Biomarker responses in caged carp (Cyprinuscarpio) and native collected fish (Leporinus obtusidens) in the Río de la Plata Estuary, Argentina
Abstract
Punta Lara is located in the Río de la Plata estuary near industrial areas contaminated mainly by organic pollutants. In this work, the responses and status of hepatic biomarkers were studied in juvenile carp (Cyprinus carpio) by means of a 21-day field exposure in cages and collection of juvenile native fish (Leporinus obtusidens) at Punta Lara. The analyzed hepatic biomarkers were: enzymatic activity of glutathione-S-transferase (GST), catalase (CAT) and superoxide dismutase (SOD), lipid peroxidation level using the thiobarbituric acid reaction (TBARS), and CYP1A protein expression, condition factor (CF) and liver somatic (LSI) index. Taking into account oxidative stress responses, SOD activity was increased in both species, while CAT was increased in C. carpio and decreased in L. obtusidens; TBARS levels indicated that oxidative damage was possibly exerted only in L. obtusidens. Biotransformation responses mediated by CYP1A were observed in both species, while GST activity was induced mainly in carps. Considering morphometric indices, CF and LSI were significantly increased in carps while CF decreased in native species. The anthropogenic pollution detected in this study in Punta Lara was associated with differences in biomarkers on both fish species, although a different pattern of response was observed. © 2012 Wiley Periodicals, Inc. Environ Toxicol 29: 950–960, 2014.
INTRODUCTION
The Río de la Plata is a funnel-shaped, coastal plain estuary with a total surface area of 30,000 km2. Despite the vast dimensions and dilution capacity of the estuary, the numerous urban and industrial areas located along the coast in Argentina have had a negative impact on this water body (Colombo et al.,2005). Considerable amounts of chemicals are continuously discharged into the estuary, which is in line with the high abundance of persistent toxic substances found in the water, sediment, and biota. The coastal locality of Punta Lara is located between two important urban centers (Buenos Aires and La Plata) which together have a population of about 12 million people and is situated near a large-scale petroleum refinery and several petrochemical plants (Colombo et al.,2006). The presence of different xenobiotics in Punta Lara include aliphatic hydrocarbons (AHs), polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dioxins, benzofurans, and heavy metals has been shown to be mainly associated with sediment, suspended particulate matter (SPM) and biota (Colombo et al.,2000, 2005, 2006). The composition of the hydrocarbon mixtures in the sediments reflects the relative contributions of different natural and anthropogenic sources: biogenic, diagenic, petrogenic, and pyrogenic. In highly populated and industrial areas, the anthropogenic inputs are the predominant source of pollution, whereas in distant areas the great majority of hydrocarbons have a natural origin (Colombo et al.,1989).
Biomarker responses are early warning signals of pollution that could be efficiently used as tools for the assessment of adverse biological effects of pollutants even at low or sublethal concentrations (Fent,2004). Biomarkers are frequently used in field studies focused on the evaluation and validation of the responses of fish to pollutants (van der Oost et al.,2003). Most often these studies involve fish collected from contaminated environments and their biological responses are further compared with those of fish from reference sites. Besides chemical pollution, biomarker responses may also be affected by factors such as migration in search of food or to spawning grounds, or the movement of individuals regarding the avoidance of contaminants. In this context, in situ caged-fish experiments proved to be a realistic approach and allowed a more controllable knowledge of the site of interest and duration of the exposure period (de la Torre et al.,2000; Oikari,2006; Almroth et al.,2008a).
In this study in order to minimize eventual sex-related variability in biomarker responses only sexually immature fish were used. Cyprinus carpio was chosen as a test organism because of its high tolerance and resistance to handling stress (Oikari,2006). In Argentina, it is an exotic species widely distributed in different water bodies. Caged C. carpio have been previously used in field assays to assess environmental contamination (de la Torre et al.,2000; Smolders et al.,2007, Scarcia et al., 2012). The “boga,” Leporinus obtusidens, is a native species that inhabits lentic as well as lotic environments. In Argentina, it is widely distributed in Paraná River basin as well as in the Río de la Plata estuary and its tributaries (Liotta,2005) and is frequently used for consumption. L. obtusidens was chosen for this study as a test organism because of its sensitivity in detecting the possible effects of environmental pollutants (Silveira Moraes et al.,2004).
Persistent hydrophobic chemicals and heavy metals are incorporated by aquatic organisms through different routes and these compounds may either bioaccumulate or undergo biotransformation in order to be excreted. The biotransformation of these compounds is a process that occurs mainly in the liver and to a lesser extent in other tissues. On the other hand, reactive oxygen species (ROS) are generated during the metabolism of numerous pollutants present in the aquatic environment, leading to oxidative stress in organisms. Fish, like mammals, possess a well-developed antioxidant defense system to alleviate the toxic effects of ROS (van der Oost et al.,2003; Di Giulio and Meyer,2008). This system includes antioxidant enzymes such as the superoxide dismutase (SOD), and catalase (CAT) and none enzymatic defenses mainly represented by reduced glutathione. ROS increase the oxidation of polyunsaturated fatty acids (PUFA), including lipid peroxidation (LPO) of those present in cell membranes.
The objective of the present study was to assess the hepatic biomarker responses of juvenile fish after exposure in cages (C. carpio) and sampling a native species (L. obtusidens) in a coastal area of the Río de la Plata (Punta Lara) affected by anthropogenic activities. For this purpose, the enzymatic activities of glutathione-S-transferase (GST), CAT and SOD, LPO level by means of the thiobarbituric acid reaction (TBARS), and expression of the protein CYP1A, were analyzed. In addition, the morphological indices condition factor (CF) index and liver somatic index (LSI) were determined.
MATERIALS AND METHODS
Study Experimental Area
The study was performed in Punta Lara (34° 49′ 0″ S; 57° 58′ 60″ W), in the Río de la Plata estuary during summer 2007. Punta Lara is located in Buenos Aires province, downstream from Quilmes city and close to a petrochemical port of La Plata City.
Experimental Design
Juveniles of C. carpio (n = 20) with a body weight (b.w.) of 3.9 ± 0.5 g and a total length of 6.8 ± 0.2 cm (mean ± SEM) were acclimatized to laboratory conditions for 7 days and then randomly assigned to either the experimental or reference group. Fish were obtained from a commercial supplier and during acclimatization period they were kept under laboratory conditions with 12L:12D photoperiod, continuous flow of non-chlorinated freshwater, fed daily with commercial food 1% b.w. (see Table 1). This assay consisted in exposing a group of caged juveniles of C. carpio to the environmental conditions of Punta Lara (experimental site) and another group to those of Las Flores stream (reference site, RSI). In each site, ten carps were simultaneously kept for 21 days in perforated polypropylene cages with a mesh size of 8 mm in diameter, with biomass density of 1.9–2.0 g b.w./L. The cages were ballasted and placed with the lowest part of each at 10 cm above the sediment surface. The upper part of Las Flores stream is considered representative of many Pampean watercourses (Feijoó et al.,2011) and was selected as a reference site (34° 27′ 30″ S; 59° 03′16″ W) because of its low anthropogenic impact (Ronco et al.,2009; see Table 2).
| Physicochemical Parameters | Laboratory Conditions | Las Flores | Punta Lara | MPQ |
|---|---|---|---|---|
| Temperature (°C) | (23–25) | (24–28) | (23–31) | |
| pH | (8.1–8.2) | (6.8–8.2) | (7.0–7.6) | |
| Dissolved oxygen (mg O2/L) | (6.9–8.4) | (6.2–8.4) | (6.2–7.4) | |
| Conductivity (μS/cm) | 669–670 | (699–809) | (303–494) | |
| Hardness (mg CaCo3/L) | – | (100.1–130.13) | (70.07–90.09) | 350a |
| Suspended particulate matter (mg/L) | – | 11.6 | 32.4 | |
| Soluble reactive phosphorus (mg/L) | (0.04–0.13) | (0.43–0.61) | (0.22–0.29) | – |
| Ammonium (mg N-NH4+/L) | (0.02–0.59) | (0.02–0.04) | (0.03–0.12) | 1.13b |
| Nitrites (mg N-NO2−/L) | (0.02–0.05) | (0.02–0.06) | (0.04–0.19) | 0.06b |
| Nitrates (mg N-NO3−/L) | – | (0.13–5.0) | (0.2–2.46) | 45a |
- Data are shown as the range (minimum–maximum) of values obtained for each parameter. MPQ: maximum permitted quantities according to a Law no. 19587 on the hygiene and safety of drinking water and b Law no. 24051 for protection of freshwater life.
| Environment | Grain Size Composition (%) | PCBs (μg/g) | PAHs (μg/g) | AHs (μg/g d.w) | Indexes | Metals (mg/g) | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | OM | UCM | Totals | Alk/Iso | LMW/ HMW | CPI | Cr | Cu | Zn | Pb | Ni | ||||
| P.L | 29.8 | 59.7 | 10.5 | 3.5 | 0.05 | 0.05 | 49.73 | 59.66 | 0.1 | 0.1 | 1.5 | 0.05 | 0.005 | 0.035 | 0.007 | 0.005 | This study |
| P.L / S.a | 21.2 | 51.2 | 27.6 | 4.1 | 0.63 | 76.8 | 79.16 | 0.3 | 0.1 | 2.1 | Colombo et al.,1989 | ||||||
| 0.039 | Colombo et al.,2005 | ||||||||||||||||
| 0.35 | Colombo et al.,2006 | ||||||||||||||||
| C.a | 0.061 | Colombo et al.,2005 | |||||||||||||||
| 31.8 | Colombo et al.,2006 | ||||||||||||||||
| O.C–R.S | 21 | 43 | 36 | 22.9 | 555 | 2230 | 2876 | 0.7 | 2.2 | 1.4 | Colombo et al.,1989 | ||||||
| 0.998 | Colombo et al.,1990 | ||||||||||||||||
| 18.6 | 43.4 | 37.5 | 21.5 | Total Hydrocarbons: 22361 | 0.0025 | 0.0558 | 0.387 | 0.1247 | 0.0397 | Ronco et al.,2009 | |||||||
| M.H | (1.8– 6.0) | (71.9– 82.9) | (11.1– 22.9) | (11.5– 13.9) | (1.83– 90.4) | (11.1– 55.9) | (21.1– 119.7) | (0.9–1.1) | (0.08– 0.17) | (0.06– 0.11) | (0.17– 0.38) | (0.04– 0.11) | (0.029– 0.032) | Muniz et al.,1998 | |||
| G.b | (0.08– 7.75) | da Silva et al.,2007 | |||||||||||||||
| 0.184 | Santos de Souza et al.,2008 | ||||||||||||||||
| L.F | 33.2 | 30 | 37 | 8 | Total Hydrocarbons: < 200 | 0.0155 | 0.0081 | 0.032 | 0.0222 | 0.0023 | Ronco et al.,2009 | ||||||
| SQG | 0.034 | 0.468 | 0.037 | 0.036 | 0.123 | 0.035 | 0.018* | CCME, 1999–2002 | |||||||||
- PL: Punta Lara (study area); S.a: areas surrounding Punta Lara; C.a: Central area of Río de la Plata (Argentina); OC–RS: Oeste Channel – Río Santiago (Argentina); MH: Montevideo Harbour (four sampling sites), Río de la Plata Estuary (Uruguay); Gb: Guanabara bay (Brazil); L.F: Las Flores stream (reference site, RSI); OM: organic matter; PCBs: totals polychlorinated biphenyls; PAHs: polycyclic aromatic hydrocarbons; AHs: aliphatic hydrocarbons; UCM: unresolved complex mixture; Alk/Iso: index (∑ n–C14–n–C18 /∑ five isoprenoids); LMW/HMW: low/high molecular weight hydrocarbon index; CPI: carbon preference index; SQG: Sediment Quality Guidelines; CCME: Canadian Council of Ministers of the Environment.
Juveniles of L. obtusidens (n = 20) with a b.w. of 13.7 ± 1.8 g and total length of 10.3 ± 0.6 cm (mean ± SEM) were used as native test organisms. Fish were collected in the same experimental site using a trawl net and then taken to the laboratory and immediately processed. Boga controls were obtained with the help of local fishermen from an uncontaminated area of the middle Paraná River located in Corrientes province (28° 16′ 11″ S; 59° 01′ 44″ W) and then transported alive to the laboratory (RSII). This site was chosen because of the absence of reference areas in Buenos Aires where this native fish can be captured. In order to minimize stress from transportation, fish were acclimatized in the laboratory for 7 days under conditions indicated in Table 1 with a summer photoperiod (16L:8D) and then sampled for biomarker measurements. It has to be mentioned that although adult wild specimens of C. carpio are usually present in Punta Lara area, no juvenile carps could be collected during the conducted assay.
Sample Processing
After the caging or sampling period, fish were immediately transferred to the laboratory in aerated containers and anesthetized with MS-222 (3-aminobenzoic acid ethyl ester methane sulfonate salt, Sigma-Aldrich, St. Louis, MO). Body weight and total length of each individual were recorded and then sacrificed by an incision close to the spinal cord and behind the operculum (CCAC,2005). The liver was removed, weighed, and stored at −80°C until processing. Liver samples were individually homogenized in buffer pH 7.4 (0.1 M NaH2PO4; 0.15 M KCl; 1 mM ethylenediamine tetra acetat-EDTA; 1 mM DTT; 10% vol/vol glycerol), with a Teflon-glass homogenizer (Nilsen et al.,2003) until tissue disintegration was complete. The homogenates were centrifuged at 10,000 × g for 15 min at 4°C, the pellet was discarded and the supernatant fraction (SF) was separated. The enzymatic activity of CAT, SOD, GST, and CYP1A expression were evaluated in the SF fraction.
Condition Indices and Biochemical Biomarkers Determinations
Two morphometric indices were calculated: the CF, as body weight (g)/total body length (cm)3 (Bagenal and Tesch,1978) and the LSI, as (liver weight (g)/body weight (g)) × 100 (Sloof et al.,2006).
CAT (EC 1.11.1.6) activity was determined with the method of Beers and Sizer (1952), which is based on H2O2 decomposition over time at a wavelength of 240 nm. The aliquots of SF were incubated in sodium phosphate buffer pH 7.2 (50 mM) containing H2O2 20 mM. The activity of CAT was expressed as μmoles of H2O2 consumed/minute/mg of protein.
The determination of SOD (EC 1.15.1.1) activity was based on the indirect method, involving the inhibition of the cytochrome c reduction by competition with SOD for the superoxide anion radical (O2.−), which is formed by the xanthine/xanthine oxidase system (McCord and Fridovich,2004; Bainy et al.,1996). The concentrations of the reagents were: potassium phosphate buffer pH 7.8 (50 mM), EDTA solution 100 μM, cytochrome c 10 μM, xanthine 50 μM, and xanthine oxidase (0.019 units). Absorbance was recorded at a wavelength of 550 nm. The results were expressed as units of SOD/mg of protein, with one unit (U) of SOD activity defined as the amount of enzyme which causes 50% inhibition of cytochrome c reduction per minute (Bainy et al.,1996).
The activity of GST (EC 2.5.1.18) was measured by spectrophotometry according to Habig et al. (1974), using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The latter is conjugated by GST isoforms in the presence of reduced glutathione (GSH), which absorbs at 340 nm. The assay conditions were as follows: sodium phosphate buffer pH 6.5 (0.1 M), GSH 10 mM, and CDNB 20 mM. Enzymatic activity was expressed as μmol of GS–CDNB conjugate formed/min/mg protein.
All measurements were made at least in duplicate, using analytical-grade reagents. The enzymatic activities were determined at 25°C and referred to protein content determined by the method of Lowry et al. (1969), using serum bovine albumin as standard.
LPO was measured as malondialdehyde (MDA) by TBARS, under acidity and heat conditions. The chromogen formed was measured by fluorometry according to Ohkawa et al. (2006) and Oakes and van der Kraak (2003). An aliquot of liver from each individual was homogenized 10% wt/vol in 1.15% KCl with 35 μM butylated hydroxytoluene (BHT). The 10% homogenate was added to a mixture composed of 8.1% sodium dodecyl sulfate (SDS), acetic acid 20% (pH 3.5), TBA 0.8% and BHT 35 μM solutions. The tubes were incubated in a thermostatized water bath at 95°C for 1 h. After cooling, Milli-Q water and n-butanol (99.4 %) were added and the mixture was mixed by inversion. Finally, the mixture was centrifuged at 2000 × g for 10 min at 15°C to separate the organic layer, which was measured by fluorometry at an excitation wavelength of 515 nm (slit width 10 nm) and an emission wavelength of 553 nm (slit width 5 nm). The MDA concentration was expressed as nmol TBARS/g of tissue and tetramethoxypropane (TMP) was used as an external standard.
Immunodetection of CYP1A Protein Using Western Blot Analysis
The presence of CYP1A protein was evaluated by immunodetection using anti-CYP1A polyclonal antibodies obtained previously (de la Torre et al.,2003). Briefly, cracking buffer (500 mM 2-mercaptoethanol, 250 mM Tris pH 6.8, 50% glycerol, 10% SDS, 0.5% bromophenol blue) was added to SF aliquots of the samples and solved through SDS-polyacrylamide gel electrophoresis (12% PAGE-SDS, Ausubel et al.,2005). Then, proteins were transferred onto nitrocellulose using a transfer buffer (50 mM Tris pH 7.5, 196 mM glycine, 20% methanol). The nitrocellulose membrane was incubated in blocking buffer (5% (wt/vol) non-fat milk powder in phosphate-buffered saline solution). Then, the membrane was sequentially incubated for 1.5 h with anti-CYP1Ar mouse serum and with a commercial anti-mouse antibody conjugated to alkaline phosphatase (Sigma Aldrich, St. Louis, MO), diluted 1:2000 and 1:20,000 in blocking buffer, respectively. Bound antibodies were visualized using 5-bromo-4-chloro-3-indolyl-phosphate (0.165 μg/mL) and nitro blue tetrazolium (330 μg/mL) substrate precipitation (Promega, Madison, WI). Finally, the membrane was digitalized and the intensity of the bands was quantified using Image J 1.44p software (National Institute of Health, USA).
SF samples of liver from carps and bogas treated with β-naphthoflavone (BNF) were included as standard references to check the stability of the measurement conditions throughout the sample set. Carp and boga samples were obtained by intraperitoneal injection of 50 mg/kg BNF in corn oil (positive control). Fish were kept under the laboratory conditions described in Section “Sample processing.” Livers were sampled 48 h post-injection and processed as described previously; carps were also sampled at 24 h.
Water Physicochemical Parameters and Sediment Characterization
The physicochemical water parameters were measured in surface water samples taken from Punta Lara, Las Flores, and aquaria. Samples were collected in three opportunities by duplicate and transported to the laboratory. The analysis included hardness, soluble reactive phosphorus, ammonium, nitrites, nitrates, and SPM. All measurements were made in duplicate following standard procedures (APHA,2005). In addition, temperature, pH, dissolved oxygen concentration, and conductivity were measured in situ using Hanna Instruments electrodes (98240 HI; 9142 HI; 933301 HI).
In Punta Lara, the composition and the presence of metals, AHs, PAHs, and total PCBs were determined in surface sediment samples from the uppermost oxygenated layer (0–2 cm). The organic matter content of the sediment was determined by ignition loss and the sediment was characterized by the percentage composition in terms of sand, silt, and clay using sieve and pipette methods (Depetris,1995). The presence of metals in the sediment (total chrome, copper, nickel, lead, and zinc) was determined after acid digestion of the samples using standardized colorimetric methods (Hach Corp., Germany). The aliphatic and aromatic fractions were separated by silica column chromatography and each fraction was analyzed by high-resolution gas chromatography in a Shimadzu chromatograph (Colombo et al.,1989; Lavarías et al.,2005). The PCBs were analyzed by high-resolution gas chromatography using a DB-5 capillary column of 30 m and electron capture detectors (HRGC/μECD). Total PCB quantification was based on the analysis of 41 individual congeners and solutions of Aroclors 1242 and 1254.
Based on the high complexity of the natural sediment samples, a possible anthropogenic or biogenic origin of the AHs was evaluated with alkane distribution indices (Colombo et al.,1989; Lavarías et al.,2005). In this context, these authors have defined the low/high molecular weight hydrocarbon index (LMW/HMW) as the ratio of the sum of n-alkanes ≤C20 over the sum of n-alkanes ≥ C21. In addition, the carbon preference index (CPI) is the ratio (2(C27+C29)/(C26+2C28+C30), and Alk/Iso index is given by (∑n-C14–n-C18/∑five isoprenoids).
Both water and sediment samples were kept refrigerated in glass bottles during transport to the laboratory and stored at −20°C until processing.
Statistical Analysis
The data were tested for normality and variance homogeneity with Kolmogorov–Smirnov and Levene's tests, respectively. The Student's t test was used for comparisons between the experimental and control groups for each fish species. Differences were considered significant at a level of p < 0.05 (Zar,1996).
RESULTS
Condition Indices and Biochemical Biomarkers Measurements
C. carpioin situ exposure in Punta Lara promoted a significant increase in LSI and CF, the former being particularly higher (45%) than the reference values (Fig. 1). The hepatic antioxidant enzymes of fish from Punta Lara were found to be increased as compared to reference fish, particularly CAT (51%); in addition, GST also showed an important response (40%). No significant changes in the levels of TBARS were observed in fish from Punta Lara as compared to those in fish from Las Flores stream (RSI) (Fig. 1). No mortality was recorded in any of the experimental groups during the assayed period.
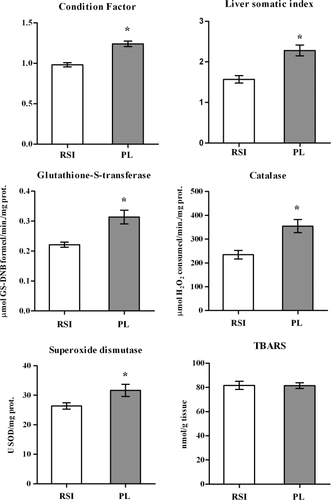
Condition indexes and biochemical biomarkers of C. carpio juveniles exposed in cages placed above the sediment surface for 21 days in Punta Lara (PL) and Las Flores stream (RSI). Values of bars indicate mean ± SEM; (n = 10). (*) indicates significant differences from the RSI (p < 0.05).
L. obtusidens (bogas) captured in Punta Lara showed no changes in the LSI; however, there was a significant decrease in CF (10%) (Fig. 2). As regards the antioxidant responses, CAT denoted a significant decrease (24%) whereas SOD denoted an increase of 47% in the enzymatic activity of fish collected in Punta Lara respect to the reference site (RSII); at the same time, TBARS levels increased in the bogas of the experimental site. On the other hand, no differences were observed in GST activity of bogas collected from both sites (Fig. 2).
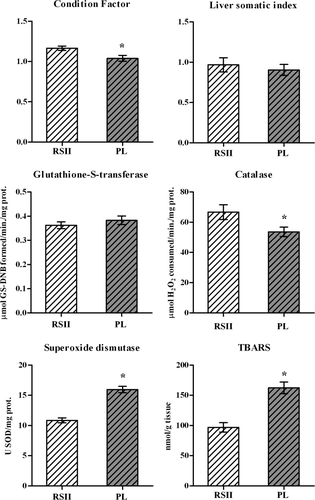
Condition indexes and biochemical biomarkers of L. obtusidens juveniles collected from Punta Lara (PL) and references site (RSII). Values of bars indicate mean ± SEM; (n = 10). (*) indicates significant differences from the RSI (p < 0.05).
Immunodetection of CYP1A Protein
The Western blot analysis showed the presence of immune response to cytochrome P450 1A in BNF-treated fish (positive control, β) and in fish from Punta Lara (PL), whereas no protein recognition by the antibody was observed in fish from the reference sites (RSI and RSII) (Fig. 3). The apparent molecular weight of the major band coincided with that reported for fish CYP1A (55–65 kDa). The intensity of bands for fish from Punta Lara was similar (boga) or 40% lower (carp) than the intensity observed for 48-h BNF-treated fish [Fig. 3(A,B)]. Besides, for carps exposed in Punta Lara the expression of CYP1A was 2.6 times higher than 24-h BNF-treated fish [Fig. 3(A)]. The results shown in Figure 3 represent samples from one fish from each in situ exposed and sampled group since similar results were obtained for the rest of fish from every group (data not shown).
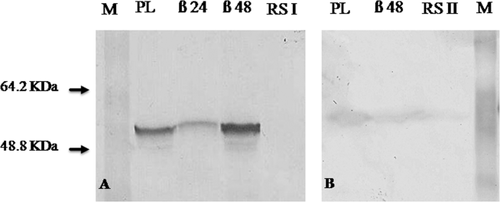
Western blot analysis of CYP1A expression of C. carpio (A) and L. obtusidens (B). SF samples from fish exposed and collected in Punta Lara (PL) and references sites (RSI, RSII); β24, 48: SF samples obtained 24 and 48 h after injection with BNF (50 mg/kg); M: molecular weight marker (Page Ruler™. Prestained Protein Ladder, Fermentas). A: PL and RSI (150 and 160 μg protein); β24 and 48 (105 and 120 μg protein). B: PL and RSII (103 and 83 μg protein); β48 (113 μg protein). Each line represents samples from one fish from each in situ exposed and collected group.
Water Physicochemical Parameters and Sediment Characterization
Table 1 shows the water physicochemical parameters of Las Flores stream, Punta Lara, and aquaria during the initial laboratory period. Field sites had rather similar values of temperature, pH, and dissolved oxygen concentration, but in Punta Lara the conductivity was lower and revealed higher ammonium and nitrite concentrations, SPM, and hardness values. Las Flores stream values are within the limits of Law no. 24051 and those allowed by Law no. 19587 on the hygiene and safety of drinking water.
Table 2 shows the characterization of the sediment, total concentration of PCBs, PAHs and AHs alkane distribution indices, and the presence of heavy metals in the surface sediment layer in Punta Lara. According to the grain-size analysis, based on the relative amount of the main textural classes (sand, silt, and clay), the sediment in Punta Lara is sandy silt, with a predominance of fine grain-size (59% silt). The analysis of the surface sediment layer in Punta Lara revealed the presence of total PAHs and PCBs.
Alkane indices allow an approximate identification of the sources of hydrocarbons in the sediment. According to Colombo et al. (1989) and Lavarías et al. (2005), the LMW/HMW ratio is close to 1 for algae, plankton, and crude oil, whereas it is lower than 1 for sedimentary bacteria, marine animals, and higher plants. In the present study, this index was 0.1. The Alk/Iso is another index used to evaluate the presence of oil and the biodegradation of n-alkanes, with low values suggesting a high degree of oil degradation and high values suggesting a low degree of oil degradation; in our case, a low value (0.1) was found. CPI values close to 1 suggest the presence of petrogenic hydrocarbons, and values from 3 to 6 indicate biogenic hydrocarbons (vascular plants) or uncontaminated sediments (Colombo et al.,1989). The surface sediment layer in Punta Lara showed a CPI close to 1 (1.5).
The surface sediment layer in Punta Lara contained heavy metals (Table 2), of which only chrome exceeded the guidance values recommended by the CCME (1999–2002).
DISCUSSION
At present, fish biomarker parameters have gained increasing importance as tools for toxicity assessment in aquatic environments for monitoring programs (Sanchez and Porcher,2008). Despite this widespread global use, until now in Argentina few studies are available reporting freshwater environmental quality using this assessment tool. To our knowledge this is the first report using a biological approach that evaluates the environmental quality of Río de la Plata estuary by means of fish biomarker responses.
About condition indices, we observed a different response in both species. In caged carps, an increase in the CF of fish exposed in Punta Lara near areas with domestic and industrial discharges was observed. It has to be mentioned that sampled caged carps intestine had feces revealing exposure to sedimental particles in both experimental and reference sites. The increase of CF values were similar to those obtained with fish collected from a river affected by the sugar processing industry (Tejeda-Vera et al.,1996) and in fish from a site polluted by high levels of PAHs (Almroth et al.,2008b). In both cases, the authors suggested that higher food availability may have compensated for the environmental changes caused by pollution. An increase in LSI may be due to an increase in liver weight or a decrease in total weight. On the basis that the weight gain of caged carps exposed in Punta Lara was reflected in an increase in CF, the increase in LSI could be attributed to an increase in liver weight. The LSI index is commonly used to evaluate fish condition and provides some information on the metabolic status of the liver (Bagenal and Tesch,1978). High values of LSI resulting from liver hypertrophia have been associated with hydrocarbon exposure (Tejeda-Vera et al.,1996). In agreement with these results, Fernandez-Jover et al. (2007) showed an increase in CF and LSI, in a field-cage assay of fish exposed to organic polluted waters. On the other hand, a decrease in CF and no changes in the LSI index were observed in bogas collected from the same experimental site where carps were held. These results are coincident with those observed by Ozmen et al. (2000) in carps collected from sites polluted with different contaminants including metals, although other authors have found no relationship between this index and environmental pollution in fish.
The presence of a variety of PAHs and coplanar PCBs in aquatic environments is known to induce cytochrome P450 1A in fish. BNF is a known PAH inducer of cytochrome P450 1A activity in different fish species, e.g. turbot (Hartl et al.,2007) and carp (de la Torre et al.,2006). In the present study, the exposed carps in Punta Lara showed high levels of protein CYP1A, suggesting phase I biotransformation processes mediated by CYP1A, which were most likely promoted by the presence of PAHs in the study area (see Table 2). A similar response was observed in the L. obtusidens specimens collected from Punta Lara. Besides, Bozcaarmutlu et al. (2009) and Hartl et al. (2007) reported similar results in different contaminated sites or in fish used in experiments with polluted sediments from Cork Harbor (Ireland).
An increase in GST activity has been reported in field assays by exposure to organic pollutants (Amado et al.,2006; Bozcaarmutlu et al.,2009) and in laboratory experiments using different inducers, e.g. PAHs (van der Oost et al.,2003; Ascar and de la Torre,2005; Scarcia and de la Torre,). The results obtained with caged specimens of C. carpio are in agreement with those of Huang et al. (2005), who found induction of GST activity in individuals of the same species collected from a polluted site of the Yellow River in China. However, in this work, a non-significant GST induction was found in the bogas collected in Punta Lara; this response was also reported by Almroth et al. (2008b) in fish collected from sites affected by PAHs. On the other hand, bogas collected in Punta Lara showed a non-significant induction of GST activity; this response was also reported by Almroth et al. (2008b) in fish collected from sites affected by PAHs.
Aerobic organisms, including fish, have developed an antioxidant defense system to cope with the potential deleterious effects of ROS generated during aerobic respiration and resulting from exposure to xenobiotics. In this context, many pollutants such as PAHs (benzo(a)pyrene, 3-methyl colanthrene), halogenated hydrocarbons (PCBs, dioxines), and metals (Cd, Cu, Fe) were considered by Di Giulio and Meyer (2008) as potential ROS producers. In the present study, the in situ exposure of C. carpio specimens induced an increase in the activity of both hepatic enzymes, CAT (51%) and SOD (20%). Exposure of fish to hydrocarbons often leads to increased activity of CAT and SOD, parts of the defense mechanism against oxidative damage (Stanic et al.,2003). A remarkable increase in the activity of CAT was also found in mullets captured in six different locations of the West Black Sea Coast of Turkey along three consecutive years and was attributed to ROS generation by pollutants (Bozcaarmutlu et al.,2009). In addition, increased GST in exposed carp may also catalyze the conjugation of GSH with electrophilic compounds and LPO derivatives such as 4-hydroxynonenal. The exposure of C. carpio in Punta Lara did not change hepatic MDA levels, as indicated by TBARS. Similar results were reported by Almroth et al. (2005) for eelpouts from a chronically polluted area affected by dredging and oil spill. However, these authors found high levels of TBARS in cods from the same area (unpublished data). Besides, Avci et al. (2005) found no significant differences in MDA hepatic levels between Silurus glanis individuals collected upstream and downstream a petroleum refinery. The similar MDA levels found in carps from Punta Lara and Las Flores stream may indicate the absence of LPO in the liver; this is possibly related to the sustained increase in the antioxidant defenses (mainly CAT and SOD) shown after in situ exposure in Punta Lara. In collected native fish, we found a different pattern in oxidative stress responses. Juvenile L. obtusidens sampled in Punta Lara denoted a significant increase in SOD activity (47%) and a decrease in CAT activity (24%). SOD is a key enzyme representing one of the most important enzymatic defenses against oxygen radicals. In this study, SOD activity of bogas living under environmental conditions of Punta Lara showed to have an important role dealing oxidative stress; however augmented hepatic levels of TBARS could indicate that an oxidative condition was still present in these fish. Increased hepatic TBARS levels have been previously reported in carp inhabiting a site polluted by urban and industrial wastes (Falfushynska and Stolyar,2009).
It is interesting to mention that biomarker responses observed in carps of reference site were consistent with a field baseline of biomarkers previously assessed, where four sampling moments and two reference sites were considered (see Scarcia et al. 2012). Briefly, expressing baseline of each biomarker (n = 40) as mean ± standard deviation, observed variability of condition indices were 1.3 ± 0.3 (FC) and 1.8 ± 0.5 (LSI). Taking into account biochemical biomarkers, variability of GST, CAT, and SOD activities were 0.16 ± 0.04; 183 ± 56; and 17± 6 respectively; TBARS levels were 89 ± 28 (see section “Condition indices and biochemical biomarkers determinations” for biomarker units). In this context, reference values found in this study fall within the expected range for each biomarker.
The quality of water and sediments in the southern coastal fringe of the Río de la Plata estuary has been well characterized based on different physicochemical parameters, and abundant information on the levels of persistent organic compounds and heavy metals can be found (Colombo et al.,1990, 2005, 2006; FREPLATA,2003). Water quality is poor at sites near sewage discharges, with high levels of ammonium-nitrogen (0.4–1.5 mg/L) and phosphates (1.3–1.7 mg/L) (FREPLATA,2003). In the present study, the values recorded in Punta Lara were well below the former ones, although nitrite exceeded the guidance levels for protection of aquatic life. On the other hand, in Punta Lara, the dissolved oxygen concentration was not critical and was similar to that at the reference site. In general terms, high oxygen levels are observed in the entire region of the Río de la Plata estuary as a result of wind action (FREPLATA,2003).
The levels of total AHs, PAHs, PCBs, and trace metals in the sediment in Punta Lara are consistent with values reported previously for the Río de la Plata estuary (see Table 2). In turn, the values of PAHs in Punta Lara were lower than those recorded in Uruguayan and Argentine coastal areas of the Río de la Plata estuary (Muniz et al.,1998; Colombo et al.,2006, respectively) and in a tributary, the Santiago River (West Channel), which receives inputs from the largest petroleum refinery in Argentina (Colombo et al.,1989). The levels of total AHs in Punta Lara were similar to those previously recorded in nearby areas (Colombo et al.,1989) however, it is worthy to mention that a high percentage of UCM (80%) registered in Punta Lara is indicative of chronic degraded petroleum. The alkane indices determined in this study in Punta Lara indicated that, although the terrestrial plants contributed to the hydrocarbons in the sediment, they derived mainly from petrogenic sources. The concentration of total PCBs found in this study in the surface sediment of Punta Lara exceeded the value recommended by the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life (0.034) (CCME, 1999–2002). The high concentration of PCBs and other organochlorine compounds present in the sediment of these areas in the Río de la Plata estuary has been attributed to a high content of silt, clay, and organic matter acting as important reservoirs of persistent toxic substances (Colombo et al.,1990). In this study, the organic matter content and total PCB concentrations in the sediment in Punta Lara was similar to values reported for these areas (Colombo et al.,1989, 2005). In regard to the metals present in the sediment, only chrome showed values higher than the sediment quality guidance values for the protection of the aquatic biota suggested by CCME (1999–2002). The sediment in the West Channel (Ronco et al.,2009) and in the Uruguayan coast of the Río de la Plata estuary (Muniz et al.,1998) shows metal concentrations higher than those in Punta Lara. Another aspect to be considered is the presence of pollutants adsorbed to SPM, which is the fraction most bioavailable to aquatic organisms. In this context, Colombo et al. (2005, 2006) found a high total particle flux and a high sedimentation rate in the coast of Río de la Plata estuary, north of Punta Lara reporting higher PCBs and PAHs concentrations in the settling particles than the mean concentration in the surface sediment. In this study, the SPM values of Punta Lara were similar to those reported by Colombo et al. (2005) and higher than those obtained in Las Flores (Table 1).
The persistent organic compounds may bioaccumulate in aquatic organisms through the ingestion of suspended particles and through the diet (biomagnification) (van der Oost et al.,2003). Studies performed in the Río de la Plata estuary, Argentina, have revealed bioaccumulation of pollutants in many species, including C. carpio and L. obtusidens (Colombo et al.,2000). These authors observed that the most representative pollutants in these fish were certain organic compounds, among which the most abundant were hydrocarbons and PCBs, suggesting a continuous input of these potentially bioaccumulable compounds. In the same study, the authors reported less accumulation of pollutants in muscle of L. obtusidens sampled upstream, in Río Tigre. This could be explained by geographic variations and by the low levels of lipid content and feeding habits compared to those of C. carpio and a detritivorous species (Prochilodus lineattus). It is interesting to mention that invading mussels such as Limnoperna fortunei and Corbicula fluminea, profusely distributed in the Río de la Plata, have become one of the main food items of the boga (Penchaszadeh et al.,2008) and that the presence of mussels in polluted environments could lead to an enhanced bioaccumulation of organochlorine compounds in fish (Colombo et al.,1990).
Finally, biomarker responses found in caged carps and collected boga could be an evidence of the biological effects probably promoted by the environmental exposure to PAHs, PCBs, and metals found in the surface sediment, suspended particle matter, and food items present in Punta Lara. In addition, L. obtusidens responses also provided more ecological information of adverse effects on native species and complemented the information obtained with C. carpio. On the basis of these results, further field studies will be conducted in Punta Lara and areas of interest for monitoring purposes in the Río de la Plata estuary.
CONCLUSION
The battery of biomarkers assayed showed that anthropogenic pollution was associated with differences in biomarkers on both L. obtusidens and C. carpio juveniles, although a different pattern of response was exerted. In addition, this biomarker field approach provided valuable information about the environmental quality of Punta Lara and on the impact of persistent pollutants on resident organisms.
Acknowledgments
Authors are grateful to the Grupo de Biología Molecular del Instituto de Biotecnología, CICVyA-INTA for their assistance in molecular techniques.




