Controlling the Porosity of Starch Hydrogels with Poly(Methyl Methacrylate) (PMMA) Beads
Abstract
Starch is a natural, biodegradable polymer that can be used to prepare hydrogels with various applications in food, pharmacy, medicine, agriculture, etc. In this study, a method of preparation using poly(methyl methacrylate) (PMMA) beads to control the porosity of starch hydrogels is proposed. The hydrogels are crosslinked with trisodium trimetaphosphate and dried in a vacuum oven. Results show that increasing the amount of PMMA beads result in higher porosity hydrogels ranging from ≈35% for hydrogels where no PMMA beads are used to ≈88% for hydrogels where the mass ratio of PMMA beads to starch is 10:1. Higher porosity hydrogels have a higher equilibrium water content and swelling degree, but lower mechanical properties. All hydrogels have a low solubility (<≈5%) and a high gel fraction (>≈90%) percentage. Upon degradation in α-amylase at 37 °C, low porosity hydrogels (prepare with 0:1 and 1:10 PMMA beads:starch) degrade within 30 min, while high porosity hydrogels (prepare with 1:1 and 10:1 PMMA beads:starch) degrade within 3 weeks. The release of a dye that is incorporated into the hydrogel walls follows similar kinetics. Therefore, the use of PMMA beads is an efficient method to control starch hydrogel's porosity and properties.
1 Introduction
Hydrogels are three-dimensional polymeric networks that can absorb high amounts of water, swell, and retain the absorbed water. They can be classified based on their source material, physical properties, method of preparation, ionic charge, biodegradability, method of crosslinking, and response to stimuli and have numerous technological applications.[1] Porosity is a very important property of hydrogels since it is correlated to the amount of water that is absorbed in the hydrogel. Various techniques have been employed to create porosity in materials, e.g., solvent casting, particulate leaching, gas foaming, phase separation, electrospinning, porogen leaching, fiber mesh, rapid prototyping, and freeze drying, and several of these have been applied to hydrogels as well.[2]
Starch hydrogels are nontoxic, biocompatible, and derived from an abundant and cheap raw material; their applications include among others biomedical (wound healing), tissue engineering (bone and cartilage tissue engineering), drug delivery, agricultural (water storage and fertilizer retention), food industry (packaging), electrical (water-resistant cable coatings), personal hygiene (absorbent for water in personal care products), and water treatment (heavy metal ion and dye absorbent).[3] Starch hydrogels can be prepared via physical blending with biopolymers or synthetic degradable polymers, graft copolymerization, direct crosslinking, and radiation-induced crosslinking.[3] Among crosslinking agents, trisodium trimetaphosphate (TTP) is nontoxic and one of the most frequently used in the food industry.[4] TTP has been used for crosslinking natural polymers over the past decades.[5]
Porosity in starch hydrogels is achieved using different techniques, such as the formation of ice crystals during freezing and freeze drying[6] or freeze-thaw cycles,[7] salt leaching,[8] and microwave vacuum drying.[9] Nevertheless, in almost all cases, the application of these techniques does not allow control over porosity. There are a few cases where porosity was controlled in starch hydrogels. Starch extrusion produced granules and their porosity was shown to increase by decreasing feed moisture, increasing extrusion temperature, and decreasing the screw speed.[10] The porosity of starch granules was increased when increasing the concentration of different hydrolases,[11] but hydrogels prepared from these starches did not generally vary in porosity, since freeze-drying was used.[6] Finally, increasing the concentration of polyvinyl alcohol in polyvinyl alcohol /starch hydrogels increased the porosity of starch hydrogels from ≈20% to ≈80%, due to the hydrophilic nature of polyvinyl alcohol and the subsequent attachment of a greater amount of water molecules which led to the formation of larger ice crystals.[7] Similarly, during reactive extrusion of starch hydrogels, increased porosity was achieved when carboxymethyl cellulose and TTP were added and this increase was attributed to the increased viscosity of the crosslinked starch/carboxymethyl cellulose system that affected air bubble nucleation and growth; nevertheless, the achieved porosity was low, ≈12%.[12]
To confront this problem, we decided to use poly(methyl methacrylate) (PMMA) beads as porogen. PMMA beads have been used to create porosity in TiO2 scaffold layers[13] and titania films,[14] yttria-stabilized zirconia ceramics,[15, 16] indium tin oxide electrodes,[17] hydroxyapatite plates,[18] and alkali-activated pastes prepared starting from refractory wastes.[19] Nevertheless, to the best of our knowledge, this is the first time that PMMA beads are used to create porosity in starch hydrogels and, obviously, the first attempt to control the porosity of starch hydrogels with this method. We hypothesize that by varying the amount of PMMA beads concerning the amount of starch, starch hydrogels with different porosity and subsequently different properties can be created. Finally, we prepared porous starch—acridine orange hydrogels to study the release of the dye upon degradation and thus simulate the release of a substance that would be incorporated within the hydrogel walls.
2 Methods
2.1 Preparation of Starch Hydrogels
To prepare the hydrogels, a 10% w/w suspension of starch in water was mixed with the crosslinking agent TTP (the amount of TTP was 20% w/w of the amount of starch) at 45–50 °C for 30 min. The PMMA beads were added to the mixture at a mass ratio of 0:1, 1:10, 1:1, and 10:1 to the amount of starch. Finally, a few drops of a 2 M NaOH solution were added to induce gelation (TTP crosslinking of polysaccharide chains takes place under an alkaline medium[5]) and the hydrogels were incubated at 4 °C for 2 days. Cylindrical samples (1 cm × 1 cm) were cut using a cork borer and dried in a vacuum oven at 60 °C for 1 day. The PMMA beads were removed by immersing the hydrogels in chloroform, which dissolved PMMA (three times for 45 min and two times for 30 min) and dried in a fume hood at ambient temperature overnight. Finally, the samples were immersed in commercial grade ethanol for 30 min and air-dried.
To prepare porous starch–Acridine orange hydrogels, 10% of the water used for the starch suspension was replaced with 0.1% w/v aqueous solution of Acridine orange.
2.2 Starch Hydrogels Morphology
The morphological features of the dry hydrogels were assessed through a desktop scanning electron microscope (SEM) (Phenom ProX, Thermo-Fisher Scientific, USA), equipped with an optical microscope with a magnification range of 20–135× and a SEM with a thermionic CeB6 source. The specimens were attached on a metal stub with double sided carbon tape (Ted Pella, USA) and inserted in the scanning electron microscopy's vacuum chamber using a special charge reduction sample holder. The sample holder was designed to reduce sample charging and eliminate extra sample preparation of nonconductive samples. The dedicated software package (ProSuite PC, Thermo-Fisher Scientific, USA) was used for the instrument control.
To visualize the hydrated hydrogels, Acridine orange-stained hydrogels were immersed for a few minutes in cryogel and sections were cut in a freezing microtome (Cryotome FSE, Thermo, USA). Sections were visualized using a confocal laser scanning microscope (LSM 700, Carl Zeiss, Germany) at an excitation wavelength of 488 nm (Intensity: 66.2%, Pinhole: 36.5 µm, Master gain: 943 [for 1:1 and 10:1 hydrogels] and 673 [for 0:1 and 1:10 hydrogels], Digital gain: 1.6 [for 1:1 and 10:1 hydrogels], and 1.1 [for 0:1 and 1:10 hydrogels], Digital offset: 0.00).
2.3 Porosity Measurement
2.4 Equilibrium Water Content (EWC) and Swelling Degree (SD) Measurement
2.5 Mechanical Testing
Hydrogels were hydrated for a few minutes and compressed until 40% strain with a head speed of 1 mm s−1 using a texture analyzer (TA.XTplus, Stable Micro Systems, UK). Stress–strain curves were plotted, and Young's modulus of elasticity was calculated by the slope of the elastic region of the curve. The elastic region of the curve was considered the initial points of the straight part of the curve where a linear trendline with R2 > 0.95 could be fitted.
2.6 Solubility (SP) and Gel Fraction (GFP) Percentage Measurement
2.7 Total Dye Content
Starch–Acridine orange hydrogels were dissolved in 10 mL of 1 M HCl and 1 mL samples were centrifuged for 10 min at 12 000 × g (Hermle Labortechnik, Wehingen, Germany). The fluorescence was measured with a microplate reader (Synergy 2, Biotek, USA) at an excitation and emission wavelength of 485 and 528 nm, respectively. To calculate the fluorescence due solely to Acridine orange, hydrogels without the dye were treated similarly and their fluorescence was subtracted from the fluorescence of starch–Acridine orange hydrogels. The amount of dye was calculated from calibration curves where the fluorescence of known concentrations of Acridine orange was measured. Finally, the results were normalized by dividing the amount of dye in each starch–Acridine orange hydrogel with the weight of each dry starch–Acridine orange hydrogel.
2.8 Enzymatic Degradation and Dye Release
To measure the amount of dye released, starch–Acridine orange hydrogels were treated similarly and 0.2 mL of the solution was removed and centrifuged for 10 min at 12 000 × g at selected timepoints. Fluorescence and amount of dye released were measured and calculated as described in Section 2.2.6. Finally, the percentage of total dye released was calculated by dividing the amount of dye released by the total dye content.
2.9 Statistical Analysis
At least three samples were used per assay. Statistical analysis of data was performed by analysis of variance (ANOVA) with pairwise Tukey comparisons. Differences between the groups with p < 0.05 were considered statistically significant.
2.10 Results and Discussion
2.10.1 Starch Hydrogels Morphology
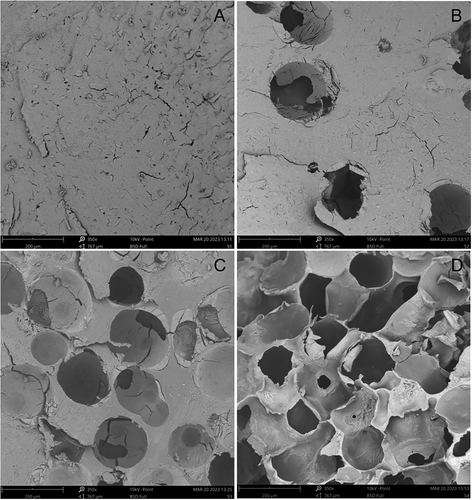
Scanning electron microscopy revealed that different porosities were achieved depending on the amount of PMMA beads that were added to each hydrogel treatment (Figure 1). 0:1 hydrogels, where no PMMA beads were added during preparation, had an interpenetrating network of small pores (ranging from a few µm to a few tenths of µm) of irregular size (Figure 1A); these pores were created as water evaporated through the starch mass in the vacuum oven. As the ratio of PMMA beads to starch increased to 1:10, pores with sizes ≈100 µm appeared (Figure 1B); these pores were formed by the PMMA beads, but small pores still existed on the walls of the hydrogels. A further increase of the ratio of PMMA beads to starch to 1:1, resulted in an increase in the number of large pores, while small pores disappeared from the walls of the hydrogels (Figure 1C). The explanation for the disappearance of small pores could be that it was easier for the water to evaporate through the large pores of the void network created by the PMMA beads, which at this point had reached a sufficient porosity, than through the starch mass. Finally, in 10:1 hydrogels, which were prepared with the highest ratio of PMMA beads, the number of pores maximized and the walls became much thinner (down to a few µm) (Figure 1D) showing the increased porosity of these hydrogels. It should be noted that the hydrogels where PMMA beads were added (PMMA beads: starch 1:10, 1:1, and 10:1) had large pores of similar size. The pore size achieved in the present study compared to the studies where the porosity of starch hydrogels was controlled with other methods was higher than the cases of freeze-thaw cycles[7] and freeze drying,[6] but lower than in the case of reactive extrusion.[12]
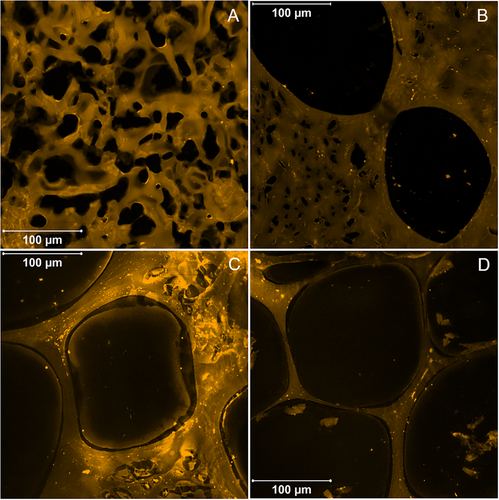
Upon hydration, starch hydrogels swelled and their pores increased in size (Figure 2). The increase was more prominent for the small pores observed in 0:1 (Figure 2A) and 1:10 hydrogels (Figure 2B). The large pores that were created by the PMMA beads did not increase dramatically and were >150 µm in general. As in the dry state, the thickness of the walls decreased when increasing the amount of PMMA beads and the thinner walls were observed in 10:1 hydrogels (Figure 2D). Regarding hydrogels prepared without PMMA beads, in our previous study,[26] starch complexes hydrogels had much larger pores with a larger size distribution (≈100–600 µm). This difference is attributed to the different method of drying (vacuum oven drying in this study versus freeze-drying in the previous one). The larger pore size caused by freeze-drying compared to oven drying has been observed in hydrogels made of different types of materials and has been explained by the structure packing induced by internal stresses due to high temperatures during oven drying.[27, 28]
2.11 Starch Hydrogels Properties
The properties of starch hydrogels prepared with the different ratios of PMMA beads: starch are presented in Figure 3. As expected, an increase in the ratio of PMMA beads: starch (0:1–1:10–1:1–10:1), resulted in higher porosity (35.4 ± 2.1%, 45.0 ± 3.4%, 67.0 ± 3.1%, and 88.1 ± 1.8%, respectively) (Figure 3A), because of the larger void volume created by the presence of more PMMA beads within the starch matrix. Consequently, this larger void volume results in an increase in the water that is absorbed by the hydrogels, expressed either as EWC (Figure 3B) or SD (Figure 3C), and a decrease in Young's modulus of elasticity (Figure 3D). The starch complexes hydrogels of our previous study,[26] that were prepared without PMMA beads and freeze-dried had a much higher porosity (70.0 ± 6.6%) than that of hydrogels prepared without PMMA beads and vacuum oven dried (0:1 PMMA beads: starch). As mentioned before, freeze-drying resulted in not only larger pore size, but also higher porosity than oven drying in other types of hydrogels.[27, 28] What is interesting is that, although starch complexes hydrogels had a slightly higher porosity than that of hydrogels prepared with 1:1 PMMA beads:starch, their EWC and SD were slightly lower than those of hydrogels prepared with 1:1 PMMA beads:starch. More striking is the fact that their Young's modulus of elasticity (56.630 ± 17.903 kPa) was notably higher than that of hydrogels prepared with 1:1 PMMA beads: starch (14.657 ± 3.265 kPa), indicating that the drying method affected not only the porosity, but the rigidness of the hydrogels walls as well. Of course, it should be taken into account, that in the previous study the material was starch–myristic acid complexes, while in this one is only starch, and, since, it is known that complexation of starch increases the degree of crystallinity,[29] the observed differences could be attributed to some extent to this as well.
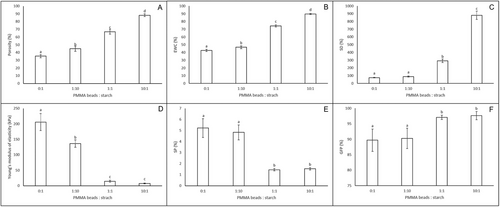
Compared to other studies where porosity was controlled in starch hydrogels, the starch hydrogels of the present study covered a similar range of porosities as starch hydrogels where porosity was controlled through ice crystal formation[7] and much higher than starch hydrogels where porosity was controlled through air bubble nucleation and growth.[12] Nevertheless, the SD achieved in the starch hydrogels of the present study is much higher than in both the aforementioned cases, showing an advantage of the proposed methodology to create and control pores in starch hydrogels.
The SP and GFP of starch hydrogels prepared with the different ratios of PMMA beads:starch are presented in Figure 3E. All starch hydrogels had a low SP (<≈5%) and a high GFP (>≈90%). However, in both cases, the starch hydrogels could be grouped into two categories: the low porosity hydrogels (prepared with 0:1 and 1:10 PMMA beads: starch) which had a higher SP and lower GFP and the high porosity hydrogels (prepared with 1:1 and 10:1 PMMA beads: starch) which had a lower GP and higher GFP and this grouping tendency was also observed during enzymatic degradation (Section 3.4). The high GFP is in good agreement with other studies where starch hydrogels were crosslinked with TTP.[12]
2.12 Total Dye Content
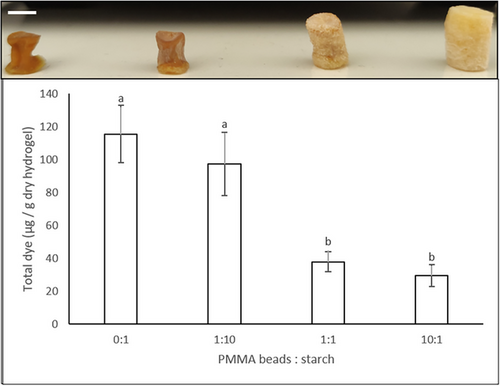
To simulate the incorporation of a substance within the starch hydrogel walls the Acridine orange dye was added in the starch suspension before hydrogel preparation. As can be seen from Figure 4 (top), starch–Acridine orange hydrogels prepared with 0:1 and 1:10 PMMA beads:starch were more colored than hydrogels prepared with 1:1 and 10:1 PMMA beads:starch and this can be explained by the larger dimensions of the later hydrogels, as a result of the larger void volume created by the presence of more PMMA beads. When the total dye content was measured and normalized per weight of each type of hydrogel, the hydrogels could be divided into two groups: the high dye content (0:1 and 1:10 PMMA beads:starch) and the low dye content (1:1 and 10:1 PMMA beads:starch) hydrogels, the former containing ≈3 times more dye than the later Figure 4 (bottom).
2.13 Enzymatic Degradation and Dye Release
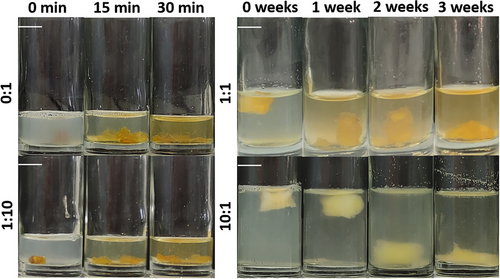
To study the enzymatic degradation, starch hydrogels were incubated in α-amylase at 37 °C. As can be seen in Figure 5, the hydrogels were again divided into two groups based on their degradation behavior: the fast-degrading hydrogels (prepared with 0:1 and 1:10 PMMA beads:starch) that degraded within 30 min and the slow-degrading hydrogels (prepared with 1:1 and 10:1 PMMA beads:starch) that degraded within 3 weeks. It would be expected that the higher porosity of hydrogels prepared with 1:1 and 10:1 PMMA beads:starch would result in a higher DR due to the largest area of starch exposed to α-amylase. On the contrary, they were these hydrogels that had the lower DR. We hypothesize that it was the ethanol treatment step during hydrogel preparation that rendered these hydrogels insoluble. Indeed, it has been shown that the ethanol treatment dehydration caused the crystallization of starch to some degree and prevented starch gels from pore collapse[30]; we have observed a similar behavior, i.e., non-ethanol treated hydrogels dissolved immediately upon hydration. Therefore, the larger area of starch that was exposed to ethanol in these high porosity hydrogels resulted in more material rendered insoluble even in the core of the hydrogel and thus a lower DR.
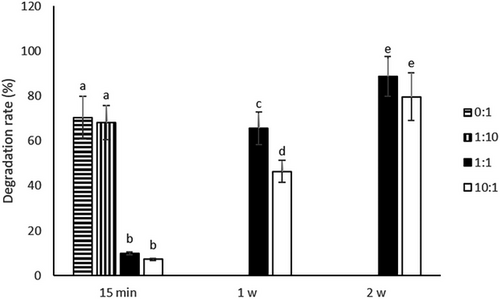
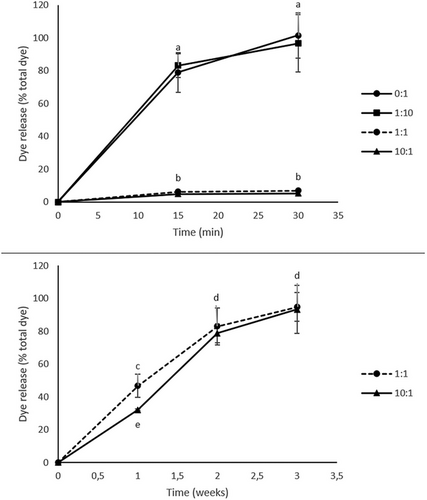
One of the most common applications of hydrogels is substance release.[31] To simulate the release of a substance that would be incorporated within the hydrogel walls, starch–Acridine orange hydrogels were incubated in α-amylase at 37 °C. Figure 6 shows the progress of the enzymatic degradation of starch–Acridine orange hydrogels and the subsequent dye release. As discussed in the previous paragraph, the hydrogels can be divided into two groups: those that dissolved quickly achieving a short-time release (starch–Acridine orange hydrogels prepared with 0:1 and 1:10 PMMA beads:starch) (Figure 6) and those that dissolved slowly achieving a long-time release (starch–Acridine orange hydrogels prepared with 1:1 and 10:1 PMMA beads:starch) (Figure 6). These results were quantified by measuring the amount of dye that was released and expressing it as the percentage of the total dye content of each type of hydrogel (Section 3.3) (Figure 7). It can be seen that starch–Acridine orange hydrogels prepared with 0:1 and 1:10 PMMA beads:starch released almost all the dye within 20 min (Figure 7, top), while starch–Acridine orange hydrogels prepared with 1:1 and 10:1 PMMA beads:starch released almost all the dye within 3 weeks (Figure 7, bottom). Nevertheless, even the 3 weeks achieved for the long-term release hydrogels (starch–Acridine orange hydrogels prepared with 1:1 and 10:1 PMMA beads:starch) cannot be compared to starch complexes hydrogels prepared without PMMA beads and freeze-dried of our previous study,[26] that had not dissolved after more than 1 month of incubation in α-amylase. Once more, the drying method (vacuum oven vs freeze-drying) as well as the lower crystallinity (starch hydrogels vs starch complex hydrogels) affected the properties and behavior of the hydrogels and resulted in faster degradation.
3 Conclusions
Different amounts of PMMA beads were successfully used during the preparation of starch hydrogels to vary the porosity. Indeed, increasing the amount of PMMA beads resulted in hydrogels with higher porosity and, consequently, lower mechanical properties. The achieved porosity played a critical role in the DR of starch hydrogels, i.e., low porosity hydrogels degraded quickly, while high porosity hydrogels degraded slowly, and this, subsequently, affected dye release from starch hydrogels. This is the first time that control over the porosity of starch hydrogels is shown to affect properties such as degradation and substance release. Therefore, the aforementioned method of preparation is effective in controlling the architecture and properties of starch hydrogels and this is of utmost importance in applications such as the controlled release of bioactive substances (nutrients, antioxidants, and drugs).
4 Experimental Section
Materials
Native maize starch was purchased from Tate & Lyle, Greece, PMMA beads (average MW 35 000, particle size 50–150 µm approximately) and Acridine orange from Acros Organics, New Jersey, USA, sodium hydroxide pellets from Lach-Ner, Neratovice, Czech Republic, chloroform from Riedel-de Haen, Seelze, Germany, cryogel from Sakura, Alphen aan den Rijn, The Netherlands. All other chemicals were purchased from Sigma, St. Louis, USA.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




