Humidity-Enhanced NO2 Gas Sensing Using Atomically Sharp Edges in Multilayer MoS2
Abstract
Ambient humidity poses a significant challenge in the development of practical room-temperature NO2 gas sensors. Herein, atomically precise zigzag edges are employed in multilayer MoS2, fabricated using electron beam lithography and anisotropic wet etching, to achieve highly sensitive and selective gas-sensing performance, that is, humidity-tolerant at elevated temperatures and humidity-enhanced at room temperature under ultraviolet illumination. Notably, exposure to 2.5 parts per billion (ppb) NO2 at 70% relative humidity under ultraviolet illumination and at room temperature resulted in a 33-fold increase in response and a 6-fold faster recovery compared to 0% relative humidity, leading to response values exceeding 1100%. The optimized samples demonstrate a theoretical detection limit ranging from 4 to 400 parts per trillion (ppt) NO2. The enhanced NO2 sensing capabilities of MoS2 edges have been further confirmed through first-principles calculations. This study expands the applications of nanostructured MoS2 and highlights its potential for detecting NO2 at sub-ppb levels in complex scenarios, such as high-humidity conditions.
1 Introduction
Detecting NO2 is crucial in modern society, as it is a common industrial byproduct of any high-temperature combustion in nitrogen containing environments.[1, 2] NO2 interacts with water and volatile organic compounds (VOCs) to contribute to the formation of acid rain and photochemical smog. Prolonged exposure to even low concentrations, such as 1 part per million (ppm) of NO2, can lead to the development of chronic bronchitis, respiratory-related diseases, and ocular discomfort.[2, 3] Furthermore, NO2 is extensively used in the production of nitric acid, a substance that finds a widespread use.[4] A pulmonary condition, known as asthma, is often associated with elevated levels of nitric oxides (NO2 and NO) in the exhaled breath. While the exhaled nitric oxide (NO) concentration typically ranges from 20 to 25 parts per billion (ppb) in healthy individuals, those with asthma exhibit elevated levels ranging from 35 to 50 ppb, regardless of age.[5, 6] Recognizing these health risks, the United States Environmental Protection Agency (U.S. EPA) has set a regulated limit for exposure to NO2 at 53 ppb.[7] Consequently, there is a pressing need for reliable sensors capable of detecting minute concentrations of NO2 below the regulatory threshold.[7, 8]
Various methods have been employed to detect NO2 at ppb levels using MoS2. MoS2 is a semiconducting material exhibiting a thickness-dependent bandgap (1.2–1.8 eV), high surface-to-volume ratio, a wide spectrum of light absorption from ultraviolet (UV) to near-infrared (near-IR), as well as excellent catalytic and gas-sensing properties.[9-11] Edges of MoS2 have attracted research interest as favorable sites for gas adsorption, surpassing their basal plane counterparts. This phenomenon stems from the existence of vacancies (and other defects) supporting high binding energies and charge transfer values for target gas molecules.[12-14] It has been observed both theoretically and experimentally that the adsorption of NO2 molecules is favorable at MoS2 edges in comparison to other gases such as H2, CO, NH3, H2S, CO2, and CH4.[12-14] Various advanced methods for improved gas sensing using MoS2 include decoration of the latter with metal nanoparticles (NPs), fabricating van der Waals heterostructures, and morphology engineering. For example, Kim et al. functionalized a solution-based MoS2 surface with different noble metal NPs.[15] The selective character of MoS2 sensors may be modified by doping with metal NPs in order to target a specific gas. Yong et al. reported UV light-activated NO2 sensing using Au NPs-decorated MoS2 thin films,[16] where the presence of Au NPs enhanced the sensing response by a factor of two compared to bare MoS2 films. Furthermore, the response was tripled under UV illumination, highlighting the synergistic effect of Au NPs and UV activation.
Long et al. proposed a MoS2/graphene heterostructure for ultrasensitive NO2 detection. At room temperature (RT), this hybrid structure exhibited a 50 ppb NO2 detection.[17] Another study demonstrated ultrasensitive trace detection of NO2 with an impressive limit of detection (LOD) of 50 parts-per-quadrillion (ppq) using a MoS2/ZnO heterostructure.[18] The mass generation of photo-excited electron–hole pairs, coupled with efficient charge separation, created favorable conditions for achieving exceptional NO2 detection sensitivity.
A notable advantage of MoS2 heterostructures is their rapid charge separation, which has the potential to facilitate development of gas sensors with fast response and recovery times.[19] Although these strategies could pave the way to developing ultra-sensitive and selective NO2 sensors, they introduce fabrication complexity in device manufacturing. Furthermore, the use of noble metal NPs is potentially costly.
Recently, gas sensors based on edge-enriched MoS2 have emerged as a promising prospect due to a higher surface area and more active edge sites in comparison to an unpatterned flake. Real-world devices and applications, such as transistors, catalysts, photodetectors, and gas sensors based on nanopatterned transition metal dichalcogenides (TMDs), especially MoS2, can experience significant improvements in their properties through edge enrichment.[13, 20-24] Cho et al. compared gas adsorption on three distinct morphologies of MoS2 (horizontally aligned (HA) MoS2, vertically aligned (VA) MoS2, and a mixture of HA and VA MoS2) grown by a combination of radio frequency (RF) sputtering and rapid sulfurization.[13] The authors showed theoretically that zigzag edges in MoS2 provide an 18 times higher binding energy for NO2 molecules adsorption compared to the basal plane. The experimental and theoretical investigations indicated that edge-enriched MoS2 has a superior sensitivity for NO2 gas molecules. Agrawal et al. proposed chemical vapor deposition (CVD) grown mixed in-plane and edge-enriched MoS2 flakes.[14] The proposed structures showed a selective and sensitive nature for NO2 sensing at room temperature. Li et al. used the hydrothermal method to prepare hollow, solid, and smooth MoS2 nanospheres. The sensing area for NO2 was doubled, and the response was enhanced 3.1 times in hollow MoS2 in comparison to smooth nanospheres. Edges in MoS2 can be either zigzag or armchair, among which the zigzag edges are thermodynamically more stable and most promising for gas molecule adsorption.[25] However, in most morphology-driven NO2 sensors, it is challenging to accurately control the precise edge orientation.
The top-down nanopatterning can substantially modify the structural, optical, and electrical characteristics of TMDs materials. These properties are strongly influenced by changes in the atomic-scale structure and defects.[24, 26, 27] Numerous methods, such as atomic force lithography, plasma treatment, block copolymer lithography (BCP), thermal annealing, laser writing, and seed-assisted growth, have been used previously.[28-33] However, these approaches lead to reduced accuracy and sharpness, random orientation, uneven patterning, complex fabrication stages, and limited control over the size and shape of the nanostructures. In seed-assisted patterning, for instance, the resulting TMD materials may exhibit random orientation and face a continuous risk of contamination from seeds throughout the growth process.[32, 34] The laser writing and thermal annealing methods potentially eliminate the necessity for masks and photoresists, simplifying the nanopattern design.[35, 36] Similarly, BCP lithography has emerged as a promising strategy for nanopatterning. However, in all these processes, the patterns are typically randomly orientated, not precisely controlled, disordered, and contain structural defects.[23]
In addition to the aforementioned challenges, it is important to recognize that the relative humidity (RH) commonly present in the atmosphere poses a significant obstacle to the development of real-time gas sensors using semiconducting materials. The water concentration in ambient air (atmospheric pressure, 25 °C) is 6,280 ppm at 20% RH and 25,740 ppm at 80% RH levels, respectively.[37] Moreover, in real-life scenarios, the humidity levels are strongly dependent on environmental conditions, such as wind, rainfall, temperature, as well as day-night and seasonal oscillations. At room temperature, water adsorption leads to significant fluctuations in baseline resistance with variations in humidity.[38-40] Additionally, exhaled human breath contains substantial RH levels, which presents a challenge for reliable chemiresistive gas sensor operation. A commonly employed approach to overcome these challenges is elevated temperatures. Furthermore, various approaches to develop humidity-tolerant chemiresistive gas sensors, such as doping with metal NPs (Pd, Pt, Ag, Au), element doping (Ce, Pr, and Tb), integration with hydrophobic materials (polydimethylsiloxane (PDMS), prepared polyaniline (PANI), graphite nanoflakes), etc. have been previously employed.[37, 41-47] Although such methods improved the anti-humidity ability of the respective gas sensors, each mentioned approach added complexity, hindering their practical use.
Here, we report sub-ppb-level NO2 sensors based on nanopatterned multilayer MoS2 exposing precise atomically sharp zigzag edges. The nanopatterned MoS2 resulted in honeycomb nanomesh structures fabricated by optimizing the process reported earlier.[26] The method provides a uniform, precise, and controllable mesh of MoS2 nanoribbons with widths ranging from a few hundred nanometers (nm) to sub-10 nm (see Figure 1b–d). We studied the concentrations of NO2 in the range of 2.5–10 ppb, below the threshold limit set by the U.S. EPA. The density of exposed edges is proportional to the hexagonal holes’ density and their dimensions. We systematically studied the density-dependent NO2 sensing in several honeycomb MoS2 nanomesh samples. The NO2 response was best at 200 °C in the optimized honeycomb MoS2 nanomesh structures due to numerous exposed, uniform, precise zigzag edges, combined with optimal electrical performance. The studied samples demonstrated selective NO2 sensing (against CO, CH4, H2, and C2H6) and demonstrated an extraordinary limit of detection ranging from 4 to 400 ppt, depending on the exact mode of operation. In addition, we investigated room-temperature operation of our sensors under high RH levels (up to 70%) with and without UV illumination. Surprisingly, at RT the sensors showed a significantly enhanced response to NO2 in a high-humidity environment. Under UV illumination, this humidity-enhanced response exceeds 1100% upon exposure to 2.5 ppb NO2. Importantly, the humidity-enhanced sensing behavior is universal and was observed for other tested gases, including CO, CH4, H2, and C2H6, albeit at much lower response levels than for NO2. This universal humidity-enhanced behavior suggests that the mechanism of the enhanced sensor performance is also universal, and likely involves competition between H2O and O2 adsorbed on the MoS2 surface under the influence of UV illumination. Our density functional theory (DFT) calculations confirm that NO2 adsorption is significantly influenced by the presence of edges and sulfur vacancies. Thus, the honeycomb MoS2 nanomesh samples, studied here, offer an optimal configuration, featuring a high density of sulfur vacancy sites along the multilayer hexagon rims. This unique structure enhances the NO2 adsorption capacity, making the honeycomb MoS2 nanomesh particularly effective for chemiresistive gas-sensing applications in high-humidity conditions.
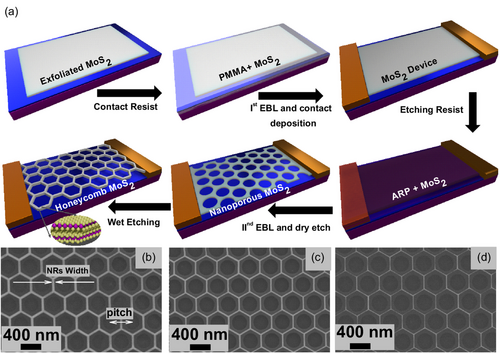
2 Results and Discussion
2.1 Nanofabrication, Morphology, and Structure of Honeycomb MoS2 Nanomesh Sensors
Figure 1 illustrates the fabrication process for honeycomb MoS2 nanomeshes in multilayer MoS2. A comprehensive exploration of the fabrication process is presented in the experimental section and elsewhere.[26] Using this method, we obtain hexagonal holes with atomically sharp zigzag edges. The scanning electron microscope (SEM) images of highly precise, uniform, and large-area honeycomb MoS2 nanomeshes formed by numerous holes are shown in Figure 1b–d. The center-to-center distance (pitch) between the two neighboring circles (defined by electron beam lithography (EBL) and dry etching) was kept fixed at 400 nm while the circle's radius varied from 280 to 300 nm in steps of 10 nm. The nano-ribbons achieved by this unique method are down to sub-10-nm width with ultrahigh precision, as depicted in Figure 1b–d.
A similar set of honeycomb MoS2 nanomeshes was prepared for the chemiresistive gas-sensing assessment. The SEM images of the fabricated sensing devices are shown in Figure 2a–d. We designed the different testing devices on the same multilayer flake (30–40 nm thickness determined by profilometer) to minimize potential variations in electrical signal that may occur due to thickness, local defects, and fabrication processes and ensure a reliable comparison. In total, we fabricated four devices with channel lengths of 20 μm and widths of 13 μm, each. Among these four devices, three contain various honeycomb MoS2 nanomeshes, while one remains unpatterned. The nomenclature for the fabricated devices is as follows: the unpatterned device is labeled as H0, while the patterned devices are denoted as H1, H2, and H3. To test the density-dependent NO2 sensing, the initial size of the circles was varied. The density-dependent honeycomb meshes of H1, H2, and H3 devices were generated from the initial circular holes of 450, 250, and 100 nm radii, with nanomesh pitches – 1450, 820, and 325 nm, respectively. The SEM images of the devices are shown in Figure 2a–d and illustrate precise, clean, and ultra-sharp fabrication of honeycomb MoS2 nanomeshes with numerous zigzag edge sites available at the rims of hexagonal holes. Step-by-step optical images illustrating the nanofabrication process, starting from exfoliation and ending with honeycomb MoS2 nanopatterning are shown in Figure S1, Supporting Information. The SEM and optical images illustrate that the fabrication process does not damage MoS2 within the remaining areas of the flake or leave photoresist residues on the sensing elements. The sensor chip mounted on the Pt100 temperature sensor and heater with a Transistor Outline (TO8) header is shown in Figure S1e, Supporting Information. The header with a sensor chip was installed inside our home-built sensing chamber and subsequently used for gas-sensing experiments.
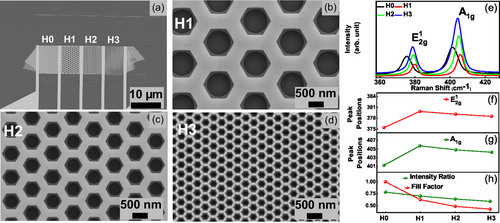
The precise zigzag nature of MoS2 hexagon was confirmed previously in detail using high-resolution transmission electron microscopy (HR-TEM).[26] Here, we performed Raman spectroscopy on patterned devices (H1, H2, and H3) and unpatterned device H0. Raman spectroscopy serves as a powerful and non-destructive technique, enabling the study of film orientation, doping, and the number of layers.[48-50] Two distinct Raman modes, corresponding to the in-plane vibrations () and out-of-plane vibrations (A1g) of S and Mo atoms, have been observed, as depicted in Figure 2e. Both and A1g peaks exhibit a blue shift upon nanopatterning compared to the unpatterned device, see Figure 2f,g. The A1g peak is sensitive to changes in electron concentration and the blue shift in A1g could signal a p-type doping in patterned devices.[48, 49] A more comprehensive discussion of p-doping observed through electrical measurements is provided in subsequent sections. However, we also observe that both Raman peaks exhibit a slight redshift with increasing hexagon density (devices from H1 to H3). The redshift in the and A1g modes can be attributed to terrace-terminated defects and an increase in MoS2 edge density.[50, 51] The difference between the and A1g peak positions, which is sensitive to the material thickness, is around 25 cm−1 for all devices, implying the preservation of the device thicknesses with etching. Since the and A1g modes correspond to in-plane and out-of-plane vibrations, respectively, the peak intensity ratio of (/A1g) can be used to identify the exposed edges in MoS2.[52] In our case, the peak intensity ratio is also decreased from 0.78 to 0.58 (in an H0–H3 row), indicating a clear increase in the number of edges with nanopatterning.
To confirm the increase in MoS2 edge content, we calculated the fill factor based on SEM images. The fill factor is defined as the ratio between the effective MoS2 area of the device to the area of the unpatterned device. The calculated fill factor is 1, 0.62, 0.48, and 0.42 for devices H0, H1, H2, and H3, respectively. These values quantify the increase in the edge content with the increasing hexagon density, see Figure 2h. The Raman signal originating from the A1g mode or from edges is increased simultaneously with the decrease in the fill factor. The detailed chemical composition and phase structure data, such as X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (EDX), have been performed by the manufacturer[53] on 2H-MoS2 crystals from which we have exfoliated our MoS2 flakes. Furthermore, in our previous studies, various high-resolution scanning transmission electron microscopy and electron energy loss spectroscopy (STEM-EELS) elemental mapping experiments of dry- and wet-etched WS2 and MoS2 have been conducted.[26, 54]
2.2 NO2 Gas Sensing
We now turn our attention to the gas-sensing performance of our devices. The NO2 sensing experiments were conducted using a synthetic air background (80% O2 and 20% N2). We tested four different concentrations of NO2, ranging from 2.5 to 10 ppb, at two different operating temperatures of the sensor heater: 200 °C and RT. The gas-sensing performance was evaluated at a fixed bias voltage, V, via two-probe resistance measurements for all four devices. The initial current–voltage (I–V) analysis revealed a notable trend: an increase in p-doping levels correlated with the rise in hexagon density within the fabricated devices. Detailed I–V characteristics are provided in Figure S2, Supporting Information.
2.3 NO2 Sensing at 200 °C
The NO2 sensing response at 200 °C without humidity is shown in Figure 3. Here, the response (R, measured in %) is defined as the ratio of change in the resistance upon exposure to NO2 (Rgas) to the base resistance in synthetic air (Rair): R(%) = . The NO2 gas was injected for 600 s and then turned off to allow for recovery in the background environment for all measurements. The left axis of each graph displays the calculated variation in response, while the right axis shows the corresponding variation in resistance.
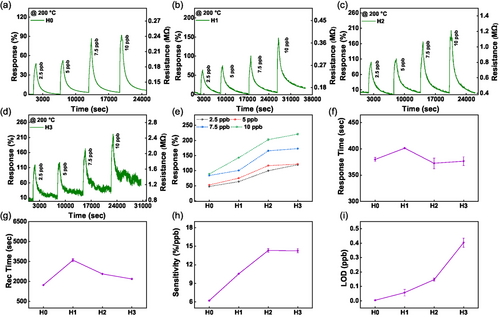
In Figure 3a–d, the baseline resistance is increased from 0.12 MΩ for the unpatterned device to 0.8 MΩ for H3. This is attributed to the concurrent rise of the p-doping due to the increased density of hexagonal holes in the honeycomb MoS2 nanomeshes, as discussed in SI. The observed increase in resistance with NO2 exposure suggests that NO2 is acting as an electron acceptor from n-type MoS2. Moreover, the change in resistance due to NO2 exposure is also concurrently increasing with the density of honeycomb MoS2 nanomeshes. The obtained response to 2.5 ppb NO2 is 48, 64, 100, and 120% for devices H0, H1, H2, and H3, respectively. These results demonstrate that our devices are highly sensitive even to trace amounts of NO2. The response value indicates fewer available NO2 adsorption sites in the unpatterned device, whereas in the nanomeshes, these sites’ density increases, as shown in Figure 3e. When the devices are exposed to NO2, the NO2 gas is accepting electrons from the n-type MoS2 surface due to its strong oxidative nature.[13, 55] As the hexagonal hole density increases from device H1 to H3, the number of zigzag edge sites also increases. The zigzag edges have higher chemical activity and strong propensity for gas adsorption in comparison to MoS2 basal plane (as we demonstrate in the DFT section below). This makes them highly attractive sites for NO2 adsorption.[11, 12] Another interesting outcome observed in Figure 3a–d is the increase in resistance noise in devices H0 to H3. The low signal-to-noise ratio poses an unavoidable obstacle in the trace detection of gases. Taking this into consideration, we have selected sensor device H2 as the optimal nanopatterned device, combining high sensitivity with low noise, and unpatterned device H0 as the control device for future optimization. Sensing parameters, including response (%), response time, and recovery time, are depicted in Figure 3e–g. The response time is determined by measuring the time interval between the sensor's transition from 10% to 90% of its response range. Similarly, the recovery time is characterized by the duration between the sensor's response shifting from 90% to 10% of its range. The response time ranges from 380 to 402 s, while recovery time falls in the range of 1500–3600 s for a 2.5 ppb NO2 cycle of each device. It is worth noting that the baseline resistance is nearly recovered to its initial level in synthetic air for each device at high operating temperatures, confirming the good recovery kinetics of each device. The sensitivity (S, measured in %/ppb) is also an important factor and it is given by the slope of response (%) and concentration (, ppb), .[56]
The calculated sensitivity profile is shown in Figure 3h. It is evident that for the unpatterned device H0, the sensitivity is the lowest while reaching the highest values for the honeycomb MoS2 nanomesh device H2, and saturating for device H3. The elevated sensitivity values imply a maximum change in response (%) can occur per ppb NO2 exposure. Moving on, we calculated the limit of detection (LOD) for each device, as illustrated in Figure 3i. The LOD was calculated using the following equation: , where S is the slope given by the response versus theconcentration curve. The RMSnoise can be calculated using: . Additional details about fitting LOD data are provided in Figures S3 and S4, Supporting Information.
Remarkably, at 200 °C, the unpatterned device H0 exhibited the lowest LOD of 4 ppt, although its sensitivity was the lowest. At the same time, a honeycomb nanomesh device H3 showed an impressive LOD of 400 ppt combined with exceptional sensitivity, a notable achievement compared to the state-of-the-art (comparison between various sensing platforms is discussed below). The low LOD of H0 can be attributed to the high signal-to-noise ratio in the unpatterned device compared to honeycomb patterned MoS2 devices H1–H3.
2.4 Humidity-Tolerant and Selective NO2 Sensing at 200 °C
Humidity is a critical issue in developing gas sensor applications. Typically, the baseline resistance is greatly affected by the presence and variation in humidity levels. Thus, real-world gas sensors are difficult to obtain under variable humidity.[57-59] Considering this, we have tested our sensors for the different RH levels, such as 0, 30, 50, and 70%. The gas-sensing performance for the unpatterned device H0 and honeycomb MoS2 nanomesh device H2 at 10 ppb NO2 and 200 °C against variable humidity has been assessed. As depicted in Figure 4a,b, both devices remain almost unaffected by variations in humidity levels. Interestingly, we observed that both devices exhibited slightly enhanced NO2 responses in the presence of humidity. When 30% humidity was inserted in the chamber, there was a sudden rise in the response, shown as the dotted circle for both devices in Figure 4a,b. However, with a further increase in RH levels, we did not observe any substantial variations in baseline resistance. The unpatterned device H0 maintained a stable baseline, while the honeycomb nanomesh device H2 exhibited a gradual decrease in baseline resistance over the same period due to faster recovery under humidity (for additional details, see Section S6.0.6, Supporting Information).
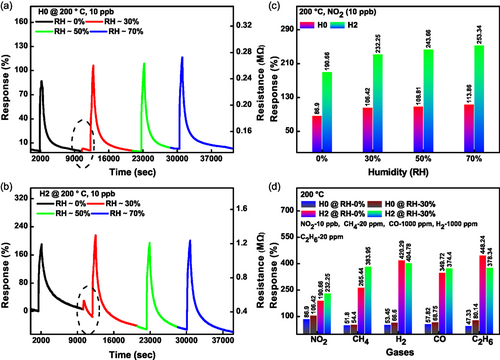
A bar graph comparison of calculated response (%) for both devices is shown in Figure 4c. The honeycomb nanomesh device exhibited higher NO2 sensing performance under different humidity variations. The detailed sensing mechanism of NO2 interaction with unpatterned and honeycomb MoS2 nanomeshes under a humid environment is discussed in detail later in the mechanism section in Supporting Information.
Selectivity is another important aspect of developing a versatile gas sensor. In Figure 4d, we present the selectivity experiments for different gases such as NO2, CH4, H2, CO, C2H6 under 0% and 30% humidity. The unpatterned device H0 showed nearly the same response for all mentioned gases. However, the nanopatterned device H2 showed exceptional selectivity for NO2 under 0% and 30% humidity. Remarkably, the concentrations of other gases were in the 20–1000 ppm range, while NO2 was present at the 10 ppb level. The difference between the responses therefore exceeds 1000 times in favor of NO2. This confirms the selective nature of the honeycomb nanomesh devices for NO2 gas sensing. Based on these results, we conclude that our devices are highly sensitive and selective toward trace detection of NO2 even at high relative humidity levels (see Figure S5 and S6, Supporting Information for complete sensing cycles).
2.5 Room Temperature NO2 Sensing under UV Illumination: Excellent Recovery and Humidity-Enhanced Response
An alternative to the heating approach to counteract the effects of moderate humidity levels is material surface activation by UV light. UV activation is efficient in forcing desorption of O2 and H2O from MoS2, which facilitates interactions of NO2 with the material surface due to higher availability of adsorption sites.[60, 61] We performed NO2 sensing experiments under continuous humidity exposure for a wide range of RH from 0% to 70% (NO2 sensing results at RT without UV illumination, that is, in the dark, are discussed in detail in Figure S7, Supporting Information). The broad sensing profile is shown in Figure 5a. As previously, we used four NO2 concentrations – 2.5, 5, 7.5, and 10 ppb, tested at each humidity level. The gray, green, blue, and red curves denote 0, 30, 50, and 70% humidity, respectively. The inset in Figure 5a shows the change in MoS2 resistance under the UV light illumination at different humidity levels. It can be seen that the baseline resistance initially drops but quickly recovers (over a period of ≈20 min). Thus, the effect of humidity is mitigated by the UV illumination with time. The baseline remains stable even under harsh rises in RH, confirming the potential use of MoS2 nanomeshes for real-world gas-sensing applications. Furthermore, Figure 5b–e illustrates humidity-enhanced NO2 sensing. Specifically, the response to 2.5 ppb NO2 is increased 33-fold (from 34% to 1120%) upon RH rise from 0% to 70%. The achieved response values were extremely high for such a low NO2 concentration with humidity variation, see Figure 5f. They outperform the highest responses at such low NO2 concentration previously reported for any bare MoS2-based sensors (see Figure 6f and Table S1, Supporting Information for detailed comparison). The recovery time also decreased significantly nearly 6-fold at fixed concentrations, while varying the humidity from 0% to 70%, as shown in Figure 5g. The calculated recovery times for a 10 ppb concentration are 4904.8, 1282, 1280.1, and 835.5 s at 0, 30, 50, and 70% humidity, respectively. A similar decrease in recovery time has been observed for other fixed concentrations while varying the humidity from 0% to 70%. We have also tested the unpatterned device H0. These measurements are shown in Figure S8, Supporting Information. The inset of Figure 5f shows the NO2 response under the identical set of humidity conditions for unpatterned device H0, which showed a decreased response with humidity and an overall poor performance. Since the baseline resistance fluctuation is very high due to variation in humidity in this case, we consider the relative response (%) of the signal. The relative response is determined by subtracting the response value when NO2 is turned on from the response value achieved when NO2 is off. The baseline resistance was not recovered under UV exposure of unpatterned device H0, unlike the nanomesh device H2, which showed both complete and sped-up recovery. Furthermore, we tested selectivity for various gases under the humid environment (see Figure 5h). We observe improved gas-sensing performance for all the studied gases. However, the patterned device H2 exhibits an especially selective and sensitive behavior upon exposure to NO2. These results further confirm the room temperature, UV-activated, and humidity-enhanced performance of our chemiresistive gas sensor, which paves the way toward real-world gas-sensing applications under high-humidity conditions.
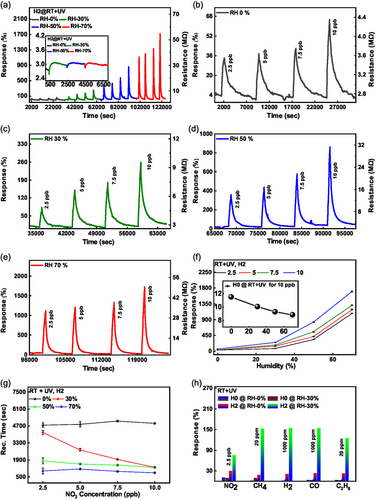
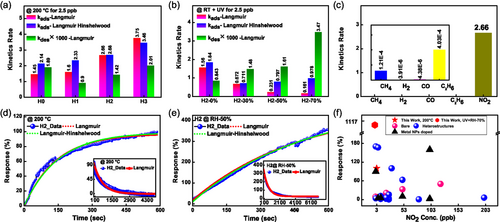
In these equations, is the maximum response of the sensor for the cycle, R0 is the response of the sensor during the recovery cycle when NO2 gas is turned off. kads and kdes are the adsorption and desorption kinetic rates, respectively. K is the adsorption capacity of the sensor, defined as the ratio of kads and kdes, . is the applied NO2 concentration (2.5 ppb in our calculations). The calculated parameters are depicted in Figure 6a,b. At 200 °C, the kads and kdes increase with an increase in the density of hexagons for patterned devices. The higher kads means stronger interaction of NO2 gas molecules with the sensing surface. Notably, kads is 1.84 times higher for honeycomb hexagon device H2 (2.66 ppm−1 s−1) than the unpatterned device H0 (1.45 ppm−1 s−1). The enhanced kads reveals the importance of edges in superior NO2 gas sensing. Initially, kdes is high for the unpatterned device H0; however, it decreases for the honeycomb hexagon device H1, indicating strong adsorption of NO2 gas molecules in the presence of edges. The value of kdes is further increased for devices H2 and H3 due to the higher availability of favorable sites for NO2 adsorption. The fitting has been performed assuming that NO2 follows the mass action law, with the response being proportional to the amount of adsorbed NO2 gas. The desorption energy and charge transfer values of NO2 on patterned devices may differ from those on unpatterned devices, as suggested by the DFT calculations presented below. This can also be inferred from the reduced kdes rate when transitioning from device H0 to H1. The patterned devices have similar adsorption and desorption energies but offer a higher number of adsorption sites for NO2. Consequently, the kdes rate increases further from device H1 to H3.
In Figure 6a, kdes for each device have been multiplied by a factor of 1000 for clarity. We note that the Langmuir model fits our experimental findings very well. Therefore, we extended the fitting to NO2 sensing under variable humidity and with UV illumination. We observed that kads rate is decreased with an increase in humidity while kdes rate is increased. The fitted kdes rate for the hexagonal honeycomb device H2 under 70% humidity (3.47 × 10−3 s−1) is 4.13 times higher than the 0% humidity (0.84 × 10−3 s−1). This confirms the improved recovery under humidity with UV illumination. However, at the same time, the kads rate for the hexagonal honeycomb device H2 under 70% humidity (0.16 ppm−1 s−1) is 9.75 times lower than 0% humidity (1.56 ppm−1 s−1). The NO2 response is the highest under 70% RH and with UV illumination, which implies that the Langmuir model is not ideal to fit the experimental findings under humidity.
In the Langmuir model, the kads and kdes can be further explained in terms of activation energy at a particular temperature.[63, 64] Furthermore, when the sensor is exposed to light, the adsorption and desorption constants start to depend on the illumination conditions. Additionally, humidity significantly affects the kinetic rates, which needs to be taken into account during the fitting. Hence, a complex interplay between these factors may explain why the Langmuir model does not satisfactorily fit the experimental data under humidity and UV illumination.
To test the specificity of our gas sensors, the adsorption kinetic rates for different gases, including CH4, H2, CO, C2H6, and NO2 were obtained using the Langmuir model, see Figure 6c. Here, we used 20 ppm cycle gas response for CH4 and C2H6, while 1000 ppm cycle response was used for H2 and CO. The corresponding kads for NO2 is 2.66 ppm−1 s−1, which is at least three orders of magnitude higher than the other measured gases. This confirms the ultra-selective nature of hexagonal honeycomb device H2 for NO2 gas.
Furthermore, we compared our results with other reports where ppb level NO2 detection has been tested experimentally. This comparison is displayed in Figure 6f. The comparison has been carried out using certain sensor categories, including bare MoS2, MoS2 heterostructures with other materials, and metal nanoparticle-doped MoS2. The corresponding data of response (%), concentration, and temperature from each reference are shown in Table S1, Supporting Information. We note that our honeycomb hexagonal sensors, which fall into the category of bare MoS2 structures, exhibit superior performance compared to other MoS2-based sensors, even those with more complex configurations like metal NP doping and heterostructures.
2.6 Density Function Theory Calculations
In this section, we complement the measurements with first-principle calculations (see Methods for details) based on DFT of adsorption energies and charge transfer to an NO2 molecule adsorbed on MoS2. These calculations are performed on both the basal plane supercell and the one-dimensional ribbon supercell, considering the three scenarios most relevant to our study. Specifically, we examine NO2 adsorption on pristine MoS2, at a sulfur vacancy defect site (S-vacancy site), and at a sulfur vacancy site with an oxygen substitution (O-defect site). The latter two scenarios are particularly pertinent to our research, as the tested sensing devices are highly susceptible to sulfur vacancy generation due to the fabrication processes, which include both dry and wet chemical etching. Furthermore, these S-vacancy sites are highly prone to oxygen adsorption from the environment. The honeycomb MoS2 nanomesh can be considered as hexagonal nanoribbons; this is especially clear for thin MoS2 structures shown in Figure 1b–d. For computational efficiency, we consider only monolayers either in the form of a 5 × 5 supercell or a one-dimensional 6 × 4 ribbon supercell. The structures are shown in Figure 7a,b respectively, where we marked the placement of the defect as the dotted circle. Thus, in total, we consider nine adsorption geometries. First, these include NO2 adsorption above the basal plane of a pristine structure, above an S-vacancy, and an O-defect. Second, for the MoS2 ribbon, we consider these three cases next to either of the zigzag edges: the Mo edge () terminated with S dimers or a S edge ().[66] An NO2 molecule was placed next to the marked site and the structure was relaxed using the optPBE-vdW functional until the maximum force on each atom was less than 0.02 eV Å−1.
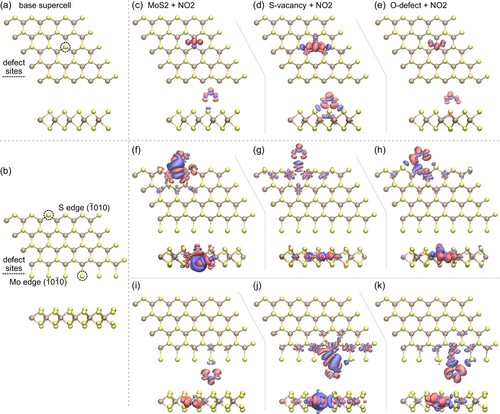
The final geometries with relaxed NO2 adsorption are plotted in Figure S9, Supporting Information. We focus on two important parameters: adsorption energy and charge transfer. The adsorption energy of each case is calculated as , with negative values indicating adsorption is energetically favorable. Herein, the obtained adsorption energies are static, that is, at 0 K with the zero point and thermal energies not included, as these have been shown to be small in comparison to the vdW contributions.[67] The energies are reported in Table 1 and all are significantly negative,[68] indicating efficient adsorption of NO2 in all cases. We further analyze the charge redistribution in the systems upon NO2 adsorption based on the Bader charges.[69] The positive value indicates that the charge is transferred from MoS2 to NO2. The results confirm that NO2 plays the role of an acceptor, in every case taking up a sizable amount of charge from MoS2, as illustrated in Table 1. This charge transfer is also visualized in Figure 7, where charge accumulation can be seen at the position of the NO2 molecule with an accompanying depletion in the MoS2. For the ribbon configuration, we analyze both Mo- and S-terminated edges, which are predominantly located at the perimeter of the honeycomb pattern. Consequently, S-vacancy and O-defect sites emerge as the most relevant at the hexagonal perimeter. Our devices were fabricated from a 2H-MoS2 crystal having AB stacking, which suggests the presence of both types of defect sites in multilayer MoS2 honeycomb nanomesh structures. This stacking configuration further enhances the potential for diverse defect interactions, contributing to the overall sensing performance. Achieving ideal pristine Mo and S edges and defect-free basal plane supercells is nearly impossible in practical settings due to the inherent limitations of our fabrication processes, which involve both dry and wet etching techniques. Thus, it is likely that in real experiments, the most favorable adsorption sites are S-vacancies and O-defects on one-dimensional edges. The calculated charge transfer is nearly doubled for one-dimensional edges for both Mo- and S-terminated edges in comparison to the basal plane. Moreover, the binding energy is higher for O-defects in all cases.
| 5 × 5 monolayer supercell | ||||||
|---|---|---|---|---|---|---|
| Ideal | S-vac | O-def | ||||
| ΔE [meV] | −263 | −235 | −287 | |||
| Δq [e] | 0.12 | 0.26 | 0.11 | |||
| 6 × 4 monolayer ribbon | ||||||
|---|---|---|---|---|---|---|
| Mo edge () | S edge ( | |||||
| Ideal | S-vac | O-def | ideal | S-vac | O-def | |
| ΔE [meV] | −243 | −260 | −357 | −1050 | −196 | −382 |
| Δq [e] | 0.21 | 0.57 | 0.31 | 0.53 | 0.12 | 0.26 |
We now turn our attention to the correlation between the DFT results and our experimental findings. For chemiresistive sensors, charge modulation due to exposed gas molecules is a crucial factor, as it ultimately translates into an electrical signal. A higher charge transfer from MoS2 to NO2 results in an enhanced NO2 sensing response. At room temperature, we find that O-defect sites are predominantly available in the basal plane supercell and in both Mo- and S-terminated ribbon configurations. The combined charge transfer of both edges is substantially higher for the honeycomb MoS2 nanomesh structure compared to the O-defect sites of the basal plane. Our RT NO2 sensing experiments conducted without UV activation corroborate this enhanced performance. Specifically, the honeycomb MoS2 nanomeshes (H2) exhibited nearly doubled NO2 sensing response compared to the unpatterned device (H0), albeit with incomplete recovery. Furthermore, under thermal or UV activation, weakly adsorbed oxygen molecules are desorbed, facilitating NO2 adsorption at the most favorable sites. Specifically, the S-vacancy and O-defect sites at the edges of honeycomb MoS2 nanomesh devices exhibit charge transfer values of 0.57e and 0.31e (at Mo-edge), respectively, making them highly effective for NO2 sensing.
2.7 Discussion of Sensing Mechanism
In this study, we employed MoS2 as the active sensing material for the detection of NO2. MoS2 nanomeshes were fabricated using flakes mechanically exfoliated from a commercial n-type 2H-MoS2 crystal.[53] Due to dry and wet etching, the resulting nanomeshes acquire a certain level of p-doping, which does not change the overall n-type nature of MoS2. The operational principle underlying the detection of NO2 involves the perturbation of charge carriers (hence, resistance) in MoS2 due to the surface charge transfer interactions between the MoS2 and NO2 molecules. The NO2 concentration, flow rate, and the MoS2 surface stoichiometry influence the sensor's resistance. The interaction between NO2 and MoS2 varies under different operating conditions. Therefore, the sensing mechanism involves a complex interplay of physical and chemical processes, making it challenging to define precisely. Given this complexity, a comprehensive understanding of the sensing mechanism requires further investigation beyond the scope of this study. In this section, we briefly summarize the critical aspects of MoS2 nanomeshes sensing behavior and suggest directions for future research. Detailed discussion is provided in the Supporting Information (section “Sensing mechanism”).
The oxidative wet etching and environmental factors (gas atmosphere, humidity, temperature, light) significantly alter the MoS2 surface, potentially capping edges and sulfur vacancies with oxygen molecules or even forming O-defect sites. The nanomesh MoS2 devices (H1, H2, and H3) feature an abundance of edge sites, possibly capped with oxygen. Enhanced NO2 adsorption and charge transfer at the MoS2 edges, particularly in the honeycomb H2, improve device response, affecting resistance even at single-digit ppb NO2 levels.
In dry air and in the dark, molecular oxygen likely dominates the surfaces of all proposed devices, thereby diminishing the adsorption sites for NO2. Despite this, the 2.5 ppb NO2 response in the patterned device H2 reaches 8%, exceeding the 4% value for the unpatterned device H0. Additionally, the strong binding between NO2 and MoS2 leads to incomplete recovery at room temperature in the dark, as illustrated in Figure S10, Supporting Information.
To achieve efficient and reversible NO2 detection, we first raise the temperature to 200 °C. NO2 has higher binding energy to MoS2 than O2, so the elevated temperature facilitates the removal of oxygen adsorbed on the surface and creates more available sites for NO2 adsorption. At 200 °C, MoS2 nanomeshes show a significantly higher response than the unpatterned device H0, indicating that the edge sites are advantageous for NO2 sensing. Moreover, the elevated temperature allows for full sensor recovery, which is not possible at room temperature.
In dry air, our second approach leverages UV illumination to both remove the adsorbed oxygen from the surface and enhance NO2 adsorption. The photogenerated electron–hole pairs are crucial in modulating the adsorption and desorption thermodynamics and kinetics of both O2 and NO2 molecules. This effect is especially pronounced in the MoS2 nanomesh H2, which exhibits higher NO2 sensitivity than its H0 counterpart. Therefore, UV illumination is critical for amplifying NO2 detection.
In dark and highly humid environments, the NO2 sensing mechanism becomes complex, mainly due to the significant influence of water vapor, as elaborated in the SI. The presence of water vapor alters NO2 detection through interactions with surface-bound hydroxyl ions (OH−) and protons (H+). These interactions may lead to the formation of surface-bound nitrate ions (NO3−(ads)), which are characterized by their elevated binding energy and enhanced charge transfer on MoS2.[70, 71] This process enhances the ability to detect NO2 in humidity-rich conditions, even in the dark.
The most compelling results of our investigation were achieved under high humidity and UV illumination at RT. Under these conditions, the MoS2 nanomesh H2 device demonstrated a universal enhanced sensing response across all tested gases, including NO2, CH4, H2, CO, and C2H6. In contrast, the unpatterned device H0 showed degraded performance when exposed to humidity, highlighting the exceptional role of zigzag edges in the H2 device's enhanced gas-sensing capabilities. This universal behavior suggests that the adsorption and charge transfer of all gases are higher in the presence of humidity for the MoS2 nanomesh device H2. Furthermore, it implies that the nanomesh surface has the most available sites for gas molecule adsorption.
While the precise mechanism behind the enhanced sensing performance remains elusive, we hypothesize that the synergy between humidity and UV light optimally cleans the surface. Specifically, UV illumination plays a dual role, removing adsorbed oxygen and preventing the accumulation of surface-bound hydroxyl ions (OH−) and protons (H+). This hypothesis is supported by the observed stable baseline resistance even at high RH levels at RT. Therefore, the exceptionally clean surface of our MoS2 nanomesh H2 device significantly enhances its sensing response across all tested gases. Additionally, we cannot rule out the possibility of enhanced adsorption and charge transfer between the exposed gas molecules and theMoS2 nanomesh H2 surface in the presence of adsorbed water. The enhanced NO2 adsorption and charge transfer in the presence of humidity have been previously reported using DFT.[72]
3 Conclusion
In conclusion, we have successfully demonstrated the fabrication of controlled, uniform, and sharp zigzag edge-enriched hexagons in multilayer MoS2. We developed room-temperature ultra-sensitive, ultra-selective, and humidity-enhanced NO2 gas sensors. The sensor response to 2.5 ppb NO2 reaches 99% at T = 200 °C and 1120% at room temperature under UV illumination with 70% relative humidity. We showed that honeycomb MoS2 exhibits superior NO2 sensing capability due to the high number of zigzag edges, as supported by DFT calculations. Our devices achieved an impressive parts-per-trillion detection limit even at high relative humidity. Our work lays the foundation for developing nanostructured MoS2 as real-life NO2 sensors operable at room temperature, under UV light illumination, and in harsh humidity conditions.
4 Experimental Section
Electrical Contacts Fabrication
MoS2 flakes were mechanically exfoliated from HQ-graphene crystals[53] using polydimethylsiloxane (PDMS) stamps and then transferred onto n-doped 285 nm SiO2/Si substrate. The different hexagon devices were fabricated onto identical thickness flakes. The thickness of the flakes was measured using the VEECO profilometer. Finally, the Autocad software was used to design the circular hole arrays and electrical contacts. The flakes with substrates were first covered with a thick layer of 300 nm of 950 PMMA A4 photoresist (MicroChem, USA) using the spin coating technique. The e-beam lithography was employed using the JEOL JBX 9300FS lithographer at 100 kV accelerating voltage and 30 nA current. The lithographically patterned design was developed using 1:3 MIBK:IPA mixture for 2 min and 20 s. The Cr/Au (5/200 nm) metals were evaporated by Lesker PVD 225 e-beam evaporation system (Kurt J. Lesker Company, Germany), followed by overnight liftoff in acetone.
Honeycomb Mesh Nanopatterning
After fabrication, we covered the device with a 500 nm thick layer of ARP 6200.13 e-beam resist (Allresist GmbH, Germany). Again, the e-beam lithography was employed using the JEOL JBX 9300FS at 100 kV accelerating voltage and 2 nA current. The lithographically patterned design was developed using n-amyl acetate for 1 min 15 s. The reactive ion etching has been performed in Oxford Plasmalab 100 system (UK) using CHF3 plasma. The dry etching conditions were as follows: CHF3 and carrier gas (argon) flow were 50 sccm and 40 sccm, respectively. The radio frequency power was 50 W. The MoS2 etching rate was ≈10 nm min−1. The devices were cleaned using 1165 remover, acetone, and isopropyl alcohol (IPA) for 5 min each. The wet etching process was performed in the mixture of H2O2:NH4OH:H2O with a volumetric ratio of 1:1:10 at a slightly elevated temperature.[26]
Instrumental Characterization
The SEM imaging was performed using the Ultra 55 FEG SEM at the acceleration voltage of 3 and 4 kV. The Raman spectra were collected at room temperature using the WITEC Alpha 300R equipped with a 532 nm laser with 1800 L mm−1 grating.
Gas Sensing Experiments
The gas-sensing measurements were performed in a home-built sealed chamber, using 20% O2 and 80% N2 (dry synthetic air) as the background gas. The total volume of the sensing chamber where the sensor is exposed to target gases was 0.89 mL. The source bottle, which was used to produce NO2 pulses, contained 100 ppb NO2 in a background of N2, with a purity of N6.0. Gas mixing was performed using a gas mixer setup comprising mass flow controllers (MFCs, Bronkhorst). Lower NO2 concentrations were achieved by diluting the original 100 ppb NO2 using pure N2. The total flow rate was fixed at 100 mL min−1. The other gases were used at different concentrations to adjust the flow rate. The methane and ethane source bottles contained 50 ppm of the respective gases mixed in synthetic air with a purity of N6.0. The hydrogen and carbon monoxide source bottles contained 2500 ppm again mixed in synthetic air with a purity of N6.0. The target gases were set on during measurement for 600 s to measure the response. The two separate MFCs were connected to nitrogen gas and were controlled independently. Typically, the dry synthetic air background is the mixture of 20% O2 and 80% N2 without humidity. The humidity in the system was introduced by adjusting the N2 gas in the background using the second MFC connected with the gas washing bottle with Dreschel Bottle Head. For example, the 30% relative humidity background is the mixture of 20% O2, 30% N2 coming from the gas washing bottle, and 50% N2 directly from the source line. The target gases were introduced by adjusting the N2 flow from the source bottle with or without humidity. Similarly, we have introduced the 50% and 70% humidity in the environment. The 355 nm UV LED was used to test the device under UV light. The sensing temperature was controlled using a ceramic heater (Heraues GmbH, Germany). Electrical testing has been performed using the single channel source measure unit (SMU, Keithley 2601 SourceMeter).
DFT Calculations
The DFT calculations were carried out using the open-source GPAW package,[73, 74] which utilizes the projector-augmented wave method. We used the optPBE-vdW functional to treat the exchange-correlation potential and van der Waals interactions.[75] For the basal plane calculations, we used a 5 × 5 × 1 supercell of 1H-MoS2 with 15 Å of vacuum above and below the monolayer to avoid interlayer interactions. The Kohn–Sham orbitals were expanded using a plane wave basis set with an energy cutoff of 500 eV, we used a 5 × 5 × 1 Monkhorst–Pack grid for the k-mesh and a grid spacing of less than 0.2 Å. The MoS2 ribbon was constructed from a pristine 6 × 5 monolayer by removing the first row of Mo atoms, leaving an S-terminated ribbon on both sides. The vacuum for the ribbon was set to 10 Å above and below, as well as to either side of the edges. For these calculations, we used a 5 × 1 × 1 Monkhorst–Pack grid for the k-mesh, while the other parameters were the same. For all calculations, we used Fermi–Dirac occupation number smearing with a factor of 20 meV and line-shape broadening of 50 meV.
Initially, we relaxed the pristine 5 × 5 monolayer and the 6 × 4 ribbon until the maximum force on each atom was smaller than 5 meV Å−1. Next, the relaxed structures were modified by introducing the two types of defects. These structures were subsequently relaxed using the same conditions. The final relaxed structures (with and without defects) had NO2 added to the vicinity of the defect (or, respectively, where it would be) and relaxed until the forces were less than 0.02 eV Å−1.
Acknowledgements
This work was performed in part at Myfab Chalmers and Chalmers Materials Analysis Laboratory (CMAL). A.V.A. is thankful to SIO Grafen's 2D Graduate network for the mobility Grant to conduct experiments at Linköping University. A.V.A, A.Y.P, and T.O.S. acknowledge funding from the Olle Engkvist Foundation (grant no. 211-0063), 2D-TECH VINNOVA competence center (Ref. 2019-00068), Chalmers Excellence Initiative Nano, and Knut and Alice Wallenberg Foundation (KAW, grant no. 2019.0140). T.J.A. acknowledges funding from the Polish National Science Center, project 2019/34/E/ST3/00359. J.E. acknowledges the Strategic Innovation Program Swelife a joint venture of Vinnova, Formas and the Energy Agency (grant no. 2023-03874), and the Convergence Accelerator Program (Track L – Real-World Chemical Sensing Applications), funded by the Swedish Research Council (grant no. 2023-07219), and Sweden's Innovation Agency, Vinnova (grant no. 2023-04186), in collaboration with the US National Science Foundation (NSF). The DFT computations were enabled by resources provided by the Swedish National Infrastructure for Computing (SNIC) at PDC partially funded by the Swedish Research Council (grant no. 2018-05973) and by the Interdisciplinary Center for Mathematical and Computational Modelling UW (ICM-UW), grant #G55-6.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Abhay V. Agrawal: conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); writing—original draft (lead). Alexander Yu. Polyakov: investigation (supporting); validation (supporting); writing—original draft (supporting). Jens Eriksson: investigation (supporting); methodology (supporting); resources (equal); software (equal). Tomasz J. Antosiewicz: formal analysis (lead); methodology (lead); software (lead). Timur O. Shegai: conceptualization (lead); funding acquisition (lead); supervision (lead); writing—original draft (lead); writing—review & editing (lead).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




