The first Silurian trilobite with three-dimensionally preserved soft parts reveals novel appendage morphology
Abstract
The first Silurian trilobite known with soft parts preserved, a Dalmanites species, is described from the Herefordshire Lagerstätte. Biramous appendages and much of the alimentary system are evident. High-fidelity three-dimensional preservation reveals a novel arrangement of the exopod, in which successive filaments are connected by a presumed membrane. This morphology explains a misinterpretation of the exopod as supporting spiral structures, originally reported nearly 150 years ago. Comparison with other trilobite limbs indicates that the exopod morphology of Dalmanites is present in other members of Phacopida. The function of the exopod is considered to be primarily respiratory.
The Herefordshire (Silurian) Lagerstätte (Briggs et al. 1996) has yielded exquisitely preserved three-dimensional (3D) fossils that have added significantly to our knowledge of the palaeobiology, relationships, and evolutionary history of diverse major groups (Siveter et al. 2020). Surprisingly, despite their abundance in many Silurian strata, trilobites are rare in this deposit. Trilobites were one of the most varied clades of arthropods to diversify after the Cambrian evolutionary ‘explosion’ (Paterson et al. 2019), with a rapid mid-Ordovician radiation (Adrain et al. 1998) and more than 20 000 described species (Adrain 2008). Appendages are known in only c. 30 of these (Zeng et al. 2017). The alimentary system of trilobites is also rarely preserved. We report here the first example of a Silurian trilobite with appendages and alimentary system preserved. It belongs to a large family, Dalmanitidae, for which soft tissues were previously unknown, and which has been listed (Harrington et al. 1959) as the type family for Trilobita. Remarkable details of the limbs reveal a novel morphology and resolve a controversy about allegedly spiral features of trilobite appendages that were first described nearly 150 years ago (Walcott 1877, 1881, 1918, 1921).
Material and method
Herefordshire Lagerstätte fossils occur as calcitic infills in concretions within fine volcaniclastics deposited sub-aqueously (Orr et al. 2000). The single specimen of Dalmanites was ground at 20 µm intervals and then digitally imaged. SPIERS software (Sutton et al. 2014; Spencer et al. 2020) generated a 3D virtual fossil for investigation. Datasets from the serial grinding plus the 3D model in VAXML/STL format are held in the Oxford University Museum of Natural History (OUMNH) and in the Dryad Digital Repository (Siveter et al. 2021). Specimens from the Museum of Comparative Zoology, Harvard University (MCZ), and the National Museum of Natural History, Washington (USNM), were also used in this study. The terminology follows that of Whittington & Kelly (1997) and Boxshall (2004).
Systematic palaeontology
Phylum EUARTHROPODA Lankester, 1904
Subphylum ARTIOPODA Hou & Bergström, 1997
Class TRILOBITA Walch, 1771
Order PHACOPIDA Salter, 1864
Superfamily DALMANITOIDEA Vogdes, 1890
Family DALMANITIDAE Vogdes, 1890
Genus DALMANITES Barrande, 1852
Dalmanites sp.
Figures 1, 2A–S, V–Z, 3, 4
Material
A single specimen, OUMNH C.29611.
Horizon and locality
Coalbrookdale Formation, Wenlock Series, Silurian, Herefordshire, England, UK (Siveter et al. 2020).
Type species
Trilobus caudatus Brünnich, 1781; from the Coalbrookdale Formation, Wenlock Series, England. By subsequent designation of Barrande (1852).
Remarks
Dalmanites is currently loosely diagnosed and British Wenlock species require revision (Budil et al. 2008). The single specimen appears to belong with the Dalmanites species group from north-west Europe and North America (Holloway 1981; Ramsköld 1985; Budil et al. 2008) although it is distinguished by the form of its hypostomal denticulation. It may represent the type species Dalmanites caudatus from the same formation (Salter 1864; Thomas et al. 1984), but we place it in open nomenclature pending revision of British species.
Description
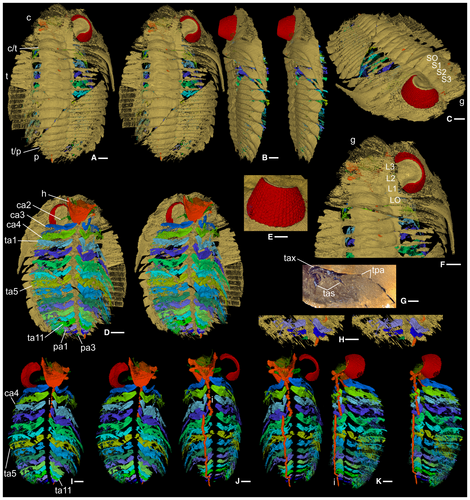
The specimen is articulated but incompletely preserved (Fig. 1A–D). The cephalon is estimated to be c. 35 mm wide at the genal spines extremity. Antennae (nominally cephalic appendage1: ca1) are not preserved. Other limbs, which occur in near-life orientation, are biramous although the endopods are incomplete distally. There are three cephalic (ca2–4), 11 thoracic (ta1–11) and at least three pygidial pairs (pa1–3) (Figs 1D, H–K, 2A–S, V–Z). Much of the digestive system is preserved (Fig. 4E, F, H, J, K).
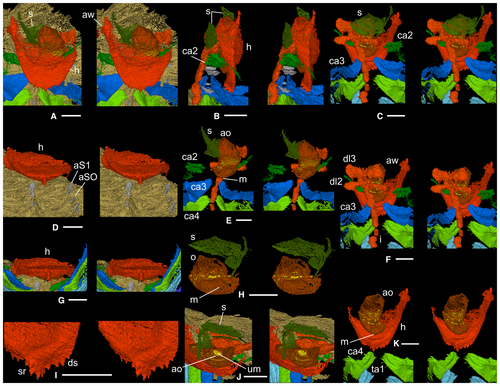
A relatively large median denticle lies on the posterior hypostomal margin, with five smaller denticles either side (Fig. 4A, D, G, I). The median denticle is the most posterior of five on a sagittal ridge on the posterior hypostomal doublure, the middle two the largest, the anteriormost two paired. The rest of the exoskeleton is typical of Dalmanites (Budil et al. 2008).
Ventrally extended apodemes (Figs 1D, 4D) are preserved in association with most of the glabellar furrows, where the thoracic articulating half ring furrows meet the axial furrow, and at segment boundaries in the pygidium. The appendages retain their relative positions, but they are taphonomically offset to the right (left when viewed ventrally) such that the appendage mid-line coincides with the right-side apodemes (Fig. 1D).
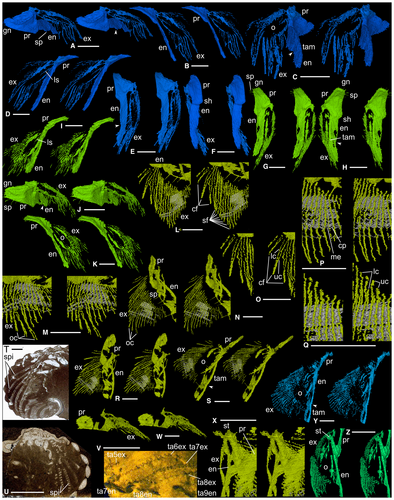
Antennae (ca1) were presumably attached dorsal to the anterior wings of the hypostome (Figs 1D, 4A, F). Appendage ca2 (Figs 1D, 4B, C, E, F) inserts dorsal to the posterior part of the median lobe of the hypostome; it is small and only fragments of endopod and exopod remain. Appendage ca3 (Figs 1D, I–K, 2A–F, 4A–C, E, F) originates above the posterior hypostomal doublure and is at least threefold to fourfold larger than ca2. The protopod is subtriangular in shape viewed dorsally; its proximal margin bears short gnathobasic spines. Distally, it is thickened and forms a posteriorly projecting shoulder (Fig. 2F). The endopod is lath-like, although this may reflect some degree of taphonomic flattening. An inflexion at about two-thirds of the length probably indicates the boundary between the first and second podomeres (Fig. 2A, C, E). The endopod anterior margin is broadly curved and slightly thickened; the posterior margin of podomere 1 bears a spine at mid-length (Fig. 2A). The exopod is an elongate ovoid in outline with an apparently thickened margin. At the long axis of the ovoid there are a few linear structures (Fig. 2B, D), the nature of which is uncertain. Thread-like elements fan out from the ovoid margin except from its more proximal part ventrally; the longest of them occur distally, and they are preferentially oriented subparallel to the ramus axis (Fig. 2A–D). Each is 5–10 µm wide and apparently subcircular to oval in cross-section, although accurate determination of their width and outline when rendering to this level of magnification is problematic. Some threads appear to form pairs, each comprising an upper and lower thread, such that when the upper surface of the exopod is viewed the distance between the upper and lower threads of a pair is low, and noticeably less than that between adjacent pairs (Fig. 2C). Appendage ca4 (Figs 1D, I–K, 2G–K, 4A, C, E, F, K) originates posterodorsal of the posterior margin of the hypostome. It is like ca3 but slightly larger; the protopod bears a slightly larger posterior spine on its gnathobasic margin and three long thin spines proximally on its posterior margin; the endopod anterior margin is thicker. Appendages ca3 and ca4 appear to be associated with apodemes beneath S1 and SO, respectively (Figs 1C, 4D); an apodeme has not been identified for ca2.
The thoracic appendages, and those posteriorly in the cephalon, are essentially similar until the thoracic mid-length, where they become progressively smaller posteriorly into the pygidium. Successive endopods stack parallel to each other; exopods overlap posterodorsally (Fig. 1K). Appendage ta5 (Figs 1D, I–K, 2L–S, V–X), an exemplar, is similar to appendages ca3 and ca4. Much of its protopod is not preserved; anterodistally its anterior margin forms a strut-like structure (Fig. 2X; see also Fig. 2Z, ta7), the dorsal surface of which forms the attachment surface to the exopod and appears cup-like, the two presumably being joined by arthrodial membrane. The first podomere bears three long straight spines posteriorly, the most proximal is the longest (Fig. 2N); the second podomere bears a single long spine proximally. On the dorsal part of the ovoid there are three successive examples of upper and lower threads joining distally to make a discrete, curved, sabre-like form (Fig. 2L, O). Individual threads are c. 40–60 µm wide. More ventrally on the exopod there are at least eight other instances where pairs of threads appear conjoined distally although they are here more tightly packed and appear straighter (Fig. 2L). The width of these pairs is difficult to constrain but is estimated to be 200–300 µm. Slightly further ventrally, where the threads are longest, they twist slightly and overlap distally without joining (Fig. 2M, N). All upper and lower threads are connected in a zigzag fashion by a c. 15-µm-thick layer. This impersistently preserved feature has been reconstructed where it is clearest (Fig. 2L–N, P–S, V), and it appears artefactually as parallel (white) lines. The zigzag geometry, as viewed normal to the longitudinal direction of the threads, is dominantly asymmetrical and notably so proximally on the exopod, with a steep-angled right-downwards slope connecting successive pairs and a shallower angled left-downwards slope connecting the two members of a pair; more distally the zigzag is more symmetrical in places and open angled.
There are the remains of four pygidial segments. Appendages pa1–3 (Fig. 1D, H), corresponding to pygidial axial rings 1–3, are small and poorly preserved (especially pa3) but appear to be undifferentiated from thoracic appendages. Other pygidial appendages may originally have been present, given the large number of pygidial axial rings (12–16) known in complete Dalmanites pygidia.
Above the hypostomal median body is an elongate, inflated, thin-walled feature (Fig. 4C, E, H, J, K) containing unstructured material (Fig. 4J), interpreted as the oesophagus and gut contents, respectively. The oesophagus narrows sharply posteriorly, ending in a transversely oval feature interpreted as the mouth (Fig. 4E, H). The oesophagus is also constricted anteriorly, where the preserved length terminates in an oval cross-section (Fig. 4E, J, K). Transversely directed gut diverticula infill glabellar lobes L2 and L3 (Fig. 4F) above the oesophagus. There is a disconnect because of preservation loss between the anterior part of the oesophagus and the L3 diverticula (Fig. 4F, K); the L2 and L3 diverticula are also taphonomically slightly displaced abaxially to the left (Figs 1J, 4C, F). The tube-like intestine (Figs 1I–K, 4B, C, E, F) runs beneath the axis from the cephalon to the end of the preserved pygidium, with taphonomic breaks along its length and an offset to the left in its posterior three-quarters. Between the diverticula and glabella are several very thin, variably oriented, sheet-like structures the nature of which is uncertain (Fig. 4A–C, E, H, J); they may represent some kind of displaced glabellar tissue lining.
Discussion
Interpretation of the exopod morphology of Dalmanites
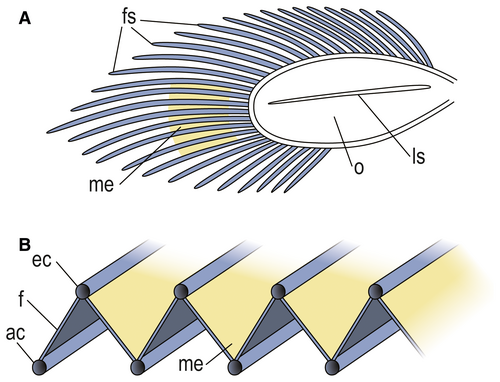
The three sabre-like elements are interpreted as exopod filaments, as are the adjacent, more tightly packed elements that comprise upper and lower ‘threads’. These threads are in the same position and similar in size to the upper and lower marginal channels of the filaments of the Ordovician Triarthrus, as described by Hou et al. (2021), and are interpreted as such. The orientation of the three sabre-like filaments indicates that they, and the other filaments, are inclined at an angle to the ovoid portion of the exopod (Figs 2M, P, Q, 3B). Thus, the thin layer (rendered as white lines) connecting upper and lower marginal channels and sloping in this direction, represents the continuous central part of each filament. The other slope of the zigzag, which connects the upper part of one filament to the lower part of the next (Fig. 3B), is more problematic. However, given that the symmetry and dimensions of the zigzags varies, implying some lateral flexibility, we interpret this slope of the zigzag as a flexible membrane connecting filaments. Those marginal channels that distally overlap and do not join (Fig. 2M, N), are presumed to represent filaments that have become taphonomically twisted and their ends disrupted, or where the distal join is not sufficiently well preserved for reconstruction. The structure of the exopod filaments and membranes may be analogous to that of an oriental fan, which takes on a zigzag form when less than fully open, the stays representing the filaments, and the material between them the connecting membranes.
Functional morphology
Bergström (1969, 1973), Suzuki et al. (2008) and Suzuki & Bergström (2009) argued that trilobite exopod lamellar setae were stiff and unsuitable for respiration, which they favoured as occurring via the thoracic ventral integument. Bruton & Haas (1999), following Manton (1977) and Whittington (1980), interpreted the function for the trilobite exopod as respiratory, as did Hou et al. (2021). The last authors described a closed, chainsaw-like circulatory system within each filament of Triarthrus, with marginal efferent (dorsal) and afferent (ventral) channels for oxygenated and de-oxygenated haemolymph, respectively, and diffusion occurring centrally on the filament between the marginal channels. The nature of the exopod in Dalmanites indicates the same type of filamentous respiration, and the connecting membrane would increase the absorption area available for diffusion. Such a connecting membrane was not reported in Triarthrus (Hou et al. 2021). This may reflect the contrast in preservation between replacement by pyrite in Triarthrus versus void filling by calcite in Herefordshire concretions, but in the current material the connecting membrane appears similar in thickness to the central (membranous) part of each filament, and if this were also the case in Triarthrus, the connecting membrane might be expected to have been preserved had it been present. The membrane connecting filaments in Dalmanites may also have enhanced a swimming function in the exopod, as suggested by Bergström (1969) and also Stürmer & Bergström (1973), although this was disputed by Bruton & Haas (1999). The ‘gill shaft’ of Phacops (Bruton & Haas 1999, text-figs 18, 22–24, 27) may be homologous to the exopod ovoid in Dalmanites. Flattened endopod podomeres have been recorded in Phacops and Rhenops (Stürmer & Bergström 1973; Bergström & Brassel 1984) in addition to Dalmanites; they would facilitate limb stacking during enrolment (Stürmer & Bergström 1973) and may prove to be a phacopide feature.
In contrast with the alimentary system preserved in many trilobites (Lerosey-Aubril et al. 2011), the specimen of Dalmanites provides good evidence for the mouth position, for a purported oesophagus, for diverticula within glabellar lobes L2 and L3, and for the intestine. The gut is preserved from the cephalon into the pygidium. The newly recognized hypostomal denticle arrangement was well placed to assist in maceration of prey items in combination with the gnathobasic spines on appendages ca3 and ca4. This is consistent with the interpretation of phacopoids as specialized predator/scavengers (Fortey & Owens 1999) using schizochroal eyes to detect prey. Nevertheless, the Herefordshire Dalmanites confirms that, unlike those in most crustaceans, trilobite limbs were not functionally differentiated along the trunk length.
The Dalmanites exopod morphology and Walcott’s spirals
In Walcott’s classic studies of trilobite appendages (Walcott 1877, 1881, 1918, 1921; Yochelson 2007), genera of the Middle Ordovician Trenton Group figured significantly, especially ground sections of the phacopides Ceraurus and Calymene (= Flexicalymene) (Fig. 2T, U). Walcott observed the exopod in section but interpreted the main shaft, in the round, as a long spiral structure with separate gill filaments (Walcott 1921, fig. 17). Both Raymond (1920) and Størmer (1939) rejected the existence of spirals, Raymond arguing that they reflected sections along filament bases at the edge of the shaft and Størmer that they are artefacts formed by ‘strands’ of secondary calcite connecting adjacent filaments in zigzag form. The appearance of the Dalmanites exopod filaments in ground slices (Fig. 2V) is similar to those in Walcott’s sections of the Trenton trilobites and provides evidence to resolve the nature of the ‘spirals’. They are an artefact of sections through the filament set, with the filaments joined by a thin, presumably membranous layer; where zigzags are evident the filaments and membrane are near normal to the plane of section (Figs 2V, 3B).
Other Phacopida with limbs preserved are from the Devonian Hunsrück Slate: Phacops, Asteropyge and Rhenops (Stürmer & Bergström 1973; Bergström & Brassel 1984; Bruton & Haas 1997, 1999, 2003). The exopod of Phacops is best known and appears similar to that of Dalmanites. Bruton & Haas (1999, p. 72) discounted their earlier (1997, text-fig. 25) indication of a connecting membrane, even though it may have been prescient. If the exopod architecture in Dalmanites was present in the Ordovician Flexicalymene and Ceraurus, as appears likely based on Walcott’s sections (Fig. 2T, U), it represents an arrangement currently known only in Phacopida and may prove to be a synapomorphy of the Order. The fan-shaped exopod of Dalmanites is similar to those of phacopide genera and differs from that in Cambrian trilobites such as Olenoides and Eoredlichia (Bergström 1969; Whittington 1975, 1980; Bruton & Haas 1999), and Ordovician Triarthrus (Whittington & Almond 1987; Hou et al. 2021), in which subparallel filaments fall in a more comb-like fashion from a long exopod shaft that supports a small filamentless, setae-bearing distal lobe.
Conclusion
The first Silurian trilobite known with soft parts preserved, a Dalmanites species, provides clear evidence for the mouth position, a purported oesophagus, diverticula within glabellar lobes L2 and L3, and the intestine. The arrangement of the denticles on the posterior margin and doublure of the hypostome is new. Biramous appendages are interpreted to show a novel arrangement of exopod filaments joined in zigzag fashion by a presumed membranous connection. This arrangement indicates that the apparent spiral structures of the exopod, first reported by Charles Walcott nearly 150 years ago, are artefacts of the same type of morphology as represented in Walcott’s sections of Ceraurus and Flexicalymene. The exopod morphology of Dalmanites is currently known only in Phacopida. The function of this ramus is considered primarily respiratory.
Acknowledgements
Sarah Joomun and Carolyn Lewis are thanked for reconstructing the virtual model, the late Tom Whitely for providing photographs of Walcott’s slides, David Evans and the late Roy Fenn for fieldwork assistance, and Greg Edgecombe and Nigel Hughes for providing insightful reviews that much benefitted the manuscript. Elissa Suphapun Sorojsrisom prepared the final version of Figure 3. The Natural Environment Research Council (NE/F018037/1), The Leverhulme Trust (EM-2014-068), Oxford University Museum of Natural History, Yale Peabody Museum of Natural History Invertebrate Paleontology Division and English Nature provided funding support.
Open Research
Data archiving statement
The raw serial-grinding data and triangle-mesh model of the specimen OUMNH C.29611 (in VAXML/STL format) are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.n2z34tmvv




