Exposure to cadmium during gestation and lactation affects development and function of Leydig cells in male offspring
Funding information: National Natural Science Foundation of China, Grant Award Number: 31271607, 81373313; the Science & Technology Major Project of Guangdong province, Grant Award Number: 2012A080201010, 2013B051000063; the Guangdong Province Higher Vocational Colleges & School Pearl River Scholar Funded Scheme.
Abstract
Toxic effects of maternal exposure to Cadmium (Cd) on Leydig cells of male offspring arises much concern recently, but its toxic effects on the development of Leydig cells and androgen synthesis have not been elucidated. In this study, female rats were exposed to Cd during gestation and lactation, and the development of Leydig cells in the first filial-generation (F1) male rats was investigated. The steroidogenic signaling pathway and biomarkers related to the development of Leydig cells were detected to disclose how maternal Cd-exposure caused reproductive damage. F1 male rats with maternal Cd-exposure gained a low relative weight of testis and declined levels of steroid hormones. Maternal Cd-exposure interrupted the development of Leydig cells with high expression of SRD5α and cell morphology of immature Leydig cells in adulthood, inhibited the activation of cyclic adenosine monophosphate/ protein kinase A signaling pathway and down-regulated the steroidogenic enzymes. These results would help to disclose the origin of male sexual dysfunction in the developmental stages of Leydig cells.
1 INTRODUCTION
Cadmium (Cd) is an environmental toxicant widely used in many occupations including electroplating, automobile manufacturing, metallurgical industry, Ni-Cd batteries, welding, and so on.1 The increase of industrial waste, urban garbage and chemical fertilizer using cause heavy metal contamination in land and water. It has been reported that Cd can accumulate in multiple organs including kidney, liver, pancreas and testis with a long biological half-life in mammals.2 Cd is considered to be one of the common contaminants which have toxic effects on male reproductive function.3, 4 The high dose of Cd caused interstitial edema, hemorrhage and necrosis in testes, accompanied by damage of sperm cells and their precursors in seminiferous tubules.5, 6 Increasing evidence has proved that exposure to Cd is relative to male infertility and the poor semen quality.7 In the study of Ji et al.,8 after investigating the effects of pubertal Cd-exposure on testicular development and spermatogenesis, they found that pubertal Cd-exposure markedly reduced the weights of testes and the level of testosterone in serum, reduced the expression of steroidogenic acute regulatory protein (StAR), P450 cholesterol side-chain cleavage enzyme (P450scc/CYP11A1), cytochrome P450 17α-hydroxylase (P45017α/CYP17A1) and 17β-hydroxysteroid dehydrogenase (17β-HSD) in mRNA and protein levels in Leydig cells.
Leydig cells are testosterone-producing cells locating in testicular interstitium among the seminiferous tubule. Four distinct stages in the development of Leydig cell have been identified: the stem Leydig cells (SLCs), progenitor Leydig cells (PLCs), immature Leydig cells (ILCs) and adult Leydig cells (ALCs).9, 10 SLCs usually appear at postnatal day (PND) 7 in spindle-shape with few smooth endoplasmic reticulums (SERs). α-Type platelet-derived growth factor receptor (PDGFRA) is expressed in SLCs. But the Leydig cell-specific markers, including 3β-HSD, CYP17A1, or luteinizing hormone receptor (LHR) are not expressed in SLCs. By PND 14, SLCs give rise to PLCs which express PDGFRA, LHR, 3β-HSD, CYP17A1, and 5α-Reductase1 (SRD5α) and very low level of 11β-HSD. PLCs are capable of synthesis of androgen. PLCs transform to ILCs from spindle-shape to oval form by PND 28. PDGFRA, LHR, 3β-HSD, CYP17A1, 11β-HSD, and SRD5α are expressed in ILCs. ILCs develop a capacity of steroidogenesis,11 but testosterone is not the major steroid production. ILCs proliferate once to produce ALCs from PND 28–56. When Compared with ILCs, ALCs contain greater amount of smooth ER and testosterone biosynthetic enzymes. ALCs express PDGFRA, LHR,3β-HSD,CYP17A1, and 11β-HSD but less level of SRD5α. In response to the stimulation of LH, ALCs are continuously producing high levels of testosterone.12
In testosterone synthesis, LH binds to the LHR, then stimulatory G proteins (Gs) is activated.13 The active G subunit subsequently binds and stimulates adenylate cyclase to raise the cyclic adenosine monophosphate (cAMP) level. StAR is targeted by protein kinase A (PKA),14 which is activated by cAMP. StAR transports cholesterol to inner mitochondrial membrane, where CYP11A1 metabolizes the cholesterol to pregnenolone. Pregnenolone subsequently is metabolized to testosterone by enzymes of the SER: 3β-HSD, CYP17A1, and 17β-HSD.12
However, effects of maternal Cd-exposure on the development of Leydig cells and androgen synthesis in the first filial-generation (F1) have not been elucidated. In this study, female rats were exposed to Cd during gestation and lactation, and the development of Leydig cells in F1 male rats was investigated. The steroidogenic signaling pathway and biomarkers related to the development of Leydig cells were detected to disclose how maternal Cd-exposure caused reproductive damage in male offspring. These results provided further understanding for the impact of maternal Cd-exposure on testicular hypoplasia in mammalian offspring.
2 MATERIALS AND METHODS
2.1 Animals and treatment
Adult male and female Sprague-Dawley rats weighing 200 ± 20 g were purchased from a qualified commercial supplier (Medical Experimental Animal Center of Guangdong Province). All rats were given standard food and tap water ad libitum, kept under conditions of controlled temperature (24°C ± 1°C), humidity (50%-60%) and a light/darkness cycle of 12/12 hours (light on at 8:00 am). After acclimatization for a week, male and female rats were mated to obtain the F1. Female rats were separated after positive identification of a vaginal sperm plug, and the day was defined as designation of gestational day zero (GD0). At GD0, pregnant rats were housed individually in plastic cages and randomly divided into 3 groups. Female animals in the control group were received water by gavage and other animals in the experimental groups were received Cd chloride (Sigma, St.Louis, MO) solution by gavage (1 mg/kg-Cd1 and 5 mg/kg-Cd5 corresponding to 1/90, 1/18 of LD50) during gestation and lactation periods. The day of parturition was designated as PND 0 and male pups were sacrificed at PND 21, 35, and 56. Some offspring were weaned at PND 21 and housed in unisexual groups on litter bases with free access to laboratory diet and drinking water. Female rats were treated from GD0 to PND21. At PND 21, 35, and 56, F1 male rats were weighed and sacrificed, and the blood samples were collected by intracardiac puncture. The testes were removed, weighed and treated as described below. All experiments were conducted according to the guidelines for animal care and use of China and were approved by the animal ethics committee of the Chinese Academy of Medical Science.
2.2 Measurement of cd concentration
Cd concentration in blood and testes were determined by atomic absorption spectrometry (AAS) with flame atomization in an air-acetylene.15 Dried tissues of testes and plasma samples were digested with a solution of nitric acid (60%) and perchloric acid (60%) in a ratio of 2:1 at 110°C for 5 hours. When fumes were white and the solution was completely clear, the samples were cooled to room temperature and diluted with distilled water. The concentration of Cd was determined by the Inductively Coupled Plasma Mass Spectrometer (Agilent 240 AA, USA). Each sample was triplicate. The detection limits for Cd was 0.2 μg/L in blood and 0.2 ng/g in wet tissue. Cd concentration in blood, testis tissues were represented as μg/L or ng/g wet tissue, respectively.
2.3 Steroid hormone levels detection
Blood samples were centrifuged at 3000g/min for 10 minutes in a refrigerated centrifuge at 4°C, to separate the plasma for hormone analysis. Levels of testosterone (T) and progesterone (P) in serum were detected by radioimmunoassay with I125 RIA Kits (Beijing North Institute of Biological Technology, Beijing, China) according to the manufacturer's instruction.
2.4 Histopathology
Testes were immersed and fixed in Bouin's fixative (75% satu-rated picric acid, 20% formalin, and 5% acetic acid) for 24 hours. After that they were dehydrated in ascending grades of ethanol before paraffin inclusion. Testicular sections (5 μm) were obtained, stained with hematoxylin and eosin, and observed by Upright Research Microscope (Nikon Eclipse Ni-U, Japan).
2.5 Immunofluorescent staining
After being washed with PBS, frozen sections of testis were permeabilized with 1 g/L Triton X-100 (Santa Cruz, CA) for 20 min, treated with 5% FBS at 37°C for 30 minutes, and then incubated at 4°C overnight with primary antibody CYP17A1 (Tab.1). The samples were thoroughly washed with PBS and then incubated at room temperature for 30 minutes with goat anti-rabbit IgG-FITC (Sigma Aldrich, USA), and with 1 mM Hoechst 33258 (Sigma Aldrich, USA) for 10 minutes, subsequently. For each analysis, negative controls were performed by the removal of primary antibody. All samples were observed by Confocal Laser Scanning Microscopy (LSM 510 META, Carl Zeiss, Germany). Leydig cell numbers were calculated per 100 tubules.16
| Source | Dilution | Manufacturer and No. | |
|---|---|---|---|
| PKA | Rabbit | 1:1000 | Cell signaling no. 4782 |
| p-ERK1/2 | Rabbit | 1:1000 | Cell signaling no. 4376 |
| ERK1/2 | Rabbit | 1:1000 | Cell signaling no. 4695 |
| CYP11A1 | Rabbit | 1:1000 | Cell signaling no. 12491 |
| StAR | Rabbit | 1:1000 | Cell signaling no. 8449 |
| 3β-HSD | Rabbit | 1:1000 | Abcam ab169785 |
| CYP17A1 | Rabbit | 1:1000 | Proteintech 14447-1-AP |
| SRD5α | Goat | 1:500 | Abcam ab110123 |
2.6 Western blotting
Tissue homogenates of testes were lysed in RIPA lysis buffer (Beyotime, China) with PMSF (Sigma-Aldrich, USA) and Protease Inhibitor Cocktail (Roche, USA) on ice. Lysates in supernatant were separated by centrifuging at 13,000 rpm, 4°C for 15 minutes. Concentration of total protein was measured by BCA Protein Assay Kit (ThermoFisher, USA). The protein samples were electrophoresed in a 12% SDS-polyacrylamide gel and electrophoretically transferred to polyvinylidene difluoride membrane. Membranes were incubated with 5% milk for 1–2 hours at room temperature and then incubated with primary antibody (Table 1) at 4°C overnight. After being washed with TBST containing 0.05% Tween-20, the membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibody for 2 hours at room temperature. The blots were detected with SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Radford, IL).
2.7 Statistical analysis
All data were presented as mean ± SD and statistical significance was determined by One-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test with statistics software (SPSS 19.0, SPSS Inc., USA). The differences were considered significant if the P value was < .05.
3 RESULTS
3.1 Cd could transfer from dams to pubs and cause a loss of steroidogenesis in Leydig cells until adulthood
Cd concentration in testes at PND 21, 35, and 56 were shown in Figure 1A. Cd concentrations were found increased in testes of rats with high maternal exposure to Cd (Cd5) at PND 21 (P = .018) and PND 35 (P = .011). F1 male rats were weaned at PND 21 to be free from continuous Cd exposure. For this reason, Cd concentration decreased from 0.065 ± 0.0072 (mg/kg) at PND 21 to 0.034 ± 0.0031 (mg/kg) at PND 35 with the increasing weight of testes in offspring of rats exposed to high dosage of Cd. At PND 56, there was no obvious difference in the concentration of Cd in rats with different treatment. Interestingly, Cd concentration in the blood had no increase (data not shown). The results suggested that Cd could transfer from dams to pubs during gestation and lactation, and accumulate in reproductive organ in male offspring.
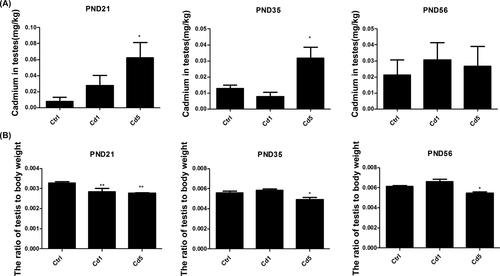
Cd concentrations in testes and weigh of testes in F1 male rats of dams exposed to Cd at PND 21, 35 and 56. A, Cd concentrations in testes. B, The ratio of testis to body weight. Data represent as mean ± SD, n = 8. *p < .05, **P < .01, compared with the control
There was no difference in body weight of rats with different treatments at PND 21, 35, and 56 (data not shown), but the accumulation of Cd in testes in the early development stage induced a decline in the weight of testes, especially in the offspring with high dosage of maternal Cd-exposure (Figure 1B, P = .008 for Cd5 at PND 21, P = .024 for Cd5 at PND 35, and P = .023 for Cd5 at PND 56).
To investigate the effects of Cd on male reproductive functions in F1 male rats, hormone levels in serum were detected (Figure 2). For lack of ability to synthesis testosterone in PLCs, level of progesterone in serum was detected in male offspring at PND 21. Rats with high dosage of maternal exposure to Cd showed a higher level of progesterone compared with those in the vehicle group (P < .001 for Cd5). Progesterone is converted to androstenedione via CYP17A1. In contrast to the high level of progesterone, level of androstenedione decreased markedly in serum of rats at PND 21 (P = .002 for Cd5). ILCs possess high levels of androgen-metabolizing enzyme activities. Thus their primary product is the androgen metabolite, 5α-androstane-3α, 17β-diol (ADIOL), still not testosterone.10 The level of progesterone reduced dramatically in serum of male offspring of mothers exposed to Cd at PND 35 (P < .001 for Cd1, P < .001 for Cd5). At PND 56, ILCs differentiate into ALCs with a decreasing activity of androgen metabolizing enzymes which results in testosterone production.17 Maternal exposure to Cd also induced a notable decline in testosterone production in male offspring (P = .029 for Cd1, P =.047 for Cd5).
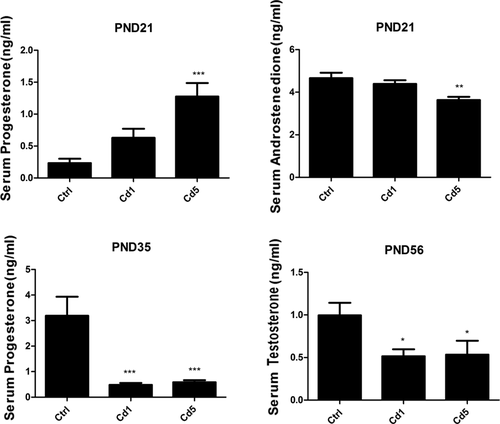
Maternal Cd-exposure affected the secretion of steroid hormones in F1 male rats. A, The progesterone levels in serum of rats at PND 21. B, The androstenedione levels in serum of rats at PND 21. C, The progesterone levels in serum of rats at PND 35. D, The testosterone levels in serum of rats at PND 56. Data represent as mean ± SD, n = 8. *P < .05, **P < .01, ***P < .001 compared with the control
3.2 Development of Leydig cells was disrupted by maternal exposure to Cd
From PND 21 to 56, Leydig cells present distinguishing characteristics in morphology. PLCs were small and long spindle-shaped at PND 21; ILCs were still long but big and became oval shape at PND 35; ALCs were round and big at PND 56 (Figure 3). Remarkably, Leydig cells still kept long and oval-shaped as the cell morphology of ILCs at PND 56 in F1 male rats of mothers exposed to Cd during gestation and lactation. The abnormal morphology suggested a delay development in Leydig cells.
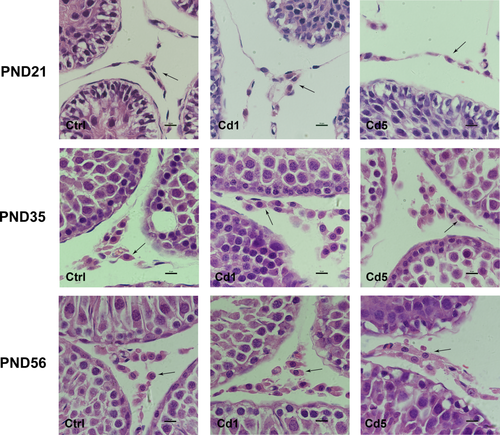
Histomorphology of Leydig cells in testis of F1 male rats with maternal Cd-exposure. PLCs were small and long spindle-shaped at PND 21; ILCs were still long but big and became oval shape at PND 35; ALCs were round and big at PND 56. Leydig cells still kept long and oval-shaped as cell morphology of ILCs at PND 56 in male offspring of female rats exposed to Cd during gestation and lactation (1000×). Leydig cells in different stages are indicated by the black arrows [Color figure can be viewed at wileyonlinelibrary.com]
ILCs secrete high levels of 5α-reduced androgens due to high expression and activity of SRD5α; ALCs have reduced levels of SRD5α in comparison to ILCs.10 The significant decreased level of SRD5α can be a biomarker to indicate the normal development of Leydig cells. In our results, the level of SRD5α kept high in testis at PND 21 and 35, and it did not differ in rats of different group. The quantity of SRD5α protein at PND 35 was higher than that at PND 21 because of the increasing cells number of ILCs (Figure 4). In rats of control group, the expression of SRD5α in testis dropped suddenly to an undetectable level at PND 56. However, in rats with high dosage of maternal Cd exposure, the expression levels of SRD5α still kept a little high at PND 56 (P < .0001, Figure 4), which meant a delay development in ALCs too.
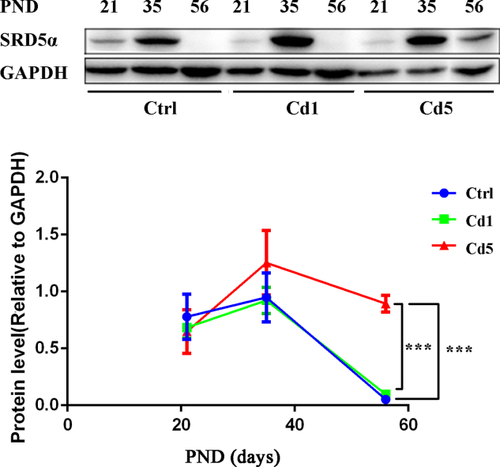
The level of SRD5α in testis of F1 male rats. In the rats of control group, the expression level of SRD5α kept declining in testis from PND 35 to 56. With high maternal exposure to Cd, the expression level of SRD5α kept a little high at PND 56. Data represent as mean ± SD, n = 4. *P < .05 compared with the control [Color figure can be viewed at wileyonlinelibrary.com]
The expression of CYP17A1, a protein specifically expressed in Leydig cells, was stained by immunofluorescence to determine whether the number of Leydig cells was reduced in male rats with maternal Cd-exposure. As shown in Figure 5, the numbers of positive cells were calculated and results showed that there was no significant difference in the number of Leydig cells in male offspring with different treatments at PND 21, 35, and 56. Although the weight of testes decreased, maternal Cd-exposure did not reduce the number of Leydig cells in male offspring, just resulted in a delay development.
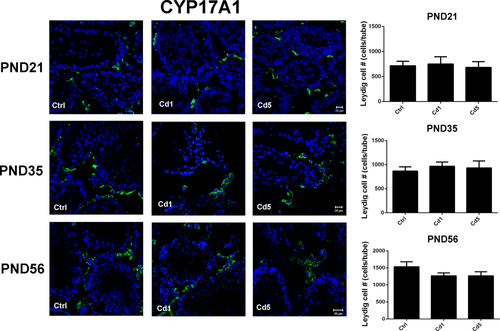
CYP17A1-positive cells stained by immunofluorescence in testes (400×). Numbers of positive cells were calculated at PND 21, 35, and 56 respectively. The normal group was as the control. Data represent as mean ± SD, n = 4 [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Reduction of steroid hormones in male offspring induced by maternal Cd-exposure was related to the inhibition of cAMP/PKA signaling pathway and steroidogenic enzymes
ERK1/2 phosphorylation and steroidogenesis may be induced by cAMP/cAMP-dependent PKA-dependent mechanisms. At PND 21, results of Western blotting revealed that the protein levels of PKA (P = .049 for Cd5) and the phosphorylation of ERK1/2 (P = .008 for Cd1, P = .021 for Cd5) were reduced (Figure 6A) in male offspring with maternal exposure to Cd. At PND 35 and 56, PKA protein levels (P = .002 for PND 35, P = .001 for PND 56) and the phosphorylation of ERK1/2 (P = .007 for PND 35, P = .012 for PND 56) were also significantly reduced in rats with high dosage of maternal exposure to Cd (Cd5) compared with those in the control group (Figure 6B,C). It indicated that cAMP/PKA signaling pathway was affected by maternal Cd-exposure in male offspring, which might be a reason for the reduction of steroid hormones.
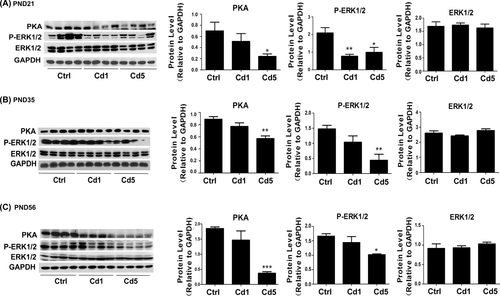
Reduction of steroid hormones was related to the inhibition of cAMP-PKA signaling pathway. A, The protein expression of PKA, ERK1/2 and the phosphorylation level of ERK1/2 at PND 21. B, The protein expression of PKA, ERK1/2 and the phosphorylation level of ERK1/2 at PND 35. C, The protein expression of PKA, ERK1/2 and the phosphorylation level of ERK1/2 at PND 56. Data represent as mean + SD, n = 4. *P < .05, **P < .01, ***P < .001 compared with the control
Cholesterol is transferred into mitochondria by StAR and become testosterone with the catalysis of CYP11A1, 3β-HSD, CYP17A1. At PND 21 (Figure 7A), maternal Cd-exposure could inhibit the expression levels of StAR (P = .013 for Cd5), CYP11A1 (P = .031 for Cd1, P = .012 for Cd5), 3β-HSD (P = .010 for Cd1, P = .011 for Cd5) and CYP17A1 (P = .047 for Cd5). At PND 35 (Figure 7B), the expression levels of CYP11A1 (P = .029 for Cd5) and 3β-HSD (P = .046 for Cd1, P = .009 for Cd5) were also down-regulated, but there was no significant alternation in the expressions of StAR and CYP17A1. At PND 56, the expression levels of CYP11A1 (P = .017 for Cd1, P = .004 for Cd5) and 3β-HSD (P = .027 for Cd1) still had a significant decline compared with those in the control group, but StAR and CYP17A1 remained no change (Figure 7C). These results suggested that steroidogenic enzymes might be influenced in male offspring by maternal Cd-exposure. CYP11A1 seemed to be most sensitive to maternal Cd-exposure in the steroidogenic enzymes. This might be another reason causing the reduction of steroid hormones in male offspring with maternal Cd-exposure.
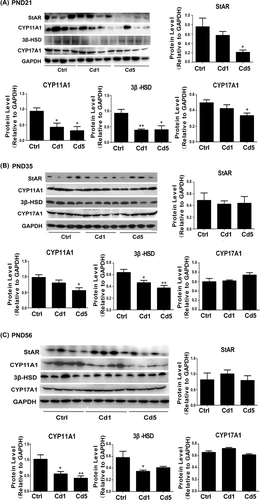
Maternal Cd-exposure suppressed the steroidogenic enzymes in testis. A, The expression of steroidogenic enzymes (StAR, CYP11A1, and 3β-HSD) in testis at PND 21. B, The expression of steroidogenic enzymes (StAR, CYP11A1, 3β-HSD, and CYP17A1) in testis at PND 35. C, The expression of steroidogenic enzymes (StAR, CYP11A1, 3β-HSD, and CYP17A1) in testis at PND 56. Data represent as mean + SD, n = 4. *P < .05, **P < .01, compared with the control
4 DISCUSSION
It has been reported that male infertility may be attributable to various environmental and occupational exposures.18. Cd is a common environmental pollutant which ranks seventh on the priority list of hazardous substances constituting security risk to the health of human and animal.19 Many studies mainly focused on the toxicity of Cd to reproductive system of adults, and demonstrated that Cd-induced testicular toxicity and suppressed the synthesis and secretion of testosterone in Leydig cells.20-22 There are a few studies focusing on the toxicity of Cd to reproductive system in offspring of mothers exposed to Cd. It showed that Cd accumulated in different organs and affected the sperm quality of male offspring.23 An increase of abnormal seminiferous tubules, a decrease in testis weight and plasmatic testosterone concentration were observed in male offspring of Cd-exposed female rats during gestation and lactation periods.24, 25 However, how maternal Cd-exposure affects the development and steroidogenesis of Leydig cells in male offspring is still unclear.
A report pointed out that the placental barrier could prevent Cd entering into the fetal tissues effectively.26 Another report suggested that low amount of Cd transferred to mice and rats through breast milk.27 Our results showed that the accumulation of Cd in testis was dose-dependent in male offspring of dams exposed to Cd. It means that Cd can be transferred to offspring via placenta and milk during gestation and lactation. Although maternal Cd-exposure was terminated after weaning, the relative weight of testis and level of serum testosterone kept decline in male offspring until adulthood. Furthermore, there was no obvious Cd to be detected in the blood of offspring. This reveals that testis is one of the main targeted organs in which Cd usually accumulates to induce damage.
In different developmental stages, Leydig cells have different shapes and express distinguishing biomarkers. PLCs are small, spindle-shaped cells that express SRD5α. ILCs become oval shape and large, possess high levels of androgen-metabolizing enzyme (including SRD5α). Consistent with the enzyme activities, mRNA level of SRD5α is higher in ILC than those in ALC.28 ALCs were round and big with continued increase in levels of testosterone biosynthetic enzymes. The significant decreased level of SRD5α might be a biomarker to indicate the normal development of Leydig cell. In this study, it found that the high dosage of maternal exposure to Cd kept the expression level of SRD5α high at PND 56. In corresponding to this, Leydig cells still kept long and oval-shaped as cell morphology of ILCs at PND 56 (adulthood). These suggest that exposure of Cd to female during gestation and lactation disrupts the development of Leydig cells and keeps them immature in male offspring. Steroidogenesis is the main function of Leydig cells which secrete different steroid hormones in different stages. PLCs contain only small amounts of SER, the organelle that houses several steroidogenic enzymes, but nonetheless are competent to produce steroids, secreting mainly androsterone.29 For the high levels of androgen-metabolizing enzymes, the primary product of ILCs is the ADIOL, still not testosterone.10 The decrease in androgen metabolism and continued increase in levels of testosterone biosynthetic enzymes culminate in the predominance of testosterone over ADIOL production in the ALCs.10 Therefore, the delay development of ALCs dramatically causes a decrease of testosterone at PND 56 in offspring of mothers exposed to Cd.
StAR, CYP11A1, 3β-HSD, and CYP17A1 are involved in the synthesis of androsterone. Many studies including ours demonstrated that heavy metals down-regulated the expression of steroidogenic enzymes StAR, CYP11A1 and 3β-HSD in Leydig cells of adults.7, 8, 30, 31 The down-regulation of StAR, CYP11A1, 3β-HSD, and CYP17A1 induced by maternal Cd-exposure was also found in different developmental stage of Leydig cells respectively in the present study. The expression of CYP11A1 displayed a sustained drop from the stage of PLC to ALC. It means that CYP11A1 is susceptible to Cd in development of Leydig cells and the down-regulation of CYP11A1 lead to a reduction of steroid hormone.
The differentiated functions of Leydig cells can be modulated by the signaling of the Guanine nucleotide binding protein (Gs)/adenylyl cyclase/cAMP/PKA pathway activated by the LHR.32-34 The activation of the LHR leads to an increase in the phosphorylation of ERK1/2.35-37 Poderoso et al.38 found that ERK1/2 was involved in hormone-stimulated steroid synthesis; ERK1/2 phosphorylation was triggered by cAMP/cAMP-PKA-dependent and -independent mechanisms. StAR is a novel substrate of ERK1/2, and mitochondrial ERK1/2, a part of the multimeric protein kinase complex can regulate cholesterol transport. In our previous study, heavy metals (Cd, manganese and lead) inhibited the activation of cAMP/PKA and PKC signaling pathway and down-regulated phosphorylation of ERK1/2 in Leydig cells.39 In this study, we also found that PKA and phosphorylation of ERK1/2 were decreased through the development process of Leydig cells in male offspring with maternal Cd-exposure. The inhibition of cAMP/PKA signaling pathway induced by maternal Cd-exposure might be another reason for the reduction of steroid hormones in male offspring.
In conclusion, this study was to investigate the effects of maternal exposure to Cd during gestation and lactation on development and function of Leydig cells in F1 male rats. Maternal Cd-exposure interrupted the development of Leydig cells with a high expression of SRD5α and cell morphology of ILCs in adulthood (PND 56), inhibited the activation of cAMP/PKA signaling pathway and down-regulated the expression of steroidogenic enzymes (StAR, CYP11A1, 3β-HSD, and CYP17A1). This study would help to disclose the origin of male sexual dysfunction in the developmental stages of Leydig cells.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.




