In vitro stimulatory effect of N-acetyl tryptophan-glucopyranoside against gamma radiation induced immunosuppression
Funding information: Defence Research and Development Organization, Ministry of Defence, Govt. of India (under Project RAKSHAK 1.2 of TP-15/INM-313)
Abstract
Radiation-induced manifestations like free radical burst, oxidative damage and apoptosis leading to cell death. In present study, N-acetyl tryptophan glucopyranoside (NATG) was assessed for its immune-radioprotective activities using J774A.1 cells. Clonogenic cell survival, cell cycle progression and cytokines i.e. IFN-γ, TNF-α, IL-2, IL-10, IL-12, IL-13 and IL-17A expression were evaluated in irradiated and NATG pretreated cells using clonogenic formation ability, flow cytometry and ELISA assay. Results indicated that 0.25μg/ml NATG exhibited maximum radioprotection against gamma-radiation (2Gy) without intervening in cell cycle progression. NATG pretreated (−2 h) plus irradiated cells showed significant elevation in IFN-γ (∼38.2%), IL-17A (∼53.7%) and IL-12 (∼58.8%) expression as compared to only irradiated cells. Conversely, significant decrease in TNF-α (∼21.6%), IL-10 (∼31.2%), IL-2 (∼23.7%) and IL-13 expression (∼17.8%) were observed in NATG pretreated plus irradiated cells as compared to irradiated cells. Conclusively, NATG pretreatment to irradiated J774A.1 cells, stimulate Th1 while diminish Th2 cytokines that contributes to radioprotection.
1 INTRODUCTION
Cancer treatment includes chemotherapy and radiotherapy as first line of treatment and thereafter, immunotherapy is used as secondary post treatment regime.1 However, chemotherapy along with radiotherapy of the cancer patients negatively impacts the overall health of cancer patients and makes them susceptible to opportunistic infections due to imbalanced immune system homeostasis. Manifestation of radiation effects include free radical burst, oxidative damage, impairment of cell repair machinery, interruption in cell cycle progression and apoptosis, etc. leading to eventual loss of cellular viability and survival.2 Stiff regulation of cell cycle progression ensures that only healthy or repaired cells should undergo cell division which otherwise could lead to harmful effects at genetic level. Stringent inspection at cell cycle checkpoints decides the fate of injured cells, which includes apoptosis.3
However, radiation-induced modifications occur through regulatory signaling cascades facilitated by a cluster of Th1 and Th2 immune response inducing-cytokines. Th2/Th1 cytokines recruitment at the site of injury, decide the pro/anti-inflammatory outcome of the regulatory signaling. The functioning of both humoral (Th2) and cell mediated (Th1) immune response is assisted by macrophages,4 which secrete various cytokines. Th1 immune response is aided by IL-2, IL-12, TNF-α, and interferon-γ (IFN-γ) cytokines. While, Th2 immune response is primarily generated by IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, and IL-17E cytokines.5, 6
IFN-γ and IL-2 cytokines contribute dynamically in the activation of cytotoxic T-lymphocytes and NK cells.7, 8 Similarly, IL-17A stimulates secretion of IL-6, G-CSF, GM-CSF, and activates dendritic cells and other immune cells.9 IL-17A expression is mainly secreted in vivo by activated T cells in response to inflammation and infections with bacterial and fungal pathogens.10 It has been reported that radiation suppresses Th1 immune response via modulation of IL-2, IFN-γ, and ΙL-17A expression from their respective secretory cells, thus, hindering IL-6, G-CSF, GM-CSF cytokines expression and by inhibiting activation of T lymphocytes and NK cells.10-12 TNF-α, a proinflammatory cytokine, promotes inflammation by upregulation of adhesion molecules, boosting production of chemokines, cell migration to infected and inflamed sites, tissue destruction and subsequent altered tissue homeostasis.13 It has been stated that upon irradiation, TNF-α is upregulated and mediates radiobiological bystander responses. Its mode of action includes activation of transcription factor NF-κB and its downstream targets include COX-2, IL-1α, IL-1β, IL-6, etc. that trigger inflammation and other cellular effects.14-16 IL-10 has been identified as an immunosuppressive and anti-inflammatory cytokine secreted by monocytes, Th2 cells, B cells, mast cells, dendritic and regulatory T cells, etc.17-19 Upon irradiation, IL-10 expression has been found to be amplified, which affects antigen presentation capacity of APCs, resulting in inhibition of Th1 cells activation in irradiated cells20 resulting in immunosuppression.
Similarly, IL-13 is an anti-inflammatory cytokine mainly secreted by cells like NK cells, mast cells, dendritic cells, eosinophils, basophils, and macrophages.21 It plays a vital role in hampering secretion of various inflammatory cytokines and activates M2 subtype of macrophages out of the biologically prevailing M1 and M2 subtype macrophages.21, 22 Radiation is reported to elevate IL-13 that induced DUOX1 expression; the primary source of sustained ROS production that causes DNA damage.23 Following low-dose irradiation, IL-13 proactivates TGF-β3 via up-regulation of iNOS that modulates redox homeostasis of the cells.24 Therefore, in view of the above cited evidences, immune response modulation/immunosuppression is one of the prime outcomes of irradiation. Thus, to combat radiation induced immunosuppression, a radioprotective agent that modulates the immune response in irradiated cells is an unavoidable requirement particularly for the cancer patients undergoing radiotherapy regime.
Radioresistant bacteria sustained their lives under extreme radiation environment. Therefore, they may have sustainable capability against radiation induced oxidative stress. Our previous study suggested that radioresistant bacteria produced secondary metabolites as a shield against radiation-induced damage and oxidative stress.11, 17, 19, 25-31 Formerly, we had isolated and characterized a novel semiquinone glucoside derivative, a remarkable secondary metabolite, from radioresistant bacteria Bacillus sp. INM-1, which demonstrated immune-radioprotective properties.11, 17, 19, 26-33 Recent findings have displayed radioprotective and immunoprotective capability of another novel secondary metabolite, N-acetyl tryptophan glucopyanoside (NATG), purified and characterized from the radioresistant bacterium Bacillus sp. INM-1.4, 34 This study is mainly focused on effects of NATG on radioprotective efficacy, regulation of cell cycle progression, and Th1/Th2 immune response homeostasis in irradiated murine macrophage J774A.1 cells.
2 MATERIAL AND METHODS
High glucose Dulbecco Modified Eagle Medium (HG-DMEM), fetal bovine serum (FBS), Penicillin-Streptomycin antibiotics, propidium iodide (PI), and RNase were obtained from Sigma-Aldrich, St Louis, MO, USA. Elisa kits for IL-2 (KB2015), IL-10 (KB2024), IFN-γ (ΚΒ2011), TNF-α (ΚΒ2052), IL-17A (KB2079), IL-13 (KB2076), and IL-12 (KB2075) were procured from Krishgen Biosciences, Mumbai, India. Triton X-100, Tris-HCl, NaCl, NP-40, EDTA, EGTA, and crystal violet were obtained from Himedia Laboratory, Mumbai, India. Phosphate buffered saline (PBS), methanol, ethanol, and protease inhibitor cocktail were purchased from Merck Millipore India, Bangalore, India. Other reagents, biochemical, and plastic wares used in the study were of standard make.
3 CELL CULTURE
Macrophage J774A.1 cells purchased from NCCS (Pune, India) were routinely cultured as adherent culture in DMEM-HG supplemented with 10% heat-inactivated FBS, 25 mM glucose, 43 mM bicarbonate, 25 mM HEPES buffer, 5000 U penicillin, and 5 mg/mL streptomycin at 37°C under 5% CO2 and 95% humidity. Cells were passaged 2–3 times per/week by scraping method for maintaining them in healthy log phase. For experiments, cells were seeded at low density (0.5 × 106 cells/mL) in petridishes (35 mm) and further experimental analysis was performed.
4 IRRADIATION OF CELLS
J774A.1 cells were irradiated in petridishes (35/60 mm) with γ-radiation at central radiation facility (CRF) established at INMAS, using Bhabhatron-II Telecobalt unit (Bhabha Atomic Research Centre, Mumbai, India) at a dose rate of 1.365 Gy/min with 80 SSD. Fresh air was circulated throughout the irradiation procedure and temperature was maintained at 25°C ± 2°C.
4.1 Cell survival and colony forming ability
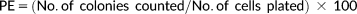

4.2 NATG immunomodulatory activity determination
Macrophage J774A.1 cells were divided into four experimental groups with 1 × 106 cells in each group. Cells in each group were treated with γ-radiation and NATG as per scheme mentioned below:
Group 1. Control: Untreated Macrophage J774A.1 cells
Group 2. Irradiation cells: Macrophage J774A.1 cells exposed with γ–radiation (2 Gy).
Group 3. NATG treated cells: Macrophage J774A.1 cells treated with 0.25 μg/mL NATG
Group 4. NATG pretreated and irradiated cells: Macrophage J774A.1 cells pretreated with 0.25 μg/mL NATG 2 hours before irradiation (2 Gy).
Followed by all treatments and desired incubations under controlled conditions (37°C, 5% CO2), cells from all the experimental groups were harvested at set time points (2, 4, 24, and 48 hours, respectively) and processed for further experimentation.
4.3 Analysis of cell cycle progression
To study the effect of NATG treatment on cell cycle progression in irradiated macrophage J774A.1 cells, cell-cycle analysis was carried out.36 Exponentially growing cells (0.5 × 106/Petridish 35 mm) were seeded and divided into four groups as mentioned above. Followed by different treatments, cells were incubated for different time intervals (i.e., 2–24 hours) at 37°C. Cells were harvested by scraping method, suspended in 200 μL PBS and fixed in 1600 μL chilled 80% ethanol (so as to reach 70% as final ethanol concentration) and left overnight at 4°C. Cells were washed twice with PBS by centrifugation at 300g for 5 minutes at room temperature. Pellets were resuspended in 0.5 mL PBS and treated with RNAase (200 μg/mL) for 30 minutes at 37°C. Further, PI (50 μg/mL) was added and incubated at 4°C for 15 minutes. Cell cycle distribution was studied with the help of FACS Calibur (Becton, Dickinson and Co., Franklin Lakes, NJ) flow cytometer using Cell Quest (version 3.0.1: Becton, Dickinson, and Co., Franklin Lakes, NJ) software for data acquisition. FlowJo software (version 7.6.2) was used for performing gating and obtaining percentage of cells in each selected gate. Singlet cell population was selected by pregating to exclude debris and doublet cells. The cell percentage thus obtained in different gates represented percentage of cells present in respective cell cycle phases and was used for further interpretation and analysis.
4.4 Sample preparation for ELISA assay
Cells of different treatment groups were harvested at post-treatment time intervals already mentioned in previous section. The cell suspension was then centrifuged at 4000g (Combi 514R, Hanil, South Korea) and the resultant cell pellet was washed with sterile PBS for further processing for cytokine analysis. Cell suspensions were treated with cell-lysis buffer (1% Triton X-100, 10 mM Tris-HCl, 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 0.2% EGTA) supplemented with protease inhibitors. Cells were then incubated on ice for 30 minutes before being centrifuged at 10 000g for 30 minutes at 4°C. Each sample supernatant was collected and stored at −20°C until further experimentation for analyses of IL-2, IL-10, IL-12, IL-13, IL-17A, IFN-γ, and TNF-α cytokine expression.
4.5 Determination of IFN-γ, TNF-α, IL-13, IL-17A, IL-2, IL-10, and IL-12 cytokine expression
IFN-γ, TNF-α, IL-13, and IL-17A cytokines expression levels in the cell free homogenate prepared from the cells treated with radiation and NATG were analyzed using ELISA kits following manufacturer instructions. In brief, 100 μL/well of standard and cell free homogenate samples adjusted to equal amount of protein, were added to the primary antibody precoated plate. The plate was sealed and incubated for 2 hours at room temperature (25°C). Samples were then aspirated and plates washed 4 times with wash Buffer (1×) and residual buffer blotted by firmly tapping the plates upside down on absorbent paper; 100 μL of diluted detection antibody solution (1:250 dilution for IFN-γ, TNF-α, IL-13, and IL-17A cytokines and 1:200 dilution for IL-2, IL-10, and IL-12 cytokines) was added in to each well, sealed and incubated for different time-intervals at room temperature (25°C; 1 hour for IFN-γ, TNF-α, IL-13, and IL-17A cytokines and 2 hours for IL-2, IL-10, and IL-12 cytokines) in accordance with the manufacturer's instructions. Followed by incubation, plates were washed again 4 times with wash buffer and 100 μL of diluted Streptavidin-HRP solution (1:250 dilution for IFN-γ, TNF-α, IL-13, and IL-17A cytokines and 1:200 dilution for IL-2, IL-10, and IL-12 cytokines) was added to each well. Plates were sealed and again incubated for 30 minutes at room temperature. Plates were then washed 4 times again with wash buffer. Then, 100 μL of TMB substrate solution was added in each well and plates were incubated in the dark for 15 minutes. Positive wells should turn bluish in color upon incubation. Chromogen formation was stopped by adding 100 μL of stop solution to each well. Final absorbance was read at 450 nm within 30 minutes of reaction termination. Total protein content in the cell lysate was estimated using Bradford method.37 Quantitative estimation of the individual cytokine expression was carried out by extrapolation from a standard curve in terms of pg/mg of protein prepared in parallel using standards supplied along with kits.
4.6 Statistical analysis
Each experiment was repeated three times and the continuous data obtained expressed as arithmetical mean ± SD. Significant variation between treatment groups was determined by one-way analysis of variance, followed by a Bon ferroni test; P values < .05 were considered to be significant.
5 RESULTS
5.1 Radioprotective efficacy of NATG in terms of clonogenic cell survival
The radioprotective efficacy of NATG at different concentrations (0.1–1 μg/mL) against gamma radiation (2 Gy) was assessed using proliferating J774A.1 cells. The colony forming ability was chosen as end point. PE of the J774A.1 macrophages was observed to be more than 80% when cultured in normal condition without any treatment. 50% cell survival (LD50) was observed in the cells irradiated with 2 Gy of gamma radiation (Figure 1).
Colony forming ability of J774A.1 macrophages after exposure to gamma radiation. Untreated control was normalized to 100% survival (80% PE). LD50 was observed to be found at 2Gy gamma radiation dose. The data represented are the arithmetical mean of three independent experiments
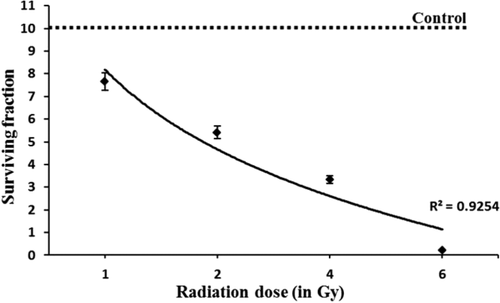
NATG treatment at all tested concentrations (0.1–1 μg/mL) exhibited a steady maintenance (∼95%–97% survival) of the cells (Figure 1). To find out the radioprotective efficacy of NATG, macrophages were pretreated with drug (NATG) and then irradiated (–2 hours) with gamma radiation (2 Gy) and survival fractions (colony forming unit) were counted. Maximal (82.7%) protection against gamma radiation was observed in the group of J774A.1 cells, which was pretreated with NATG at 0.25 μg/mL concentration as compared with the irradiated cells not pretreated with NATG (53.8%). However, beyond 0.25 μg/mL of NATG, the SF of the irradiated cells was found to decline in a dose-dependent manner (Figure 2). Therefore, 0.25 μg/mL concentration of NATG was observed to be optimized radioprotective dose for macrophages.
Radioprotective efficacy of NATG against gamma radiation induced lethality. J774A.1 cells were treated with various concentrations of NATG (0.1–1 μg/mL) 2 hours prior to irradiation (2 Gy). Colony forming ability was determined at 10–14 days by counting the number of individual colonies stained with crystal violet. Untreated control was normalized to 100% survival (80% PE). Maximal surviving dose (0.25 μg/mL) showed ∼82% survival. The data represented are the arithmetical mean of three independent experiments. *; Indicates P < .05 level of significance in NATG +Radiation treatment group versus Radiation alone treatment group
5.2 Influence of NATG pretreatment on cell cycle progression of irradiated macrophage J774A.1 cells
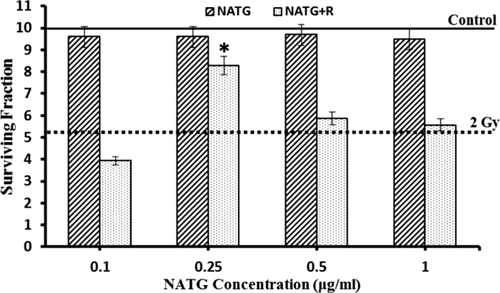
J774A.1 cells revealed an insignificant variation in G2 and S phase cells percentage (%) at 48 hours post treatment time in NATG treated cells as compared with untreated control (Figures 3 and 4A–C). However, G1 phase depicted an increase (∼10%–20%) in NATG treated cells as compared with control cells at all observed (except 4 hours) time-intervals. Also, only irradiated cells showed significant (P < .05) decline in G1 phase cells at all observed time-intervals except 24 hours when compared with control cells. Additionally, NATG pretreated plus irradiated cells group did not exhibit any decrease in G1 phase as compared to only irradiated cells at all the tested time-intervals (Figure 4).
Representative figures for flow-cytometric analysis of cell cycle progression in J774A.1 cells treated with NATG, gamma radiation (2 Gy) individually and in combined treatments. Cell cycle distribution was studied with the help of FACS Calibur flow cytometer using Cell Quest (version 3.0.1) and FlowJo software for data acquisition and analysis. One representative is the mean of three independent experiments each with six subjects
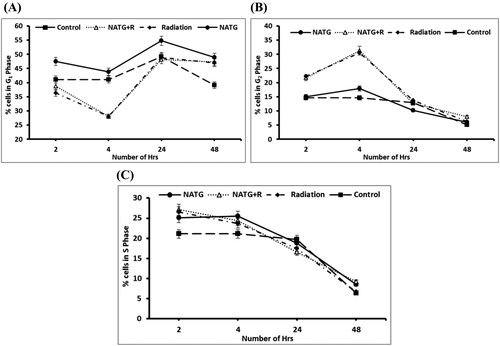
A–C, Quantitative analysis of percentage (%) of cells in G1 phase (A), G2 phase (B), and S phase (C) of J774A.1 cells subjected to NATG and gamma radiation (2 Gy) individual and combined treatments at different time intervals. The data represented are the arithmetical mean of three independent experiments
In parallel, irradiated cells or irradiated cells pretreated with NATG showed significant (P < .05) increase in G2 phase at 2 and 4 hours time points respectively in comparison to untreated control cells. Following 4 hours post treatment time, cellular percentage (%) in G2 phase of all groups was observed to decline steadily and reach equivalent to untreated control group of cells at 24 and 48 hours time periods. Further, a slight increase in S phase was observed in NATG treated J774A.1 cells as compared with untreated control cells till 4 hours post treatment time interval. Beyond 4 hours time-interval, a steady decline was observed in S phase cells in all groups and reached equivalent to untreated control group of cells onwards 24 hours (Figure 4C). Overall, it was observed that NATG pretreatment does not induce a significant effect on cell cycle regulation upon radiation exposure.
5.3 NATG provides protection against gamma radiation-induced suppression of IFN-γ expression in macrophages
Radiation (2 Gy) treatment to J774A.1 macrophages shows a steady decline in IFN-γ expression in a time-dependent manner as compared with untreated control cells (Figure 5A). A significant (P < .05) decline (57.3 pg/mg of protein) in IFN-γ expression was observed at 24 hours as compared with untreated control cells (71.4 pg/mg of protein). However, as the result of NATG pretreatment to irradiated cells, significant (P < .05) elevation in IFN-γ levels at 2 hours (84.9 pg/mg of protein) and 24 hours (79.2 pg/mg of protein) time-intervals was observed as compared with radiation alone (69.5 and 57.3 pg/mg of protein for 2 and 24 hours, respectively) group of the cells (Figure 5A). Maximum elevation in IFN-γ expression (∼38.2%) was observed at 24 hours (79.2 pg/mg of protein) time-interval in the NATG pretreated plus irradiated cells. Therefore, results of this study clearly indicated that NATG pretreatment effectively restored IFN-γ expression, which was otherwise suppressed by gamma irradiation in absence of NATG treatment in macrophages.
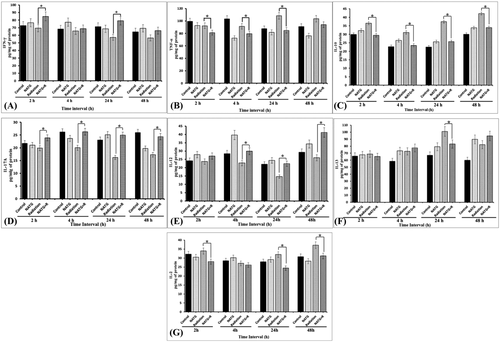
Evaluation of γ-radiation mediated fluctuations in cytokines expression and its modulation with N-Acetyl Tryptophan Glucoside (NATG) pre-treatment to J774A.1 macrophages cells. A, IFN-γ; B, TNF-α; C, IL-10; D, IL-17A; E, IL-12; F, IL-13; and G, IL-2. The data represented are the arithmetical mean of three independent experiments. *; Indicates P < .05 level of significance in NATG +Radiation treatment group versus Radiation alone treatment group
5.4 Radiation-induced elevation of TNF-α expression and its normalization by NATG pretreatment
To analyze the deleterious effects of a sublethal dose (2 Gy) of gamma radiation in macrophages, TNF-α expression levels were estimated using ELISA. As a result of radiation exposure a mild increase in TNF-α levels was observed at 24 hours (108.5 pg/mg of protein) and 48 hours (103.7 pg/mg of protein) as compared with untreated control cells (87.6 and 91.1 pg/mg of protein at 24 and 48 hours, respectively) (Figure 5B). However, NATG treatment prior to irradiation (2 Gy) induced a significant (P < .05) decline in TNF-α levels at 24–48 hours (81.3–85.1 pg/mg of protein, respectively) as compared with the irradiation only group of cells (Figure 5B). Maximum protective effect (∼21.6%) of NATG pretreatment to irradiated cells was demonstrated at 24 hours as compared with radiation-induced TNF-α expression in macrophages (Figure 5B). Therefore, present results demonstrate NATG ability to alleviate radiation-induced increased of TNF-α expression in macrophage cells.
5.5 Effect of NATG pretreatment on IL-10 expression in irradiated (2 Gy) macrophage J774A.1 cells
Sublethal irradiation was observed to enhance IL-10 expression significantly (P < .05) at 2 hours, 24–48 hours as compared with untreated control cells. Whereby, maximal (∼66.4%) induction in IL-10 expression was observed at 24 hours (42.3 pg/mg of protein) in the irradiated macrophages (Figure 5C). Conversely, pretreatment with NATG to irradiated (2 Gy) cells resulted in significant (P < .05) decrease in the IL-10 expression at 2 hours (29.4 pg/mg of protein) onwards till 48 hours (33.9 pg/mg of protein) with the maximum (∼31.2%) subsidizing effect being observed at 24 hours (25.8 pg/mg of protein) as compared with only irradiated cells (Figure 5C). Results of the present study imply that NATG has potential to normalize IL-10 over-expression in irradiated cells. However, NATG alone treatment did not affect the IL-10 expression significantly.
5.6 Immunomodulatory effect of NATG pretreatment on gamma radiation induced suppression of IL-17A cytokine in macrophages
Immunomodulatory activity of NATG against gamma radiation (2 Gy) induced ill-effects was assessed in terms of IL-17A cytokine expression analysis. Results of this study inferred that gamma radiation (2 Gy) induced a significant (P < .05) suppression in IL-17A activity at 4–48 hours (20.0–17.3 pg/mg of protein) time intervals as compared with untreated control (26.2–25.9 pg/mg of protein) cells (Figure 5D). Maximum (∼33.3%) suppression in IL-17A expression was observed at 48 hours (17.3 pg/mg of protein) in irradiated cells as compared to untreated control (25.9 pg/mg of protein) cells (Figure 5D). On the contrary, NATG pre-treated plus irradiated cells expressed a significantly (P < .05) improved level of IL-17A at all the observed time-intervals, that is, 2–48 hours (23.9–24.4 pg/mg of protein) with the maximum (∼53.7%) elevation at 24 hours (24.9 pg/mg of protein) as compared with radiation alone group of cells (16.2 pg/mg of protein; Figure 5D). Further, significant increase in IL-17 expression was also observed at 24 hours with the cells treated with NATG alone as compared with untreated control cells. Therefore, this study implies that NATG contributes to radioprotective efficacy in irradiated J774A.1 macrophages by inhibiting IL-17A cytokine expression.
5.7 Aversion effect of NATG pretreatment on gamma-radiation induced suppression of IL-12 expression in macrophages
It was observed that radiation exposure (2 Gy) caused a significant (P < 0.05) decline in IL-12 expression in macrophages at 24 hours (14.7 pg/mg of protein) as compared with untreated control cells (28.4 pg/mg of protein; Figure 5E). In contrast, it was observed that NATG pretreatment to irradiated cells significantly (P < .05) averts IL-12 suppression at all tested time-intervals, that is, 4–48 hours (29.8–41.2 pg/mg of protein) as compared to irradiated cells not pre-treated with NATG (Figure 5E). Maximal induction (∼58.8%) was observed at 48 hours in NATG pretreated plus irradiated cells (41.2 pg/mg of protein), while, radiation alone treatment suppressed IL-12 activity maximally (∼33.6%) at 24 hours (14.7 pg/mg of protein) in macrophages. These observations imply that NATG pretreatment bears the potential to inhibit radiation-induced suppression of IL-12 activity in J774A.1 macrophages.
5.8 Assessment of gamma radiation-induced fluctuations and modulatory effect of NATG treatment on IL-13 cytokine expression in murine macrophages
Effect of radiation-induced modulations and NATG pre-treatment on IL-13 cytokine expression was studied using the ELISA method. Radiation exposure showed significant (P < .05) upsurge in IL-13 expression at 24 hours (101.1 pg/mg of protein) and 48 hours (82.1 pg/mg of protein) with the maximal increase (∼50.4%) at 24 hours as compared with untreated control cells (67.2 pg/mg of protein; Figure 5F). However, NATG pretreatment to irradiated cells showed a significant (P < .05) decline in IL-13 level at 24 hours (83.1 pg/mg of protein) as compared with radiation alone treatment (101.1 pg/mg of protein) to the macrophage J774A.1 cells (Figure 5F). As the results of NATG alone treatment to J774A.1 cells, a moderate increase in IL-13 expression was observed as compared with untreated control. Thus, it can be inferred from the analysis that NATG pretreatment down-regulates the IL-13 expression in irradiated J774A.1 macrophages at 24 hours post treatment period. Whereas, no such inhibitory effect in IL-13 expression was observed at other time points.
5.9 Effect of gamma radiation and NATG treatment on IL-2 cytokine expression in J774A.1 macrophages
Results of the study indicated a non-significant increase (37.2 pg/mg of protein) in IL-2 expression in irradiated (2 Gy) macrophage J774A.1 cells at 48 hours post treatment period as compared with untreated control cells (30.8 pg/mg of protein; Figure 5G). However, NATG pretreated irradiated cells showed a significant (P < .05) decline in IL-2 cytokine expression at all the tested time-intervals, that is, 2–48 hours except at 4 hours as compared with irradiation alone cells (33.9–37.2 pg/mg of protein; Figure 5G). Maximum inhibition (∼23.7%) in IL-2 expression levels was observed at 24 hours time-interval in pretreated plus irradiated cells (24.4 pg/mg of protein) when compared with only radiation cells (31.9 pg/mg of protein; Figure 5G). These findings suggested IL-2 expression inhibitory activity of NATG only in irradiated cells (Figure 5G). While, no such inhibitory effect in IL-2 expression was observed in unirradiated but NATG treated cells.
6 DISCUSSION
Radiation exposure to immune system results in creating a discrepancy in the Th1/Th2 homeostatic balance of the body. In our previous study, it was revealed that the bacterial secondary metabolite, NATG, mainly exerts radioprotective effect by priming the macrophages via interactions with NK-1 receptor of cells.4, 34 Hence, to further evaluate the immunological implications of gamma radiation (2 Gy) induced damages, this study was designed. In present study, assessment of deleterious effects of radiation exposure and protective effects of NATG on cell survival, cell cycle progression, and expression of various cytokines belonging to Th1 and Th2 arms of immune system was studied.
Radiation-induced DNA damage and apoptosis are the major likely mechanisms implicated in clonogenic cell death of macrophages. Radiation exposure (2 Gy) triggered a loss of clonogenicity in J774A.1 cells (Figure 1). NATG at 0.25 μg/mL exhibited maximum colony formation, as compared with other tested doses, when given prior (–2 hours) to γ–irradiation (Figure 2). Such augmentation in cell proliferation rate against radiation damage could be the result of several factors, such as inhibition of free radical generation, repair of damaged DNA, protection of cell membrane and an increase in the reproducing ability of cells.38
Regulated progression of the cell cycle and cell division rate are vital for maintenance of normal growth, development and tissue maintenance. To elucidate the effect of NATG on radiation induced cell cycle arrest in G2/M phase, cell cycle progression analysis was carried out. Previous findings of our group exhibited that NATG alone pre-treatment did not induce G2/M arrest or alter cell cycle regulation in J774A.1 cells after exposure to a high dose (20 Gy) of ionizing radiation.4 Similar results were obtained in this study for exposure with 2 Gy radiation (Figures 3 and 4B). Therefore, results from the previous study validate the incidence of noninterference by NATG observed in cell cycle regulation of macrophage J774A.1 cells.
IFN-γ is secreted from macrophages and activates cytotoxic T cells and NK cells, thus instigating Th1 immune response in immune system. Previous reports validated that irradiation down regulates IFN-γ and thus leads to failure in regulating the diverse signaling components inside the cell.39, 40 In complete agreement with earlier reports, substantial decline in IFN-γ expression beyond 24 hours was observed with irradiated cells as compared with untreated control cells (Figure 5A). In contrast, significant elevation in IFN-γ levels were witnessed in NATG pretreated plus irradiated (–2 hours) macrophages at all tested time-points except 4 hours suggested that NATG induced IFN-γ activation as a probable contributing factor in protecting the cells against radiation damage. Earlier reports suggesting that augmented IFN-γ levels stimulate cell mediated immunity by prompting Th1 response39 supported findings of this study.
Irradiation causes elevation in TNF-α expression, which results in inflammation, apoptosis, and cell death by triggering NF-κB pathway.15, 16 Gamma irradiation (2 Gy) to the cells demonstrated a significant (P < 0.05) increase in TNF-α expression at 24 hours onwards as compared with unirradiated control cells (Figure 5B), suggested activation of inflammatory response in the injured cells. However, NATG pre-treatment prior to irradiation was found to help in diminishing radiation-induced TNF-α expression at all tested time-points except 48 hours (Figure 5B). Modulation of inflammatory response attributed to NATG pretreatment to irradiated cells may lead to radioprotection.
Radiation exposure resulted in significant increase in cellular IL-10 expression (Figure 5C) that may lead to immune suppression due to its Th1 cytokines down regulating activities and macrophage costimulatory molecule expression that caused inhibition of antigen presentation and cytokine secretion activity by macrophages.11, 18, 19 On the contrary, NATG pre-treatment proficiently neutralized radiation-induced upsurge in IL-10 expression at all tested time-points (Figure 5C) and thus, it may contribute to maintain immune response by stimulating Th1 pathway.
Findings of this study revealed a sharp decline in IL-17A levels in irradiated macrophages from 4 hours onwards (Figure 5D). Such outcome induced immune suppression due to radiation damage. NATG pretreated plus irradiated cells exhibited significant increase in IL-17A expression at all observed time-points suggested alleviation of exposed cells from radiation-induced demolition (Figure 5D). The conclusions drawn are reinforced by association of IL-17A with inducing IL-6, G-CSF, and GM-CSF, thus, contributing towards radioprotection as suggested by Tan et al.10
IL-2 has been instrumental in CTL and NK cells activation.41-43 IL-2 is known to be induced by irradiation. However, irradiation also reduces its utilization by mononuclear cells.44 Similar to previous reports, irradiation elevated IL-2 levels at all tested time-points (except 4 hours) suggested an inclination towards Th2 type of immune response (Figure 5G). Conversely, NATG-pretreatment to irradiated cells revealed a significant decline in IL-2 levels at all observed time-points (except 4 hours), suggested a reversal of immune balance towards Th1 response. Therefore, observations obtained suggested that NATG pretreatment to irradiated cells helps to maintain Th1/Th2 homoeostasis and thus contribute to radioprotection.
The expression and regulation of IL-12, a pro-inflammatory and Th1 immune response regulatory cytokine, is directly proportional to IFN-γ.5,11,19,21 Irradiated macrophages showed suppression of IL-12 levels at all tested time-points (except 2 hours; Figure 5E). Moreover, previous reports have indicated that dwindling IL-12 levels cause declined stimulation of IFN-γ by Th1 cells.11, 17, 19 On the contrary, NATG pretreatment to irradiated cells revealed a significant (P < .05) upsurge in IL-12 levels at all tested time-points (except 2 hours) clearly suggesting a role of NATG in Th1 immune response stimulation via IL-12 pathway.45
IL-13, an anti-inflammatory cytokine, is known increase due to radiation exposure along with ROS production and DNA damage.23 In parallel, our study indicated that irradiation triggered a steady elevation in IL-13 levels from 4 hours onwards as compared to control cells (Figure 5F). However, NATG pretreatment to irradiated cells exhibited a marginal decline in IL-13 expression at 24 hours only in comparison to irradiated cells (Figure 5F). Marginal effect of NATG on IL-13 expression in irradiated macrophages suggesting likelihood of pursuing other pathways for achieving radioprotection. Thus, mechanism of action adopted by NATG (Figure 6) to protect irradiated macrophages involves immune system (Th1 immune response) modulation, which contributes to radioprotection.
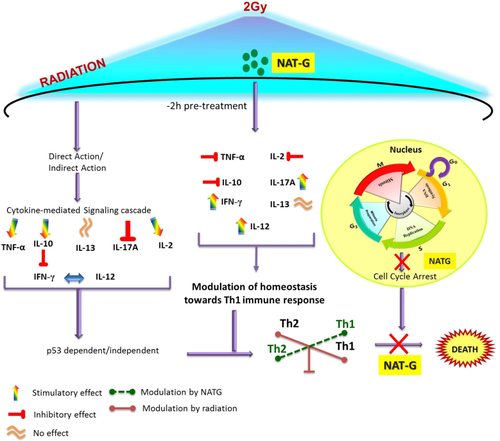
Summarized diagram depicting the most probable mechanism of action undertaken by NATG pretreatment upon radiation exposure and its effects on cytokine regulation in J774A.1 macrophage cells [Color figure can be viewed at wileyonlinelibrary.com]
7 CONCLUSION
This study suggested that gamma radiation altered the expression of a plethora of cytokines in macrophages and induced homeostatic imbalance in Th1/Th2 immune response lead to immune suppression. However, bacterial secondary metabolite NATG pretreatment to irradiated cells can instigate an immunomodulatory response against gamma irradiation. NATG mediated immunomodulatory response may comprise nonintervention in G2/M cell cycle progression, Th1 cytokines stimulation, and Th2 cytokines down regulation in irradiated cells. These outcomes potentiate the candidature of NATG in safeguarding the immune system specifically macrophages against gamma radiation-induced injuries. Further, in-depth analysis of NATG will be undertaken to unravel its mode of action at molecular level to prove its applicability as a countermeasure agent against radiation induced toxicity.
ACKNOWLEDGMENT
The authors would like to acknowledge Defence Research and Development Organization, Ministry of Defence, Govt. of India [under Project RAKSHAK 1.2 of TP-15/INM-313] for providing the financial support and infrastructural facilities to conduct the present study. Poonam Malhotra would like to acknowledge UGC for providing research fellowship.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




