Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil
Abstract
The potential environmental toxicity of zero-valent iron nanoparticles (nZVI) and three types of nanosilver differing in average particle size from 1 to 20 nm was evaluated using seed germination tests with ryegrass, barley, and flax exposed to 0–5000 mg L−1 nZVI or 0–100 mg L−1 Ag. For nZVI, germination tests were conducted both in water and in two contrasting soils to test the impact of assumed differences in bioavailability of nanoparticles. Inhibitory effects were observed in aqueous suspensions at 250 mg L−1 for nZVI and 10 mg L−1 for Ag. Reduction in shoot growth was a more sensitive endpoint than germination percentage. Complete inhibition of germination was observed at 1000–2000 mg L−1 for nZVI. For Ag, complete inhibition was not achieved. The presence of soil had a modest influence on toxicity, and inhibitory effects were observed at 300 mg nZVI L−1 water in soil (equivalent to 1000 mg nZVI kg−1 soil). Complete inhibition was observed at 750 and 1500 mg L−1 in sandy soil for flax and ryegrass, respectively, while for barley 13% germination still occurred at 1500 mg L−1. In clay soil, inhibition was less pronounced. Our results indicate that nZVI at low concentrations can be used without detrimental effects on plants and thus be suitable for combined remediation where plants are involved. Silver nanoparticles inhibited seed germination at lower concentrations, but showed no clear size-dependant effects, and never completely impeded germination. Thus, seed germination tests seem less suited for estimation of environmental impact of Ag. © 2010 Wiley Periodicals, Inc. Environ Toxicol 2012.
INTRODUCTION
Within nanotechnology, iron nanoparticles can be used for a range of different applications, including catalysts for carbon nanotube production (Wong et al., 2005), magnetic fluids (Wu et al., 1999), magnetic resonance imaging (MRI) contrast agents (Mornet et al., 2004; Jun et al., 2005), core materials in Ni-Fe batteries, and catalysts and sorbents for environmental remediation (Li et al., 2003; Zhang, 2003). All these applications are in some ways exploiting the altered properties of nanosized iron particles with respect to enhanced surface area and high surface reactivity compared to otherwise identical larger particles. In the case of remediation, zero-valent iron (nZVI) is the most relevant type of nanoiron, as its high reduction potential has proven valuable for degradation of several organic pollutants, included chlorinated compounds like TCE and atrazine (Liu et al., 2007; Satapanajaru et al., 2008).
Silver-based materials have been widely used over the last decades within medicine, electronics, photography, etc. (Ratte, 1999). Even after the widespread replacement of photographic films with numeric cameras, the annual release of silver into the environment from industrial wastes and other emissions has been high. In the US, amounts in the order of 2500 tons year−1 have been reported, out of which 150 tons ended up in sewage sludge and 80 tons have been released into surface waters (Khaydarov et al., 2009). Silver nanoparticles are currently receiving considerable attention because of their numerous applications in consumer products. By far the greatest number of consumer product applications of nanomaterials involves nano silver (http://www.nanotechproject.org/inventories), and the products range from wound dressings, socks, and other textiles to air filters, toothpaste, baby products, and house old appliances like refrigerators, vacuum cleaners, and washing machines. In most cases these are metallic silver nanoparticles deposited on exposed surfaces, while some applications exploit combined effects of particulate silver and electrochemically generated ionic silver.
The widespread use of nanomaterials has lead to an increasing amount of research on their toxicity and ecotoxicity. Data now exist demonstrating different levels of toxicity for several types of nanoparticles, such as fullerenes, carbon nanotubes, metals, and metal oxides (Lam et al., 2006; Moore, 2006; Nowack and Bucheli, 2007; Klaine et al., 2008). The majority of the toxicity studies on nanomaterials to date have concerned mammals (including mammalian cell cultures) and aquatic species, and comparatively few studies have been conducted using terrestrial species (soil invertebrates, soil microorganisms, or plants; US-EPA, 2007; Christian et al., 2008; Klaine et al., 2008).
Some data on both positive and negative effects of nanoparticles on higher plants do however exist. It has e.g., been shown that a mixture of nanoscale SiO2 and TiO2 can increase nitrate reductase activity in soybean (Glycine max), enhance its ability to absorb and utilize water and fertilizer, stimulate its antioxidant system, and apparently hasten its germination and growth (Zheng et al., 2005). Nano-TiO2 has further been reported to promote photosynthesis and nitrogen metabolism, and thus greatly improve growth of spinach at certain concentrations (Hong et al., 2005; Zheng et al., 2005; Yang et al., 2006). However, after investigating the phytotoxicity of nanoscale Al2O3 powders with or without phenanthrene coating, Yang and Watts (2005) concluded that uncoated alumina particles inhibited root elongation of corn, cucumber, soybean, cabbage, and carrot. This study attracted attention from scientists and media, and was used to claim that nanoparticles can exert a negative effect on plants (Murashov, 2007). The authors above did however not quantify Al dissolution and thus could not determine whether the observed phytotoxicity was caused by nanosized Al2O3 or aluminum ions in the aqueous solution (Murashov, 2007).
Recently, Lin and Xing (2007) tested phytotoxicity of five types of nanoparticles (multiwalled carbon nanotubes, aluminum, alumina, zinc, and zinc oxide) on seed germination and root growth of six higher plant species (radish, rape, ryegrass, lettuce, corn, and cucumber). Only Zn and ZnO particles were observed to have significant inhibition on seed germination and root growth. Most recently, Barrena et al. (2009) evaluated the toxicity of three types of nanoparticles: Au (average particle size, APS, 10 nm), Ag (APS 2 nm), and Fe3O4 (APS 7 nm) using germination tests with cucumber and lettuce, but detected no or only low toxicity at the assayed concentrations (62, 100, and 116 mg L−1, respectively).
There are thus still many unresolved issues and challenges concerning the biological effects of nanoparticles on terrestrial organisms, and particularly plants. The aim of this investigation was to provide more information on phytotoxicity of two types of engineered nanoparticles that will either be spread deliberately on soil where they are likely to interact with living plants (nZVI) or nanoparticles that are widely used and likely to enter soil through unintended spread (silver nanoparticles). For the latter, three particle types differing in average particle size was compared, as toxicity is likely to be affected by this parameter.
MATERIALS AND METHODS
Seeds of three plant species, flax (Linum usitatissimum L., cv. Electra), ryegrass (Lolium perenne L., cv. Tove), and two-rowed barley (Hordeum vulgare L., cv. Annabell) were used in these trials, and generally followed the US EPA protocol for seed germination toxicity tests (US-EPA, 1996; Yang and Watts, 2005). These plant species were chosen after a preliminary experiment based on two criteria. First, they represent both monocotyledonous and dicotyledonous plants. Second, the two monocotyledonous species have very different seeds sizes, and thus may be affected differently by the nanoparticle suspensions. Lastly, they were responsive to nZVI within the concentration range tested in a preliminary test (rejected species included red clover, white clover, and meadow fescue).
Briefly described, seeds were rinsed three times with deionized water and soaked in water or in nanoparticle suspensions for 8 h. Then 10 seeds were placed in a Petri dish with a disc of filter paper and 5 mL water or nanoparticle suspension per dish with 1 cm or more between each seed. Petri dishes were covered and sealed with tape, and incubated for 5 days at 23°C in the dark. At this time, controls had developed roots that were at least 20-mm long, and all treatments were harvested by recording germination percentage and root and shoot lengths (measured with a ruler with the plant stretched out on a filter paper).
Germination tests in soil used the same plant species and seed densities, placing seeds in Petri dishes and covering them with 50 g of air dry soil that was subsequently wetted to 75% of their respective water holding capacities using water or aqueous nanoparticle suspensions to provide final concentrations of ∼300, 750, 1500, 3000, 6000, and 15 000 mg nZVI per liter soil water. The corresponding nZVI concentration per kg soil were 100, 250, 500, 1000, 2000, and 5000 mg nZVI kg−1 soil. Treatments with water or water with 0.25% polyacrylic acid (PAA) were used as controls. The two soils used had comparable pH and organic C content, but contrasting texture (clay loam and sandy loam), and were collected at the experimental farm of the University of Life Sciences at Ås, Akershus county, S.E. Norway, air-dried and sieved (<2 mm) and subjected to traditional soil analysis (Schinner et al. 1996). Table I summarizes the physicochemical characteristics of the two soils. Petri dishes with seeds and soil were covered with 8-cm tall transparent boxes permitting shoot development and preventing loss of water, and placed in growth chamber with 16/8h light/dark cycle (∼300 μmol m−2 s−1 PAR) at 23°C. Loss of water was checked by weighing, and deionized water added when required. After 5 (for barley) or 7 days (for flax and ryegrass) the percent seed germination was recorded and the length of shoots measured (height of plant measured from the soil surface). Only seeds with shoots protruding from soil surface were considered as having germinated.
| Soil | Clay Loam | Sandy Loam |
|---|---|---|
| pH(water) | 5.34 | 5.76 |
| Water holding capacity (%) | 44.8 | 41 |
| Organic matter content (%) | 4.5 | 1.1 |
| Texture | ||
| Sand (%) | 23 | 85 |
| Silt (%) | 46 | 11 |
| Clay (%) | 31 | 4 |
The nZVI used for experimentation was produced by adding 30 mL of a solution with 71.8 g L−1 NaBH4 in deionized water drop-wise to 100 mL of a solution with 48 g L−1 of FeCl3 6H2O under vigorous stirring (Huang and Ehrman, 2007). The black particle suspension formed was retrieved from the suspension using a magnet and washed with methanol. Methanol was then removed by centrifugation at 4000 × g for 15 min and the nZVI resuspended in a similar volume of deionized water with 0.25% (v/v) polyacrylic acid (PAA), and dispersed by sonication for 15 min. The washing step was introduced to avoid confounding toxic effects of residual BH4 and consequent high suspension pH (approximate pH 9.4 before washing). Six concentrations of nZVI were used with final concentrations of 100, 250, 500, 1000, 2000, and 5000 mg Fe kg−1. pH of the suspensions ranged from 5.1 to 7.5 for the low to the high concentrations of Fe, respectively. Corresponding Eh values ranged from 350 to -50 from low to high concentrations of nZVI, respectively.
Two types of commercially available silver nanoparticles were used: Colloidal silver nanoparticles (Agcoll) in aqueous suspension with 20 mg Ag L−1 and a pH of 8.0 was purchased from Purest Colloids, Westampton, NJ (trade name “MesoSilver”). These had a particle size range of 0.6–2 nm, according to the manufacturer. The second type (Ag20) was a dry silver nanoparticle powder purchased from Quantum Sphere, Santa Ana, CA (trade name Ag-QSI), with an average particle size of 20 ± 2.5 nm (Oughton et al., 2008) and a surface area of 10–40 m2 g−1, according to the manufacturer. These particles were suspended in 0.1% (v/v) Tween 20 using an ultrasound bath, and had a pH in the range of 6–6.5. In addition to the commercial silver nanoparticles, we also used silver nanoparticles produced in our own laboratory (Ag5) through reduction of AgNO3 with NaBH4. Here, 15.7 mg AgNO3 was mixed with 136 mg Na-citrate in 80 mL deionized water, upon which a solution of 35 mg NaBH4 in 20 mL water was added drop wise under vigorous stirring. The produced particles were separated from the solution by centrifugation at 4200 × g for 15 min, after which the nanoparticles were suspended directly in an equivalent volume of water with 0.1% Tween 20 and dispersed by sonication for 15 min.
RESULTS
Preliminary tests with different seed germination protocols showed that seeds imbibed in a suspension of nano particles for 8 h and subsequently germinated in pure water had only a limited ability to demonstrate toxic effects on germination. When seeds were imbibed in water for 8 h and subsequently germinated in nanoparticle suspensions, a toxicity response was observed. This response was quantitatively similar to that observed on seeds that were both imbibed and germinated in corresponding nanoparticle suspensions (both germination percentage and root and shoot growth). We thus chose to present only results from the latter germination test protocol.
The effects of nZVI up to 5000 mg L−1 on germination of seeds in aqueous suspension are shown in Table II. There was no significant (P < 0.05) effect of nZVI on seed germination rates of any plant species at concentration up to 250 mg L−1. At 500 mg L−1, flax germination was unaffected, while barley had a germination rate of 90% and ryegrass 60%. At 1000 mg L−1 both flax and barley had 90% germination and ryegrass 0%. No seed germination of any of the plant species occurred at 2000 and 5000 mg nZVI L−1. Growth of shoots and roots from germinating seeds responded differently to nZVI, with relative inhibition of root growth generally being lower than for shoot growth (Fig. 1). Also, shoot and root growth was a more sensitive indicator of the inhibitory effect of nZVI than germination percentage, as the first adverse effects could be seen already at 250 mg L−1.
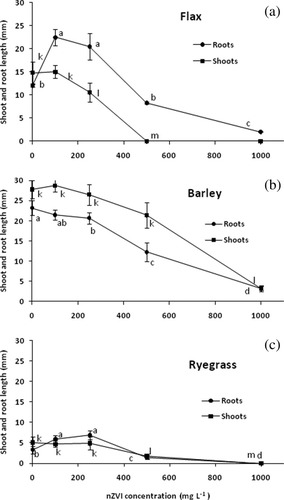
Effects of nZVI concentration on germination of seeds soaked and incubated in nano-suspensions. Graphs show root and shoot development of flax (a), barley (b) and ryegrass (c). Means associated with the same letter are not significantly different (Student's t test, P = 0.05, n = 10). Bars are SEM (standard error of the mean).
| nZVI (mg L−1) | Flax (%) | Barley (%) | Ryegrass (%) |
|---|---|---|---|
| 0 | 100 | 100 | 100 |
| 100 | 100 | 100 | 100 |
| 250 | 100 | 100 | 100 |
| 500 | 100 | 90 | 60 |
| 1000 | 90 | 90 | 0 |
| 2000 | 0 | 0 | 0 |
| 5000 | 0 | 0 | 0 |
Inhibition of seed germination in soil by nZVI, based on efficient concentration in soil water, varied according to soil texture, with the highest inhibition observed in sandy soil. Germination percentage of barley was unaffected up to ∼750 mg L−1 in clay-rich soil, while a reduced germination for barley in sandy soil was observed already at ∼300 mg L−1 (Fig. 2). Seed germination was observed in both soils up to 1500 mg L−1. At higher concentrations, no seed germination was observed for any of the species (results not shown). Ryegrass was more sensitive to nZVI than barley when germinated in soil, as observed for aqueous germination tests, but in clay soil ryegrass could still germinate (17% of the seeds) at the highest concentration (1500 mg L−1). Flax was seemingly the most sensitive to nZVI when germinated in soil, but this was partly attributed to poor ability to form shoots that grew to the surface. Flax roots were observed in many cases where no shoots appeared, a phenomenon that was also observed for germination of flax in aqueous suspension. Seed germination in soil measured as length of protruding shoots showed only slightly more detailed effects than germination percentage. Only for barley germinated in clay-rich soil could a gradual decrease in shoot length be observed between 0 and 750 mg L−1, while the germination percentage remained at 100%.
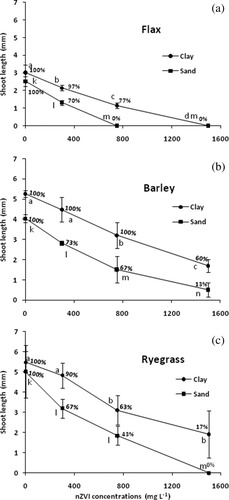
Shoot length of flax (a), barley (b), and ryegrass (c) germinated in a clay loam soil or a sandy loam soil with different concentrations of nZVI in the soil water. Tests, letters and bars as in Figure 1 (n = 30). Values associated with each data point represent average germination percentage.
The three types of silver nano particles affected seed germination differently for the three plant species tested. The smallest particle type, Agcoll had an inhibitory effect at a concentration as low as 10 mg L−1, but only with ryegrass (Table III). The intermediately sized particle type (Ag5) also had weak inhibitory effect at 10 mg L−1, which increased at higher concentrations, but this effect was only observed with barley. Barley was also the only plant species affected by the largest sized type of silver nano particles (Ag20), with a weak inhibition observed at 10 mg L−1 increasing slightly at higher concentrations. No effect on germination percentage of flax was observed for any types of silver nano particles, even at the highest concentrations.
| Ag (mg L−1) | Flax (%) | Barley (%) | Ryegrass (%) | |
|---|---|---|---|---|
| H2O | 0 | 100 | 100 | 100 |
| Tween 20 | 0 | 100 | 100 | 100 |
| Agcoll | 10 | 100 | 100 | 80 |
| 20 | 100 | 100 | 50 | |
| Ag5 | 10 | 100 | 90 | 100 |
| 20 | 100 | 80 | 100 | |
| 100 | 100 | 80 | 100 | |
| Ag20 | 10 | 100 | 90 | 100 |
| 20 | 100 | 80 | 100 | |
| 100 | 100 | 80 | 100 |
- Agcoll with a 2- to 20-nm size range distribution; Ag5 with APS = 5 nm; Ag20 with APS = 20 nm) at different concentrations on seed germination (%) of flax, barley, and ryegrass in aqueous suspensions with 0.1% (v/v) of the surfactant Tween 20.
Shoot length of germinating seeds responded in a more sensitive way to the silver nano particles tested. For Agcoll, an inhibition was observed at 10 mg L−1 for flax and ryegrass and at 20 mg L−1 for all three plant species (Fig. 3). The intermediately sized Ag5 reduced shoot growth of flax and barley at 10 mg L−1, but this effect did not occur at higher concentrations. Ryegrass had a more consistent reaction with inhibition observed for all concentrations tested. For Ag20, an inhibitory effect on shoot growth was observed for barley already at 10 mg L−1 and for all three plant species at 20 mg L−1.
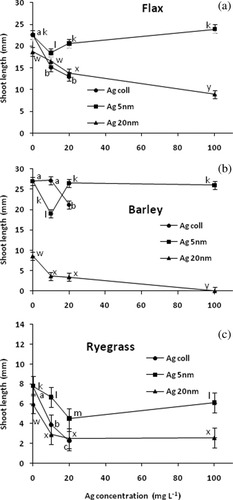
Effects of three types of silver nanoparticles on germination of flax, barley, and ryegrass seeds soaked and incubated in nanoparticle suspensions of different concentration. Tests, letters and bars as in Figure 1 (n = 10).
DISCUSSION
There is currently a need for validating existing hazard assessment methodologies for screening of possible adverse effects of engineered nanomaterials that may be released into the environment (OECD, 2008; SCENHIR, 2009). Seed germination and root elongation measurements are rapid and widely used acute phytotoxicity tests with several advantages: sensitivity, simplicity, low cost, and suitability for reactive chemicals and contaminated soil samples (Wang et al., 2001; Munzuroglu and Geckil, 2002; Lin and Xing, 2007). Their suitability for testing nanomaterials is only starting to be investigated. The present study applied different seed germination test protocols and successfully demonstrated the feasibility of using seed germination tests, both in water and in soils, as acute toxicity tests for readily suspended metallic nanoparticles.
The use of nZVI for remediation purposes has attracted much attention in the last few years, and results are promising regarding both water and soil treatments. The direct application to polluted water and soil implies intentional spreading of nZVI into the environment, but many scenarios where nZVI may be used for remediation are also likely to rely on continued attenuation mediated by microorganisms and plants. For aerobic bacteria and plants, it is important to avoid prolonged periods of anoxic conditions which may result from the reductive reactions with excess Fe0. This would favor low application rates. Our results indicate LOEC (lowest observed effect concentration) values around 250 mg L−1 in aqueous suspensions and 350 mg nZVI L−1 in soil water (equivalent to <100 mg nZVI kg−1 in soil). This is lower than the concentrations currently suggested for practical remediation purposes, which are frequently in the range of 0.5–5 g nZVI kg−1 for soil (Li et al., 2006, 2007; Satapanajaru et al., 2008) and 1–40 g L−1 for water treatments (Li et al., 2006; O'Hara et al., 2006), the latter translating e.g., to 0.1–4 g kg−1 of an aquifer with 20% pore space and a density of 2 g cm−3. Our tests were however conducted with freshly prepared and introduced nZVI, and do not account for aging effects rendering nZVI less toxic with time (Phenrat et al., 2009). Separating nZVI treatments and other biological treatment temporally would thus be likely to raise the threshold concentration for adverse effects on plants and permit higher nZVI dosages.
Nanoparticles are likely to react with constituents of environmental matrices like soil and water, e.g., due to interaction with surfaces, destabilization induced by changes in pH or salinity, etc. (Brant et al., 2007). The resulting adsorption and aggregation reactions will modify their properties with respect to mobility, and thereby their bioavailability and possibly their ecotoxicity. For this reason, we expected to see large differences in toxicity when we compared seed germination in the absence and presence of soil. To facilitate comparisons of data based on seed exposure to aqueous nZVI suspensions and nZVI amended soils, we have reported responses to aqueous concentrations at the time of application in both cases, and considered that nZVI in soil is contained in the soil water. The first negative responses observed for seeds germinated in the absence of soil occurred at 250 mg L−1 where shoot growth of flax and root growth of barley were reduced. At the next concentration level, all species and all parameters showed a negative impact. In the presence of soil, all species and all parameters were negatively affected at a concentration of 300 mg nZVI L−1 in the soil water. Unfortunately, lower concentrations were not tested, but the decrease in germination and growth appeared to be in the same range or slightly lower than that observed at 500 mg L−1 in the absence of soil. We thus conclude that exposure to and bioavailability of nZVI in soil is not reduced to any large extent, at least with the mode of application we employed (initially air dry soil, large volume with low concentration of nZVI added).
When comparing the germination rates and shoot growth observed for the two soils with contrasting texture, we still observe a substantial reduction in toxicity in the clay loam soil compared to the sandy loam soil with a far lower clay content. As clay minerals have specific surface areas surpassing even most nanomaterials (e.g., illites: 50–200 m2 g−1; smectites: 600–800 m2 g−1; Scheffer and Schachtschabel, 1984), it is clear that changes in clay content will strongly affect the surface area of a soil available for interactions with nanoparticles, and that the different responses to nZVI in these two soils are likely to be related to this parameter. This is supported by the fact that clay minerals are known to reduce bioavailability and phytotoxicity of both inorganic (Lombi et al., 2002) and organic (Roberts et al., 2007) pollutants.
Contrary to nZVI which has not previously been examined for ecotoxicological effects, silver nanoparticles are well known to be toxic to a range of organisms, mainly bacteria and aquatic species (Morones et al., 2005; Wijnhoven et al., 2009). Acute toxicity in e.g., zebrafish embryos has been observed at silver nanoparticle concentrations as low as 0.19 nM or ∼20 ng L−1 (Lee et al., 2007). In comparison, ionic silver has a toxicity threshold around 1 μg L−1 for several aquatic species (Ratte, 1999) and can affect soil bacteria at concentrations around 100 mg kg−1 (Throback et al., 2007). Few toxicity tests have been made with silver nanoparticles and terrestrial species, and to our knowledge, none with plants. Copper nanoparticles, which have many properties in common with nano silver, have been tested for phytotoxicity using Mung bean and wheat, and given LOEC values of 200 mg L−1 and EC50 values (effective concentrations reducing growth by 50%) from 335 to 570 mg L−1 (Lee et al., 2008). Our results indicated a certain impact of aqueous suspensions of silver nanoparticles at 10 mg L−1, but higher concentration did not consistently lead to further reduction in seed germination or seedling growth. Further, there were no trends indicating that smaller particles were more toxic than larger particles, as has been observed for Ag with bacteria (Choi and Hu, 2008) and for gold nanoparticles with different mammalian cell lines (Pan et al., 2007). The absence of concentration-dependant and particle size-dependant effects observed in our experiment may be due to some sort of saturation or equilibrium being reached, possibly involving an ionic component that was not distinguished in this experiment. For silver nanoparticles, confounding effects of ionic silver is common and may account for a substantial part of the toxicity response (Navarro et al., 2008). Our conclusion from the experiments with silver nanoparticles is thus that seed germination tests may be poorly suited for estimation of environmental impact of Ag. Other studies using seed germination tests have also shown that seeds of cucumber and lettuce are not sensitive to metal and oxide nanoparticles, with no detectable inhibitory effects of Au, Ag, and Fe3O4 nanoparticles at concentrations up to 62, 100, and 116 μg mL−1, respectively (Barrena et al., 2009). Similarly, Cañas et al. (2008) found only minor effects on seed germination with six plant species using functionalized and nonfunctionalized carbon nanotubes up to 1750 mg L−1. On the other hand, seed germination tests seem to be well suited to assess the ecotoxicity of zinc-based nanoparticles (Zn and ZnO), where values as low as 20 mg L−1 have given 50% reduction (Lin and Xing, 2007). The reports above all employed seed germination after or during exposure to aqueous nanoparticle suspensions, and the present report is so far the only one having attempted measurements of inhibition during exposure in soil. While nZVI is particularly relevant for such measurements due to its intended use in polluted soils, we still expect to see similar assays in the near future, as soil may have a major impact on bioavailability of other types of nanoparticles, notably hydrophobic ones. In that case, interactions with nonpolar domains of organic matter will determine their fate, and the quality and quantity of organic matter will be more important than clay content in determining their bioavailability (Ehlers and Loibner, 2006; Wang et al., 2008) and hence their ecotoxicity.




