lumens (E5.5-E8.5, 25 mg/kg bwt/d; E8.5-E10.5, 25 and 50 mg/kg bwt/d; E0.5-Delivery, 50 mg/kg bwt/d), and interstitial blood congestion (E8.5-E10.5, 25 mg/kg bwt/d).
3.3 Neonatal kidneys
Gestational effects of melamine on neonatal kidney in various gestational periods are summarized in Table 2. Significant increases in size and weight of neonatal kidney were identified after maternal exposure of melamine in both the maturation (12.5 and 25 mg/kg bwt/d) and the whole gestation periods (25 and 50 mg/kg bwt/d). No obvious dose-dependent effects were observed or any major kidney malformation noticed.
| Control group | Study group | |||
|---|---|---|---|---|
| 0 mg/kg/d | 12.5 mg/kg/d | 25 mg/kg/d | 50 mg/kg/d | |
| Maturation period: E16.5-delivery | ||||
| Kidney length, longer dimension (mm; mean ± SD) | 4.79 ± 0.29 | 4.95 ± 0.51* | 4.89 ± 0.26* | 4.80 ± 0.31 |
| Kidney length, shorter dimension (mm; mean ± SD) | 3.26 ± 0.29 | 3.36 ± 0.41* | 3.28 ± 0.26 | 3.29 ± 0.31 |
| Kidney weight (mg; mean ± SD) | 26.23 ± 4.92 | 30.56 ± 20.69* | 27.24 ± 5.34 | 24.99 ± 4.72 |
| No of sections examined | 260 | 160 | 168 | 218 |
| Blood-congested glomeruli (%) | 43 (16.5%) | 6 (3.8%)‡‡‡ | 81 (48.2%)‡‡‡ | 98 (45.0%)‡‡‡ |
| Interstitial blood-congestion (%) | 85 (32.7%) | 17 (10.6%)‡‡‡ | 77 (45.8%)‡‡ | 75 (34.4%) |
| Dilated tubules (%) | 38 (14.6%) | 11 (6.9%) ‡ | 71 (42.3%) ‡‡‡ | 70 (32.1%)‡‡‡ |
| Interstitial edema (%) | 9 (3.4%) | 6 (3.8%) | 12 (7.1%) | 59 (27.1%)‡‡‡ |
| No of glomeruli examined | 2161 | 1335 | 1257 | 1485 |
| Primitive glomeruli (%) | 774 (35.8%) | 432 (32.4%) | 458 (36.4%) | 474 (31.9%)* |
| S-shaped glomeruli (%) | 495 (22.9%) | 253 (19.0%)* | 262 (20.8%) | 295 (19.9%) |
| Transitional glomeruli (%) | 656 (30.4%) | 456 (34.2%) | 400 (31.8%) | 553 (37.2%)* |
| Mature glomeruli (%) | 236 (10.9%) | 194 (14.5%)* | 137 (10.9%) | 163 (11.0%) |
| Crystal in cortex (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Whole pregnancy: e0.5-delivery | ||||
| Kidney length, longer dimension (mm; mean ± SD) | 5.23 ± 0.39 | 5.17 ± 0.29 | 5.41 ± 0.26* | 5.28 ± 0.29 |
| Kidney length, shorter dimension (mm; mean ± SD) | 3.49 ± 0.28 | 3.53 ± 0.29 | 3.52 ± 0.34* | 3.52 ± 0.29* |
| Kidney weight (mg; mean ± SD) | 30.70 ± 4.70 | 30.69 ± 4.17 | 34.04 ± 10.41 | 32.85 ± 4.89 |
| No of sections examined | 260 | 214 | 205 | 179 |
| Blood-congested glomeruli (%) | 17 (6.5%) | 17 (7.9%) | 39 (19.0%)‡‡‡ | 31 (17.3%)‡‡‡ |
| Interstitial blood-congestion (%) | 67 (25.8%) | 128 (59.8%)‡‡‡ | 88 (42.9%)‡‡‡ | 95 (53.1%)‡‡‡ |
| Dilated tubules (%) | 18 (6.9%) | 20 (9.4%) | 58 (28.3%)‡‡‡ | 36 (20.1%)‡‡‡ |
| Interstitial edema (%) | 8 (3.1%) | 8 (3.7%) | 30 (14.6%)‡‡‡ | 23 (12.9%)‡‡‡ |
| No of glomeruli examined | 2103 | 1801 | 1862 | 1510 |
| Primitive glomeruli (%) | 699 (33.2%) | 657 (36.5%) | 608 (32.7%) | 482 (31.9%) |
| S-shaped glomeruli (%) | 481 (22.9%) | 364 (20.2%) | 390 (20.9%) | 327 (21.7%) |
| Transitional glomeruli (%) | 695 (33.0%) | 621 (34.5%) | 643 (34.5%) | 503 (33.3%) |
| Mature glomeruli (%) | 228(10.8%) | 159 (8.8%) | 221 (11.9%) | 198 (13.1%) |
| Crystal in cortex (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
- One-way ANOVA with post hoc Student t test: *P < .05, compared with 0 mg/kg, with Bonferroni correction; Chi-Square test: ‡P < .05, ‡‡P < .01, ‡‡‡P < .005, compared with 0 mg/kg.
No crystals were found in the neonatal kidney sections. There was a general increase in the incidences of congestion in glomeruli and interstitium (Figure 2), dilated tubules (Figure 3), and interstitial edema (Figure 4) in both the maturation and the whole pregnancy periods, and was significant between the test groups and the control. The relative risks, the probability of having a certain observation in the test group divided by that of the control group, increased from 0.23 to 2.73 (congested glomeruli), 0.32 to 1.05 (interstitial congestion), 0.47 to 2.20 (dilated tubules), and 1.12 to 7.97 (interstitial edema) between the lowest dose and the highest dose of the maturation groups. The relative risks increased from 1.22 to 2.66 (congested glomeruli), 2.31 to 2.05 (decreased, interstitial congestion), 1.36 to 2.91 (dilated tubules), and 1.19 to 4.16 (interstitial edema) between the lowest dose and the highest dose of the whole gestation period groups. Extra finding like thickening of the glomerular basement membrane (E16.5-delivery, 12.5 mg/kg bwt/d) and blood congestion in tubules were rarely found, but only in the whole gestational period (E0.5-delivery, 12.5 and 50 mg/kg bwt/d). Extensive hyperplasia with dilated tubules was found in 2 of the neonatal kidneys, mainly E0.5-delivery, 50 mg/kg bwt/d and E16.5-delivery, 12.5 mg/kg bwt/d.
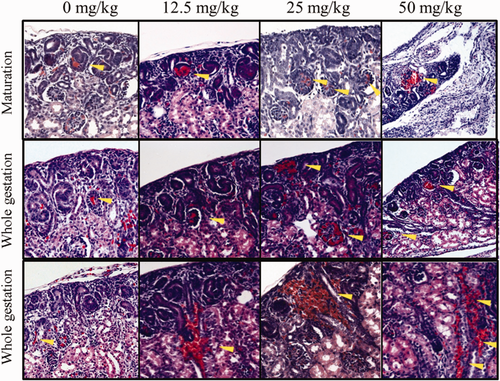
Histological change of prenatal melamine nephrotoxicity. Upper 2 panels: congestion in glomeruli in the maturation and the whole gestation groups; lower panel: congestion in interstitium in neonatal kidneys in the whole gestation group. Magnification: 20× [Color figure can be viewed at wileyonlinelibrary.com]
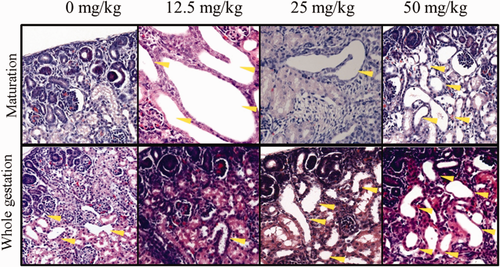
Dilated tubules in neonatal kidneys in the maturation and the whole gestation groups. Magnification: 40× [Color figure can be viewed at wileyonlinelibrary.com]
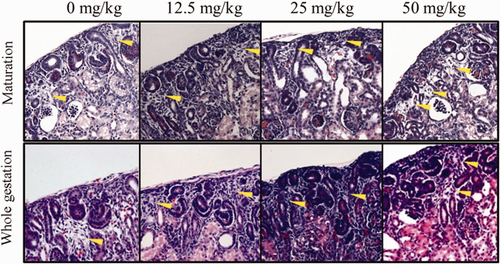
Interstitial edema in neonatal kidneys in the maturation and the whole gestation groups. Magnification: 40× [Color figure can be viewed at wileyonlinelibrary.com]
Observation of the glomerular development revealed the percentage of primitive glomeruli was generally lower in treatment groups than the control, while those of the transitional and the mature glomeruli were higher in the treatment groups, which is more prominent in the 50 mg/kg bwt/d groups, but not dose-dependently. This suggested an accelerated growth of the developing fetal kidneys under maternal melamine influence.
4 DISCUSSION
This study is looking into the gestational stage-specific effects of melamine during pregnancy. Also we have chosen the low-dose melamine so as to be more similar to the authentic contamination of dairy products and housewares.
4.1 Maternal nephrotoxicity
Under the current low-dose condition, melamine did not cause gross change in morphology in any of the developmental windows. More crystals were found in the rats of the earlier developmental periods, which may due to lower resorption rate in early pregnancy compared with the late pregnancy. Kidney stones are uncommon in normal pregnancy, that is, about 1 in 1500 and more frequently in late pregnancy43, 44; however, change in renal physiology during pregnancy can increase the rate of hydronephrosis, dilated collecting tubes, increase in kidney length and volume of kidney but decrease in kidney weight,45 which may indicate an increase in the susceptibility to challenges by any nephrotoxic substances in different gestational periods.
Observation of the dilated tubules and simple hyperplasia suggested degeneration/regeneration of the renal tubules caused by xenobiotic toxicity. Mitotic figures can sometimes be seen in the simple hyperplastic tubules, which also denoted the active regeneration of the tubular epithelium. Besides, chemical-induced nephrotoxicity can result in tubular necrosis that can be recognized by the karyorrhexis and the absence of nucleus of the tubular epithelium. The toxic effects caused by melamine under these experimental conditions without tubular necrosis suggested that the damage was comparatively mild and the renal tubules can still self-repair the damage. Crescentic glomeruli were found only in one of the maternal kidney in the of 12.5 mg/kg bwt/d of the whole pregnancy group. There has been no report on melamine-related glomerular crescents so far, which is characteristic of rapid progressive glomerulonephritis. This piece of information in our study may suggest that melamine may be able to cause glomerular injury in addition to the tubular injuries.
Recruitment of inflammatory cells was not very common in this study, which usually occurs around the ruptured tubules in acute kidney injury or the necrotic glomerulus that the Bowman's capsule is lost. Inflammatory cells were only found in the interstitium in our study in the absence of any rupture of the renal tubules or necrotic glomeruli, suggesting low-dose melamine only induced mild inflammation in the maternal kidneys.
4.2 Neonatal nephrotoxicity
In this study, absence of crystals may be due to the very low concentration of melamine in the neonatal kidneys as well as the fact that fetal kidney is not the main organ for waste removal, but placenta. Our previous toxicokinetic study on the melamine distribution, by quantifying the melamine concentration in tissue homogenates, showed that melamine did not accumulate in the neonatal kidneys collected in the study as much as that in the adult kidneys.35 As a result, crystallization of melamine in the neonatal kidney should be is rarer than that in the maternal kidney.
Kim et al.23, 24 has carried out 2 detailed studies, one with doses of melamine much higher than that in this study and another administering melamine together with cyanuric acid. Both studies covered the external, the visceral and the skeletal alterations of the affected fetuses; no histological changes were found in the fetal kidneys. In a study conducted by Jingbin et al.22 employed a lower dose of melamine, but no remarkable changes were noticed in the fetal kidneys either.
From our histological finding, we see that there was an increase in relative risks in tubular and glomerular damage of the neonates receiving melamine in utero. Interstitial and glomerular congestion suggested that there may be some renal dysfunction and glomerular damages. The very low concentration of melamine in the fetal kidney may be one of the major reasons why the histopathological changes were so rare and mild. In our study, we also assessed the kidney maturation. Although the Jingbin's study has mentioned the maturation of the glomeruli, no comparison between the control and the dose groups was make.22
Unlike human infants whose kidney development complete in utero by gestational week 36, the number of rat nephrons is still increasing after birth. The renal developmental stage of rats at birth to postnatal week 2 is similar to that of the human second and third trimesters. Many factors correlate to a decrease in number of glomeruli, for example, uteroplacental insufficiency. In this experiment, we proposed an accelerated renal development in the melamine-affected neonatal kidney. Accelerated renal development is shown to be associated with maternal renal dysfunction caused by bilateral ureteral ligation and maternal uninephrectomy.46, 47 Maternal uninephrectormy-related accelerated glomerular development and the accelerated glomerular function will lead to stimulation on the development of the proximal tubules, dilation of the tubular lumen and differentiation of the tubular epithelium of the loops of Henle.48, 49 Accelerated glomerular maturity is also observed in preterm human infants during the postnatal catch-up growth. From the autopsy of these infants, reduced number of less developed glomeruli, increased cross-section area of the glomeruli, increased number of morphologically abnormal glomeruli as well as increased in kidney-to-body weight ratio were observed.50 However, whether or not it was the result of the preterm birth or the administration of glucocorticoids before preterm delivery, and whether the increase in glomerular volume is physiological or pathological are still unknown. Even if melamine does not show direct toxicity on the developing fetus, the medical conditions on the mother per se may also have indirect nephrotoxic effects on the fetal kidneys. More detail studies are required to understand the underlying mechanism of advanced nephrogenesis under maternal low-dose melamine exposure. Nevertheless, damages to the tubular development may reduce the number of the functional nephrons later in life, which in turn may associate with high blood pressure and reduction in the insulin sensitivity.51 Long-term follow-up of the melamine-affected infants is advisable.
Higher dose of melamine may result in urinary tract obstruction as reported in the clinical findings in the period of the milk scandal.52-54 Infant urinary tract obstruction can lead to end-stage renal disease in children. The outcome depends on the time of obstruction, whether prenatal or postnatal. Prenatal obstruction altered the structure of the growing kidneys and thus their function whereas the postnatally obstructed kidneys showed normal structure.55 The gestational stage that the obstruction starts to take place also determines the outcome. When it occurs at early gestation (<day 55 of the 147-day ovine pregnancy), nonatrophic growth impairment was resulted; when the obstruction occurs at late gestation, hyperplastic and hypertrophic growth were accelerated. On the other hand, loss of renal mass was noted in postnatal renal obstruction.56, 57 Hyperplastic compensatory renal growth can be observed in the contralateral kidney opposite to a growth-impaired, obstructed fetal kidney.58 These structural changes of the prenatal obstruction include excessive interstitial connective tissue, primitive-appearing peritubular mesenchymal cells, abnormal glomeruli, cystic structures, simple interstitial fibrosis, subcapsular cystic dilation and epithelial hyperplasia.59 However, the short pregnancy course of rodents may not allow enough time to detect the nephropathology described in the ovine studies.
Although advancement of detection methods60-67 and the monitoring of food safety can help reduce the incidence of high melamine adulteration, low-level unintentional melamine contamination of the food chain by giving food animals melamine-laced animal feed, degradation of the pesticide cyromazine,68-70 migration from melamine-ware and breakdown of melamine foam may still post risk of the contact of the compound. Detection of melamine in the breast milk, whole fetus, and amniotic fluid indicated the contact of melamine through the placental and the lactational transfer, that is, the melamine contact can be as early as in utero and some of the melamine is recirculating in the amniotic fluid in rats.33-35 Histological changes of the maternal and the neonatal kidneys due to the melamine intake may be reversible but the accelerated kidney development may bring about renal problems later in adult life. However, more evidences have to be produced to fill the gap. In 2009, Hsieh's group has suggested a revision of the TDI of melamine for the vulnerable group of the infants to 0.0081 mg/kg bwt/d71; Li's group has also reported the risk of having nephrolithiasis is 1.7 times higher in the children receiving < 0.2 mg/kg bwt/d, which is the TDI recommended by the WHO in 2008.72
5 CONCLUSIONS
In this study, we conclude that in utero exposure of low-level melamine could post a risk on the kidneys of the pregnant mother as well as the developing fetuses. Melamine may cause some underlying toxic effects that the previous clinical reports have been overlooked. Longer follow-up and examination of other organ systems may be needed for the sake of the melamine-affected infants.




