Lophotrochozoa in the Cambrian evolutionary radiation and the Pelagiella problem
Abstract
Tiny snail-like conchs of Pelagiella Matthew are important in discussions of lophotrochozoan and mollusc origins in the Cambrian evolutionary radiation (CER). The limited morphological features of Pelagiella conchs have led to the ‘Pelagiella problem’: (1) poorly distinguished Pelagiella species; (2) an exceptional genus range across all Cambrian palaeocontinents and climate belts; (3) an unparalleled genus-lifespan (c. 31 myr) in the early Palaeozoic; and (4) the assumption that all Pelagiella spp. are gastropods, which compromises lophotrochozoan and mollusc phylogenies. The type species P. atlantoides is a gastropod, but we show that so-called Pelagiella species with sub-to-triangular apertures and turbiniform conchs are not referable to the genus. Re-evaluation of spectacular specimens of Pelagiella exigua Resser & Howell show it to be a polychaete with two apertural paleal chaetae fan arrays. Given that some modern sedentary polychaetes have paleal fan arrays, P. exigua is regarded as a likely member of the Sabellida Levinsen and is assigned to Pseudopelagiella gen. nov. Other Pelagiella spp. and forms with similar conchs are probably polychaetes not relevant to syntheses of early mollusc diversification. Pelagiellidae fam. nov. should be restricted to the late early Cambrian (c. 508 Ma) P. atlantoides. Pseudopelagiella exigua was unattached, probably benthic and lived with the apical conch surface on the sea floor, a mode of life similar to that of another family of the Sabellida, the sessile spirorbids that are cemented to substrate. Among numerous crown groups, the late early Cambrian eve of the CER included the polychaete Pseudopelagiella exigua and gastropod P. atlantoides.
At least 30 named species of Pelagiella Matthew, 1893, are known, with many species based on a few conchs that are commonly morphologically similar to those of other nominate forms (Parkhaev 2001, MolluscaBase 2021a). The assignment of all of these Pelagiella species to the Gastropoda Cuvier, 1795, is based on the general similarity of their conchs to that of the type species P. atlantoides Matthew, 1893, itself an early gastropod. In this report, a re-evaluation of superbly preserved specimens of P. exigua Resser & Howell, 1938, with chaetae fan arrays unknown in molluscs, emphasizes that this and other purported Pelagiella species with similar conchs have no taxonomic relationship to the gastropod P. atlantoides. This means that numerous Pelagiella spp. named in the past must be removed from the interpretation of molluscan phylogeny, and that a re-evaluation is required of lophotrochozoan evolution and the time of origin of a number of eumetazoan crown groups in the Cambrian evolutionary radiation (CER).
Interpretations of Pelagiella spp.
Almost all named Pelagiella species are based on relatively small (c. 1.0 mm wide), rapidly expanding, depressed conchs without any observable visceral attachment structures. This paucity of information has led to conflicting higher level taxonomic assignments of the genus. It has been noted that ‘there has never been any real doubt that Pelagiella was a mollusc’ (Thomas et al. 2020, p. 11), however, Pelagiella has been regarded as: (1) an annelid (Yochelson 1978) compared with spirorbids (Beus 1980; Robison 1987; Landing 1993); (2) a molluscan incertae sedis possibly not related to gastropods (Wenz 1938; Knight 1952); (3) an unassigned micromollusc (Li et al. 2016); (4) an untorted endogastric taxon related to helcionelloids (Geyer 1986, 1994; Peel 1991); or (5) an untorted endogastric taxon assigned to the class Paragastropoda (Linsley & Kier 1984). Additional higher level taxonomic assignment includes: (6) a gastropod intermediate between helcionelloid monoplacophorans and other gastropods (Runnegar & Jell 1976) given that it shows a purported partial (10%) torsion (Ghiselin 1966; Pojeta & Runnegar 1976; Runnegar 1981; Runnegar & Pojeta 1985); or (7) a torted gastropod (e.g. Parkhaev 2001, 2008; Landing et al. 2002; Thomas et al. 2020).
Type species of Pelagiella
The type species Pelagiella atlantoides Matthew, 1893 (non Matthew, 1895; fide Thomas et al. 2020; see MolluscaBase 2021a) is based on mouldic specimens. Topotype P. atlantoides (Fig. 1), up to 7 mm long, is the largest known so-called pelagiellid. It has a dextral, low discoidal conch; a smoothly elliptical, elongate aperture with minor flattening of the dorsal outline in one specimen; and c. 1.5 rapidly expanding whorls (Matthew 1893, pl. 6, fig. 8a, b; Matthew 1895 pl. 16, fig. 6a–c). As noted by Knight (1952, p. 43), P. atlantoides has an ‘oval’ aperture. Matthew (1895, p. 131, pl. 6, fig. 6a, c) recorded a ‘peculiar lip’, a thickened ridge that forms lateral and umbilical re-entrants on the visceral side of the whorl. These re-entrants are similar to the anal and siphonal notches (or sinus) notches of gastropods, and along with torsion (discussed below), emphasizes that P. atlantoides is a gastropod.
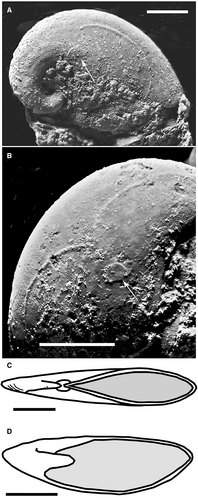
The type species’ morphology distinguishes it from other named species referred to Pelagiella. Unfortunately, the concept of Pelagiella has been distorted over the years with the assignment of many named species, particularly in the Lower Cambrian, to the genus. Most reports do not compare their Pelagiella forms with Matthew’s (1893, 1895) illustrated topotypes (Fig. 1C, D) and describe very different conchs of species that are assigned to Pelagiella; these include conchs with subtriangular or triangular (not oval) apertures; an elongate umbilical margin; and turbiniform/turbospiral, not discoidal, conchs (e.g. Runnegar & Jell 1976; Parkhaev 2001, 2008; Li et al. 2016; Thomas et al. 2020, p. 20). These features, which have resulted in a long-term ‘Pelagiella problem’, make a gastropod and even molluscan assignment of these Pelagiella species questionable (this report). (In this report, the Cambrian is divided into three formal subsystems (Lower, Middle, Upper) and subperiods (Early, Middle, Late) that are capitalized; see Landing et al. 2020.)
Pelagiella and torsion
The soft-part anatomy of Pelagiella atlantoides (i.e. Runnegar 1981; Landing et al. 2002; Thomas et al. 2020) is consistent with a gastropod. The two ovoid muscle scars close to the umbilical and lateral whorl walls (Fig. 1A, B) are comparable to the pedal muscles of Haliotis Linnaeus, 1758 (Landing et al. 2002). Haliotis has a small, post-torsional left muscle (compare Fig. 1A in P. atlantoides) and a later formed, larger (hypertrophied), right muscle (compare Fig. 1B) that functions as the main pedal muscle (e.g. Landing et al. 2002). Thomas et al. (2020, p. 13, fig. 9) complement this gastropod interpretation of P. atlantoides by showing spiral grooves, interpreted as gill attachment tracks (their fig. 9 caption) or pallial lines marking mantle attachment (their p. 13) on the lower and upper internal whorl surfaces (Fig. 1A, B).
A second Pelagiella specimen with a P. atlantoides-like, and thus gastropod-like, dorsal muscle scar is known from the Moroccan upper Lower Cambrian (Stage 4, also known as the lower Middle Cambrian; Landing et al. 2002, fig. 9). A bean-shaped scar on the upper, umbilical surface of a whorl wall seems to mark a hypertrophied pedal retractor. In discussing phosphatized Pelagiella conchs, Thomas et al. (2020, p. 13) dismissed this feature in the Moroccan specimen as a muscle scar because it does not show myostracal prism impressions, lacks a muscle track and scalloped edges, and is in the wrong place. As noted by Landing et al. (2002, fig. 9 caption), the Pelagiella sp. specimen is coarsely haematized and could not show the myostracal prism casts of phosphatized mollusc conchs. Furthermore, topotype P. atlantoides steinkerns do not show a muscle track (Fig. 1A, B), while Pelagiella sp. shows an elevated (scalloped) margin of the scar (Landing et al. 2002, fig. 9, upper edge in photograph). This scar is close to the umbilical margin, as in the topotype P. atlantoides. Thomas et al. (2020) made an unanswerable proposal that the Pelagiella sp. muscle scar should not be compared with the umbilical muscle scar in P. atlantoides but is similar to the elongate muscle track on a phosphatic cast of Pelagiella deltoides Runnegar & Jell (1976, fig. 7e, f); a form with a triangular aperture and which is questionably referable to Pelagiella.
Pelagiella atlantoides and the Moroccan Pelagiella sp. show that torsion had developed by the late Early Cambrian, as shown by two morphologically dissimilar pedal muscles comparable to those of the extant vetigastropod Haliotis (see Landing et al. 2002). Early taxa showing partial torsion are unknown from transitional helcionelloid–gastropod fossils (contra Ghiselin 1966; Pojeta & Runnegar 1976; Runnegar 1981; Runnegar & Pojeta 1985). Indeed, gradual evolution of torsion is problematical and would require partially torted forms to function with the gill(s) blocked by an occluded mantle cavity and with excretion into the mantle cavity. The selective advantage of partial torsion is unknown, and complete torsion is demonstrably advantageous (Signor 1985). Using modern gastropod larval ontogeny as an analogue, this suggests that torsion arose as an either–or development in the veliger and was the feature that defined stem gastropod or gastropod-like groups. Torsion possibly arose in some individuals in every generation early in evolution of the gastropods (Stanley 1982; Goodhart 1987).
The non-torted, non-gastropod (paragastropod) assignment of Pelagiella (i.e. Linsley 1977; Linsley & Kier 1984) relied on the interpreted functional morphology of purported pelagiellids with triangular or subtriangular apertures and other taxa with helical conchs. Pelagiellids were regarded as molluscs, but their apertures with an elongate and angular lateral whorl shoulder and somewhat elongate umbilical outline do not have counterparts in bona fide gastropods.
This paragastropod model is inappropriate for P. atlantoides because it has the soft-parts, oval aperture, and torsion of a gastropod (Fig. 1). Parkhaev (2008, p. 59) countered the paragastropod model by listing three modern gastropods that are morphologic analogues for purported Pelagiella species. They include Lacuna Turton, 1827 and Vanikoro Quoy & Gaimard, 1832, genera that have the aperture inflated low on the outer whorl margin, lack an umbilical elongation, and, thus, are not morphologic analogues of forms referred to Pelagiella (see MolluscaBase 2021b). The third genus, Lippistes Montfort, 1810, of the capulids was reported erroneously as Lepistes Hayvanlar (sic, Lebistes Peters, 1859, with Hayvanlar not an author’s name but Turkish for ‘animal’) by Parkhaev (2008). Lippistes has a high-spired conch in the type species L. cornu (Gmelin, 1791) and is not a Pelagiella analogue. Other capulid species earlier included in this genus have been transferred to other capulid genera (MolluscaBase 2021b; Fassio et al. 2015). In general, the degree of shell spiralization in capulids seems to be related to host features (Fassio et al. 2021), and cannot be considered a sound diagnostic character. Thus, Linsley & Kier’s (1984) non-gastropod assignment of purported Pelagiella species cannot be dismissed.
Conch microhistology and composition: mollusc or polychaete?
Conch microstructure and mineralogy may provide insights into the higher-level taxonomy of forms assigned to Pelagiella. Thomas et al. (2020, fig. 8) regarded the primary conch mineralogy of skeletalized annelids and molluscs (gastropods, helcionelloids and tergomyans) as calcite and aragonite, respectively. However, calcitic ‘monoplacophoran’ shells occur, and calcite layers appear in purported Pelagiella conchs (e.g. Vendrasco et al. 2015, fig. 7.3, 7.4), while annelid tubes (i.e. Serpulida) can be primarily calcitic or aragonitic (Vinn et al. 2008), meaning that mineralogy does not distinguish these lophotrochozoan phyla.
The conch histology of Cambrian mollusc-like forms includes a complex succession of distinct layers in Pelagiella madianensis Zhou & Xiao, 1984, cautiously termed a ‘micromollusc’ by Li et al. (2016). However, the latter authors show microstructures (e.g. crossed foliated lamellar and lamellar–fibrillar) known in modern members of the family Serpulidae Rafinesque, 1815, of the order Sabellida Levinsen, 1883 (see Vinn et al. 2008; their chevron and lamellar–fibrillar structures). Thomas et al. (2020, p. 13) also cited studies that show layers of perpendicularly oriented aragonite grains in so-called pelagiellids and concluded that these represent a plesiomorphic grade of organization in molluscs and hyoliths. However, identical, so-called spherulitic prismatic layers occur in modern Sabellida (family Sabellidae Malmgren, 1866) (Vinn et al. 2008), suggesting convergent histology in different phyla. An additional inter-phylum similarity is that the periostracum of so-called Pelagiella features radially oriented, sub-parallel fibres (Bengtson et al. 1990, fig. 170; Li et al. 2016) identical to the spherulitic sub-parallel fibres that form the outer wall in serpulids (Vinn et al. 2008, figs 2E, F, 3F). (In this report, ‘sabellid’ refers to the order Sabellida; the family Sabellidae is distinguished below as the ‘Sabellidae’.)
The available evidence is that the microhistology and composition of so-called pelagiellid conchs are compatible with those of the serpulid polychaetes (order Sabellida). This is consistent with the alternative assignment of Pelagiella and other Cambrian mollusc-like conchs to the annelids (Yochelson 1978; Beus 1980; Robison 1987; Landing 1993).
Taxonomy and palaeogeographical and geochronological ranges
Limited morphological features and assignment of many simple helical conchs to Pelagiella probably explain why Pelagiella has a global distribution across all palaeocontinents and climate belts (see Parkhaev 2001). The ‘genus’ is known from high temperate Avalonia and Baltica and tropical Sibiria (Siberian Platform and adjacent fold-belts in Russia and Mongolia), Kazakhstania (Malyi Karatau), West and East Gondwana, and along the Laurentian margins. This palaeogeographical distribution extended c. 330° longitudinally through the Early Cambrian tropics (Landing et al. 2013, fig. 2A; Torsvik & Cocks 2017, fig. 5a, b). A comparable great circle distance of distribution is known for far less than 0.01% of marine genera (Foote & Miller 2013, table 3, data from Paleobiology Database). This suggests that a number of genus-level or higher taxa with similar conchs may actually be included in reports of Pelagiella. Thus, the seeming global distribution of middle Early Cambrian species such as P. subangulata (Tate, 1892) that have been proposed as correlation standards (see Landing et al. 2013), probably reflects a number of local species with similar conchs.
The very generalized definition of Pelagiella also probably explains its exceptionally long chronostratigraphic range. The earliest named species of so-called Pelagiella appear in the upper Lower Cambrian (lower Stage 3; lower Atdabanian Stage of the Siberian platform) at c. 520.3 Ma (Landing et al. 2021). As listed in Parkhaev (2001), the majority of named, so-called Pelagiella species appear in the uppermost Lower Cambrian (Stage 4), with only a few species known from the Middle and middle Upper Cambrian. An upper bracket of the genus from the lowest Ordovician of Mexico (Yochelson 1968) has been revised to uppermost Cambrian (Landing et al. 2007).
With a recalculated date of c. 488.7 Ma on the middle Upper Cambrian (Landing et al. 2015), a c. 31 myr range of so-called Pelagiella species is exceptionally long. The 90% genus-lifespan expectancy in the Series 2 to Upper Cambrian is c. 4 myr (Kröger et al. 2019), and this suggests that reports on Pelagiella through this long interval actually include a number of supraspecific taxa.
Pelagiella atlantoides occurs in the lower part of this stratigraphic range. Matthew (1893, 1895) noted its rare occurrence in the middle Hanford Brook Formation in Avalonian southern New Brunswick. The associated trilobites (Protolenus elegans Zone) are confidently referable to the upper Lower Cambrian (Stage 4) (Landing & Westrop 1996; Westrop & Landing 2000; see Geyer 2019). A recalculated U–Pb zircon age of 508 Ma in the P. elegans Zone (Isachsen et al. 1994; Schmitz 2012) shows that topotype P. atlantoides geochronologically occurs in the very late Early Cambrian.
The base of the Middle Cambrian (Miaolingian Series) was reported as 509 Ma by Zhou et al. (2019), using zircon dates from the traditional lowest Middle Cambrian of Avalonian England. However, the lowest Miaolingian trilobite faunas are younger than the olenelloid- and redlichioid-bearing strata of Laurentia and East Gondwana and the lowest paradoxidid strata of Avalonia (e.g. Geyer 2019; Sundberg et al. 2020). More recent c. 506 Ma U–Pb zircon dates on uppermost olenelloid-bearing strata (Sundberg et al. 2020) confirm the late Early Cambrian occurrence of P. atlantoides. Thomas et al. (2020) referred topotype P. atlantoides to the lower Middle Cambrian based on acritarch work (Palacios et al. 2017) that had confirmed the trilobite-based correlations of the middle Hanford Brook Formation (Landing 1996; Landing & Westrop 1996; Westrop & Landing 2000). However, Palacios et al. (2017) suggested a traditional correlation with the lower Middle Cambrian of west Gondwana, which now, after IUGS definition of the base of the Middle Cambrian (Miaolingian Series; Zhou et al. 2019), is uppermost Lower Cambrian.
The non-mollusc Pelagiella exigua
Stratigraphic occurrence of Kinzers Lagerstätte and age
A Pelagiella-like species (Fig. 2) is known from spectacularly preserved P. exigua specimens from south-east Pennsylvania (Thomas et al. 2020). The occurrence is in organic-rich facies of the lower Kinzers Formation (Emigsville Member) deposited off the Laurentian Early Cambrian carbonate platform (Skinner 2005). These P. exigua specimens are distinctly older than P. atlantoides from New Brunswick.

An approximate age of these Pelagiella exigua specimens can be determined by regional correlation of the Kinzers. The Kinzers Formation is probably the lateral equivalent of the black mudstone-dominated slope deposits of the Browns Pond Formation in the Taconic allochthon, eastern New York State. The Browns Pond records the late Early Cambrian Browns Pond dysoxic/anoxic interval on the east Laurentian shelf and slope and is the second eustatic highstand of the Early Cambrian (Landing & Bartowski 1996; Landing 2012, 2013; Landing & Webster 2018).
The uppermost Browns Pond has eodiscoid trilobites (Calodiscus Howell, 1935; Hebediscus Whitehouse, 1936; Serrodiscus Richter & Richter, 1941) (e.g. Landing & Bartowski 1996) that allow correlation with the middle–upper Callavia broeggeri Zone of Avalonian North America and the Callavia Zone of Avalonian England (Westrop & Landing 2012). With a 514.45 Ma U–Pb date in the Callavia Zone (Harvey et al. 2011), Pelagiella exigua is c. 6 myr older than topotype P. atlantoides. This Stage 3–4 boundary interval correlation (i.e. Geyer 2019) is lower than the Stage 4 correlation of Thomas et al. (2020).
Thomas et al. (2010a, b, 2020) described bedding plane associations with numerous, small (to 2 mm diameter) moulds of Pelagiella exigua. These moulds show the rapidly expanding conch with the triangular cross-section commonly assigned to Pelagiella, but which is morphologically distinct from the oval aperture of P. atlantoides.
‘Pelagiella’ exigua fan arrays
Many specimens of Pelagiella exigua have two radiating bundles (fan arrays) of flexible rod-like structures (chaetae) up to 2 mm long that extend from the conch apertures (Thomas et al. 2010a, b, 2020; Fig. 2). Their composition is claimed to be of chitin (a tough, nitrogen-containing polysaccharide) by Thomas et al. (2020), but the outcrop area is east of the anthracite belt of eastern Pennsylvania, and the rods are now graphitic. An assumption of a chitin composition is followed in the comments below.
Chaetae, chitin, conchiolin, and the non-gastropod Pelagiella exigua
The ability to secrete chitin occurs in fungi and all bilaterians; in the ecdysozoans, lophotrochozoans, paracoelomates and deuterostomes (e.g. hemichordates, graptoloids and parts of fish scales). The occurrence of rod-shaped chitinous chaetae/setae is more limited, and they occur in paracoelomates (chaetognaths and protoconodonts; Szaniawski 2002) and lophotrochozoan phyla (e.g. Valentine 2004). The latter phyla comprise the lophophorates (brachiopods, phoronids, bryozoans) and eutrochozoans (echiurans, annelids, molluscs). Chaetae are absent or sparse in some sub-phylum level groups (i.e. class Hirudinea Savigny, 1822, and Oligochaeta Grube, 1850, of the Annelida Lamarck, 1809) and characteristic of other sub-phylum level groups (e.g. lingulacean brachiopods and the traditional class Polychaeta Grube, 1850, of the Annelida) (e.g. Glasby et al. 2000; Harper et al. 2017). Development of chaetae/setae may not be a synapomorphy (fide Thomas et al. 2020), and their presence in larval and adult lingulaceans (e.g. Topper et al. 2015) and throughout all life stages of the polychaetes may represent convergent evolution (Lüter 2000).
Molluscs use chitin to bind the calcium carbonate in shells, with conchiolin binding the nacre (e.g. Weiss et al. 2013; Chan et al. 2018). Modern molluscs have chitin in blunt, microscopic spines (Kölliker’s organs) in the outer body wall in juvenile octopi (e.g. Maldonaldo et al. 2019), as cores in calcareous dermal sclerites in aplacophorans (now the clade Aculifera Blumrich, 1891) and polyplacophorans (i.e. Furuhashi et al. 2009), and as sensory hairs in the polyplacophoran girdle (e.g. Leise & Cloney 1982).
Pelagiella exigua not a gastropod
No evidence from the fossil or Recent record shows long chaetae or chaetae fans limited to the anterior or apertural region in any organism confidently assigned to the molluscs. Dzik & Mazurek (2013) and Thomas et al. (2020) compared the fan arrays in Pelagiella exigua to the anterior chaetae fan arrays of polychaetes, but did not further develop the comparison. Thomas et al. (2020) seem to deflect consideration of a polychaete assignment by emphasizing that chaetae occur along the length of polychaetes. Their gastropod interpretation of P. exigua allies with a belief ‘that Pelagiella was a mollusc’ (Thomas et al. 2020, p. 11, noted above). They suggested analogues for P. exigua chaetae in the uncalcified sclerites of the Middle Cambrian ‘stem mollusc’ Wiwaxia Walcott, 1911 (which, alternatively, is also interpreted to be a polychaete; e.g. Zhang et al. 2015), and in the chitinous cores of aplacophoran (i.e. aculiferan) and polyplacophoran dermal sclerites, but they did not list homologous chitinous fan arrays or chaetae restricted to the anterior margin or aperture in molluscs. Thomas et al. (2020) compared the chaetae fan arrays in P. exigua to epipodial tentacles in vetigastropods, seemingly suggesting that they represent ‘dechitinized’ chaetae with muscles.
The only known skeletal structures that protrude from a mollusc aperture occur in the ‘scaly foot gastropod’, in which sclerites form rows of anteriorly projecting, overlapping, sulphide mineral-coated plates with a conchiolin core (Warén et al. 2003; Chen et al. 2015). These sclerites are on the foot of the snail and surround a tiny operculum.
Gastropod opercula and the Nautilus operculum are produced on the mollusc foot and include conchiolin, not chitin (e.g. Checa & Jiménez-Jiménez 1998; Furuhashi et al. 2009). Thus, an anteriorly facing structure (i.e. a fan array) would probably be produced on the Pelagiella exigua foot, if it is a gastropod, and be composed of conchiolin. Both a probable conchiolin composition of P. exigua fan arrays and the absence of anterior chitinous chaetae bundles or fan arrays in undoubted molluscs led to the conclusion that there is no synapomorphy of chitin-based structures in lophotrochozoans with P. exigua (contra Thomas et al. 2020).
Of the chaetae-bearing taxa listed above, chitinous chaetae that face anteriorly occur only in chaetognaths, protoconodonts, lingulacean brachiopods and two groups of sedentary, tube-dwelling polychaetes. The latter include the order Terebellida Dales, 1962, and family Sabellariidae Johnston, 1865, of the order Sabellida Levinsen, 1883 (see Glasby et al. 2000). Pelagiella exigua cannot be allied with the soft-bodied, apertural spine-bearing planktic chaetognaths and their apparent fossil representatives, the protoconodonts. Similarly, P. exigua is univalved, which precludes a brachiopod assignment. This leaves the original polychaete comparison of the fan arrays in P. exigua noted by Dzik & Mazurek (2013) and Thomas et al. (2020).
The annelid Pelagiella exigua
Chaetae in polychaetes
Capillary (i.e. elongate) chaetae occur in polychaetes that are errant or sessile, although many of which can move in and out of dwelling tubes. These chaetae are chitinous, simple to complex bristles that are blade, rod, and club-like to flattened, and diagnostic of polychaetes. Their distribution is along the animal (around the mouth, on the collar, along the thorax and abdomen) or they occur as two anterior fan arrays (paleal chaetae) lateral to the mouth in sessile polychaetes (e.g. Glasby et al. 2000). Thorax and abdomen chaetae form tufts or radiating bristles that arise from the dorsal and ventral parapodia lobes (e.g. Merz & Woodin 2006). Chaetae are known as far back as the late Early and Middle Cambrian on annelids that have been referred to stem groups from the Sirius Passet, Burgess Shale and Chengjiang faunas (e.g. Parry et al. 2014; Han et al. 2019; and references therein).
Paleal fan arrays in Canalipalpata Rouse & Fauchald, 1997, and Pelagiella exigua
A close comparison of the fan arrays of Pelagiella exigua exists with paleal chaetae fan arrays in two orders of the very diverse polychaete clade Canalipalpata. Notochaetae that form anterior fan arrays generated on the anterior segments are common in sedentary polychaetes, and occur in the order Terebellida, in all species of the Ampharetidae Malmgren, 1866, and Pectinariidae Quatrefages, 1866, and some genera of the Flabelligeridae de Saint-Joseph, 1894. They are absent in the order Spionida Rouse & Fauchald, 1997, but the order Sabellida has paleal chaetae in all Sabellariidae Johnston, 1865, and none in the Sabellidae Latreille, 1825, or Serpulidae Rafinesque, 1815. Some Serpulidae have collar chaetae, but the Spirorbidae Chamberlin, 1919, also included in the Serpulidae, in almost all reports, lack paleal chaetae and have only thoracic chaetigers (see reviews by Glasby et al. 2000 and references therein, family-level assignments follow Capa et al. 2011). The Sabellariidae are a problematical group of highly specialized polychaetes traditionally assigned to the Sabellida on morphological grounds (e.g. Glasby et al. 2000) but they have a weak sister group relationship to the Spionida, which lacks fan arrays (Capa et al. 2011). However, 25 of 29 morphology-based family trees suggest a traditional relationship of the Sabellariidae to the Sabellida (Capa & Hutchings 2010; Capa et al. 2012), while both the Sabellariidae and Sabellidae are interpreted as outgroups within the Sabellida (Kupriyanova et al. 2006).
The uses of paleal chetae vary. Those of the Pectinariidae are used in burrowing (Glasby et al. 2000), while the paleal chaetae in Amphicteis Grube, 1850 move the animal up and down its tube and sort food (Fig. 3; e.g. Glasby et al. 2000).
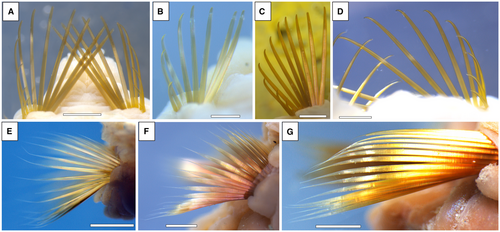
Paleal fan arrays are present in canalipalpatans that lack very strongly developed tentacular feeding apparatuses. The Sabellidae and Serpulidae (order Sabellida) have prominent tentacular crowns and no paleal fan arrays. By comparison, the Sabellariidae of the order Sabellida (i.e. Capa et al. 2011) have golden-coloured paleal fans that are longer than the tiny buccal cirri. In the Terebellida, the pectinariids have paleal fan arrays larger than the brachial and buccal tentacles, while the ampharetrids and flabelligerids have small fan arrays with relatively few buccal tentacles and branchiae (e.g. Glasby et al. 2000). No evidence of a soft feeding apparatus exists in the Pelagiella exigua specimens from Pennsylvania, but it may have had a small (or no) tentacular feeding apparatus that did not require any space if the paleal fan arrays were retracted into the conch.
Detailed similarities exist between the fan arrays of Pelagiella exigua and those of canalipalpatans. The paleal fan array in Ampharete Malmgren, 1866, has been reported as having up to 30 (Jirkov 2009, 2011) and up to 41 (I. A. Jirkov, unpub. data) chaetae, with P. exigua having as many as 50 chaetae in a fan array (Thomas et al. 2020). The paleal chaetae in A. sundevalli Malmgren, 1866 form two semi-conical fan-like clusters as in P. exigua, that face out of the tube (Schiaparelli & Jirkov 2016; Fig. 3G; see shorter fan-like paleal chaetae in other Amplicteis species in Schiaparelli & Jirkov 2016, fig. 7A, C).
Another similarity with Pelagiella exigua is that Amphicteis chaetae emerge from a bulbous tissue mass (Schiaparelli & Jirkov 2016). This attachment is similar to that recorded by Thompson et al. (2020, p. 9, fig. 7, lower margin), who noted that ‘the chaetae converge on a basal node potentially a muscle mass’.
Fan arrays in Pelagiella exigua and Amphicteis have similar sizes. Thomas et al. (2020, fig. 6) note that P. exigua chaetae are 5–10 µm wide and c. 1.3 mm long, with the fan arrays in their fig. 6 measuring 1.0–1.5 mm wide distally. The corresponding measurements in paleal chaetae of seven Amplicteis species are 7–11 µm wide and 0.75–2.2 mm long, with the fan arrays 1–4 mm wide distally (Fig. 3). One difference between the fan arrays in P. exigua and Amphicteis species is that Amphicteis individuals are significantly larger but the fan arrays are proportionally smaller.
Notochaetae that form fan arrays on anterior segments are common in sedentary polychaetes. As noted above, they occur in the order Terebellida: in the Ampharetidae and Pectinariidae (all species) and Flabelligeridae (some genera). Paleal chaetae are absent in the order Spionida Rouse & Fauchald, 1997. The order Sabellida has paleal chaetae in all Sabellariidae (included in the Sabellida, discussed above) and no paleal chaetae in the Sabellidae or Serpulidae. Some Serpulidae have collar chaetae, but the Spirorbidae, included in the Serpulidae in some reports, have only thoracic chaetigers (reviewed by Glasby et al. 2000).
Discussion
Pelagiella sensu stricto and Pelagiella sensu lato
Superbly preserved specimens of Pelagiella exigua from the late Early Cambrian (Stage 3–4 boundary interval) provide evidence for the most credible solution to the suprageneric and phylogenetic affinities of numerous forms assigned to Pelagiella, and to the Pelagiella problem. The many Pelagiella species with subtriangular–triangular apertures and turbiniform conchs are taxonomically distinct from the type species of the genus (the torted gastropod Pelagiella atlantoides; Fig. 1), and a solution to the long questioned assignment of these purported Pelagiella spp. to the gastropods or to molluscs is suggested by the presence of polychaete-like paleal fan arrays in P. exigua.
Significance of anterior chaetae in Pelagiella exigua
The two chaetae fan arrays that arise from the aperture of Pelagiella exigua are not ‘a plesiomorphic lophotrochozoan character linking molluscs to their common ancestry’ with annelids, sipunculids, brachiopods and bryozoans (Thomas et al. 2020, p. 15). Indeed, no undoubted mollusc has chitinous apertural chaetae or fan arrays. Instead, chaetae or chaetae-like structures appear as plug- or thread-like dermal spicules in the outer body wall of molluscs (e.g. Leise & Cloney 1982), although they may be convergent or plesiomorphic and their phyletic significance is problematical (Giribet et al. 2008). The simplest interpretation of the chaetae fan arrays in Pelagiella exigua is that they are similar to, and are, annelid fan arrays, an early suggestion by Dzik & Mazurek (2013) and Thomas et al. (2020) borne out by comparisons with the paleal chaetae fan arrays of Terebellida and Sabellida (family Sabellariidae) polychaetes.
The polychaete assignment of Pseudopelagiella
An apparent solution of the Pelagiella problem is allowed by the discovery of a Pelagiella exigua Lagerstätte. The gastropod paradigm for any so-called Pelagiella, with the exception of the type species P. atlantoides, is not sustainable. Persuasive homologies, including very similar paleal chaetae fan arrays in a number of families of the terebellids and one family of Sabellida (Sabellariidae), support a polychaete assignment of P. exigua and other similar Pelagiella-form species.
Paleal fan arrays and microhistology suggest an assignment of P. exigua to the non-Linnaean clade Canalipalpata, which includes the orders Terebellida and Sabellida. Recent terebellids have an agglutinated tube and chaetae fan arrays, but the order Sabellida includes taxa with fan arrays (Sabellariidae) as in Pelagiella exigua, and taxa that produce calcareous dwelling tubes (Serpulidae) with a histology similar to that in forms traditionally assigned to Pelagiella. This occurrence of Recent sabellid families with calcareous tubes and paleal chaetae in the Sabellariidae arrays suggests a tentative assignment of P. exigua to the order Sabellida, as shown herein by the designation ‘Sabellida?’. Convergence probably explains the similarity of conchs of P. exigua and other Pelagiella spp. to those of the Spirorbidae Chamberlin, 1919, which are also referred to the Sabellida (e.g. Glasby et al. 2000).
Assigning Pelagiella exigua to the Canalipalpata and to the Sabellida and allies means that the species must be removed from assignment to the gastropod genus Pelagiella. It is assigned below in the Systematic Palaeontology section as the type species of the polychaete genus Pseudopelagiella. This genus may include many species with triangular conch apertures assigned to Pelagiella and is the standard for the Pseudopelagiellidae. This new family would include many named Pelagiella spp. and morphologically similar forms such as Costipelagiella Horný, 1964. Proposal of the new family reduces the Pelagiellidae Knight, 1956 and Pelagielliformes MacKinnon, 1985 to the gastropod Pelagiella s.s. The tentative assignment of the cemented spirorbids as well as the free-living Pseudopelagiellidae to the Sabellida means that the Sabellida would include two families of coiled, calcareous polychaetes.
Alternative classifications of conchs brought to Pelagiella sensu lato
Aldanella Vostokova, 1962 appears at the top of the lowest Cambrian Fortunian Stage (Landing & Kouchinsky 2016) and is the oldest known gastropod. Topotypes of the type species A. attleborensis (Shaler & Foerste, 1888) consist of low turbiniform conchs with a broad, gastropod-like adapertural sinus (Landing 1988). Dzik & Mazurek (2013) examined similar conchs from the Siberian Platform that they assigned to A. attleborensis. One of their specimens has an irregular lump on the aperture that they regarded as phosphatized soft tissue. The lump has a tiny hollow crossed by irregularly oriented, small (c. 2 µm wide × 10 µm long) phosphatic columns that they called ‘chaetae’, and which were compared with Pseudopelagiella exigua chaetae. These ‘chaetae’ are probably phosphatized remnants of soft tissue (see Conway Morris & Chen 1992). At c. 1% of the size of P. exigua chaetae and located inside phosphatized tissue, these purported chaetae cannot be compared with those of P. exigua.
Dzik & Mazurek (2013) regarded Aldanella attleborensis as phyletically related to forms traditionally assigned to Pelagiella. This assignment illustrates the Pelagiella problem, given that Pelagiella s.s. is a gastropod and, as we conclude herein, other so-called Pelagiella spp., now referred to Pseudopelagiella, are polychaetes. They note that Siberian A. attleborensis has a short, relatively straight conch section just adapertural to what they term an embryonic shell. They termed this a ‘mucro’, which they compared to the growth habit of hyolith conchs, and then noted that the (chitinous) chaetae of P. exigua were somehow precursors to the calcareous helens in hyoliths. This argument meant that Aldanella and Pelagiella are hyoliths. Actually, A. attleborensis topotypes sometimes show a ‘mucro’ in this undoubted gastropod (i.e. Landing 1988, figs 5.2–4, 6, 7, 9, 10, 12, 15). Briefly summarized, the taxonomic evaluations of this report do not support Dzik & Mazurek’s (2013) hyolith assignment for the gastropods Aldanella and Pelagiella s.s. and for the polychaetes traditionally assigned to Pelagiella (i.e. Pseudopelagiella).
The shape and size of conchs assigned to Pseudopelagiella resemble those of a problematical group regarded as lophophorates: the Late Ordovician to Middle Jurassic Microconchida Weedon, 1991. The key differences are that microconchid conchs, which resemble those of Spirorbis Daudin, 1800, are cemented to substrates and are pseudopunctate or punctate (e.g. Zatoń & Olempska 2016), unlike those of Pseudopelagiella.
Life and growth of Pseudopelagiella
Although Pseudopelagiella was not cemented to a substrate, it may have lived essentially like a spirorbid. Its relatively thick shell (see Pelagiella subangulata (Tate, 1892) in Bengtson et al. 1990, fig. 168A, B) and seemingly relatively small paleal arrays (Fig. 2) suggest it was not a swimmer and was benthic, an uncertainty that might be answered by modelling.
If benthic, Pseudopelagiella conchs are likely to have rested on their hydrodynamically more stable convex surface with the fan arrays in the water column. The relatively uniform conch size in the P. exigua assemblages illustrated by Thomas et al. (2020) may reflect hydrodynamic sorting of an allochthonous association or an in situ spat fall. If in situ, the similar sizes may reflect crowding (e.g. Wisely 1962).
The gastropod-like shell of Pseudopelagiella may have formed, as in spirorbids, late in metamorphosis. In spirorbids, the larva settles on a substrate and secretes a curved, tubular shell that envelops the larva or is an open tube that does not cover the animal’s posterior. Within a day, the open shell is capped with a round terminus (Kupriyanova et al. 2001, p. 46–49, fig. 11D) that resembles the mollusc-like protoconch of some purported Pelagiella species (e.g. Parkhaev 2001, pl. 44, fig. 7, pl. 46, figs 1, 2). Thus, a seeming protoconch-like terminal part of a conch is not an argument for a gastropod assignment of forms traditionally assigned to Pelagiella.
Pseudopelagiella and other named species traditionally assigned to Pelagiella and similar genera differ from spirorbids in that they were not cemented to a substrate. Although the spirorbine Helicosiphon Gravier, 1907 was initially and subsequently described as unattached (e.g. Knight-Jones 1978), more recent work shows that it is attached to a substrate (Monteiro et al. 2013), similar to other spirorbines. A benthic P. exigua may have been poorly mobile given the large fan arrays and seemingly heavy shells (note the shell mould thicknesses in Thomas et al. 2000, fig. 4, centre). If the animal lay on the sea floor with the convex upper surface down, then P. exigua and cogeneric species had functionally sinistral conchs, similar to many spirorbines, and should not be illustrated as if they are dextral gastropods.
As some sabellids and spirorbines, Pseudopelagiella could not defecate through an open posterior tube end. Modern fan worms with closed tubes pass fecal pellets along a ciliated groove and expel them from the mouth area (e.g. Glasby et al. 2000). A notch on the peripheral edge of the outer whorl in Pelagiella primaeva (Billings, 1872) (see Landing et al. 2002) may represent the oral notch known in some polychaete conchs.
Polychaete Pseudopelagiella and gastropod Pelagiella in the Early Cambrian
Pseudopelagiella exigua shows that soft-bodied (Chengjiang, Burgess Shale, Sirius Passet faunas) and conch-constructing polychaetes existed by the late Early Cambrian. The suprageneric assignment of such an old form as Pseudopelagiella is problematical, but an assignment to the clade Canalipalpata and tentatively to the Sabellida seems appropriate.
This tentative assignment implies a much older appearance of the Sabellida than a suggested late Carboniferous – Permian origin (Ippolitov et al. 2014). However, the earliest taxa referable to the Sabellida prove to be far older, and appear in the earliest part of the CER, which includes the late Ediacaran (Landing et al. 2018). Moczydłowska et al. (2014) referred Sabellidites Yanishevsky, 1926, and possibly Conotubus Zhang & Lin in Lin et al., 1986, to the Siboglinidae Caullery, 1914 (previously phylum Pongonophora Johansson, 1937 and order Vestimentifera Webb, 1969). Given that siboglinids are referred to the Sabellida (Rouse & Fauchald 1997; Glasby et al. 2000; Capa et al. 2011; World Register of Marine Species (WoRMS; http://www.marinespecies.org) LSID 129096 (Siboglinidae) accessed 26 February 2021), Pseudopelagiella is not the oldest probable sabellid.
Pseudopelagiella conchs resemble those of spirorbids (discoidal–turbiniform with depressed apex; evolute–involute; generally dextral, also sinistral; aperture subtriangular–triangular–round; shell surface smooth to longitudinally ridged as in Costipelagiella, see Rzhavsky 1994). Conch shape can be merely convergent, and many presumed spirorbids in the Palaeozoic are microconchids (discussed above). Spirorbids are regarded as appearing in the early Triassic at c. 244 Ma (e.g. Vinn et al. 2008), but many small coiled forms in the Palaeozoic are still considered to be spirorbids (Ziegler 2006), with Ippolitov et al. (2014) noting that many of them remain problematical. The resemblance of the Pseudopelagiellidae conchs and spirorbids provides an alternative candidate for unassigned Palaeozoic spirorbid-like conchs.
This seemingly ‘too old’ appearance of the Sabellida must be put into the context of the occurrence of the oldest gastropods. The pre-Ordovician appearance of gastropods is disputed in some reports (e.g. Vinther 2015; Zapata et al. 2014). However, Aldanella and Pelagiella s.s. occur well down in the Cambrian and show gastropod apomorphies (univalved shell, torsion, one or two pedal muscles, apparent pallial lines showing a mantle cavity in Pelagiella). Pelagiella atlantoides and Pseudopelagiella bring the gastropods and Order Sabellida down into the ‘eve’ of the CER, to c. 508 Ma, with Pseudopelagiella probably appearing at c. 520 Ma in lower Stage 3 (Siberian Atdabanian Stage), the gastropod Aldanella appearing c. 10 myr earlier (Landing & Kouchinsky 2016; Landing et al. 2021), and the sabellid Sabellidites appearing c. 10 myr earlier than that, in the terminal Ediacaran.
Reference to stem and crown groups in the Cambrian evolutionary radiation
The gastropod Pelagiella atlantoides and apparent sabellid Pseudopelagiella exigua prompt consideration of what comprises stem group and crown group in the CER. Budd & Mann (2020) detailed that: (1) stem and crown are divisions of the total group clade; (2) preservational (and discovery) issues affect understanding of clade development; and (3) misidentification of stem group taxa takes place. To this might be added another consideration: that phylogenies, however sophisticated, are interpretations of relationships that reflect the current taxonomy and known chronostratigraphy of biotic groups, with taxonomy often reflecting the consensus of a few specialists.
Gastropods have been regarded as crown group conchiferans (e.g. Haszprunar 1988). Although teloconchs by themselves provide no evidence for suprafamilial assignment (e.g. Frýda et al. 2009), the designation of Pelagiella s.s. as a stem group gastropod (e.g. Vinther 2015; Thomas et al. 2020) is not justified. Indeed, topotype material of the type species allows close comparison with the extant crown group vetigastropod Haliotis. Similarly, paleal fan arrays and the microhistology of its calcareous conch allow comparison of Pseudopelagiella and other forms traditionally brought to Pelagiella with taxa of the crown group Canalipalpata and tentatively the Sabellida. For this reason, Pseudopelagiella shows the presence of a crown group taxon in the late Early Cambrian. The presence of the probably benthic Pseudopelagiella complements the presence of the sessile, tube-dwelling Dannychaeta Chen et al., 2020 from the late Early Cambrian of China, which is also regarded as a crown group annelid (Chen et al. 2020) and whose features are consistent with the Canalipalpata. The age of these late Early Cambrian annelids is significantly younger than the earliest known occurrence of Sabellidites, a crown group siboglinid sabellid, in the terminal Ediacaran (Moczydłowska et al. 2014).
The late Early Cambrian, c. 18–30 myr younger than the Cambrian base, featured the lowest known appearance of many higher-level taxa (colonial anthozoans, possible tabulates, echinoderm classes, graptoloid groups, trilobites). These taxa join somewhat earlier appearing forms (lingulate and rhynchonelliform brachiopods; Harper et al. 2017), and all proliferate in the Great Ordovician Biodiversification Interval (Landing et al. 2010, 2018). A number of these groups are extant and can be termed crown groups.
In addition, crown groups (orders) of the phylum/class Foraminifera have Early Cambrian or older appearances. These include the Monothalamida Loeblich & Tappan, 1961 (terminal Ediacaran), and Textulariida Delage & Hérouard, 1896, Rotaliida Delage & Hérouard, and probably Miliolida Delage & Hérouard (Pawlowski et al. 2003).
At least one-third of metazoan phyla are confidently known in the Early Cambrian (e.g. Chen & Huang 2002; Giribet et al. 2008). This comports with the late Early Cambrian marking the latest part or the end of the origin of higher level taxa of the CER (Landing et al. 2018).
What defines early members of a crown group may need consideration. Thus, specimens of the Sipuncula Rafinesque, 1814, in the Chengjiang fauna, which, but for a U-shaped gut, not a helical gut, are otherwise comparable to the extant Golfingia Lankaster, 1885. Whether these forms lack a full crown group body plan (Huang et al. 2004) or are actually crown group taxa (Eibye-Jacobsen & Vinther 2012) reflects the weight placed on specific morphologic features and the variability allowed by definitions of lower level taxonomic groups.
The appearance of Pseudopelagiella exigua and Pelagiella atlantoides in the late Early Cambrian shows that crown group polychaetes (probable sabellids) and the canalipalpatan (Dannychaeta) and gastropods existed late in the CER. This conclusion complements the existence of crown group sabellids (Sabellidites) and probable gastropods (Aldanella) in the late Ediacaran and earliest Cambrian (Fortunian), respectively. The apparent resolution of the Pelagiella problem with recognition of polychaete fan arrays in Pseudopelagiella and the re-emphasis that Pelagiella atlantoides is a gastropod contributes to the discussion of lophotrochozoan phylogeny.
Conclusion
The re-evaluation of forms commonly termed Pelagiella leads to a reconsideration of lophotrochozoan evolution and relationships in the CER. Forms known only from conchs with any similarity to so-called Pelagiella (i.e. pelagiellids as traditionally interpreted, helcionelloids, coreospirids, igarkiellids, khairkhaniids, see Parkhaev 2008) should not be slotted into early molluscan phylogeny based on a presumed mollusc/gastropod affinity. It took the discovery of exceptional specimens of Pseudopelagiella exigua gen. nov. to re-evaluate the regularly unquestioned assignment of so-called Pelagiella spp. to the Gastropoda Cuvier, 1795.
An understanding of the higher-level taxonomy and phyletic relationships of early mollusc-like fossils in the CER has been limited by the Pelagiella problem, in which tiny coiled conchs are assumed to be molluscs and gastropods: an assumption that is the basis for naming numerous, poorly differentiated species assigned incorrectly to the late Early Cambrian gastropod Pelagiella Matthew, a crown group gastropod comparable to the modern Haliotis. The long recognized unusual form of the many so-called Pelagiella spp. conchs sets them apart from Pelagiella s.s. Preservation of polychaete-like chaetal fan arrays in Pseudopelagiella exigua shows it to be a crown group canalipalpatan. Fan arrays and calcareous tubes are features in separate modern sabellid families that allow a tentative reference of Pseudopelagiella exigua, and probably many similar Pelagiella spp., to the crown group Sabellida?. This qualified assignment reflects a tentative assignment of the Sabellariidae, which has paleal chaetae, to the Sabellida. By this interpretation, the pelagiellids and spirorbids represent vagrant and sessile (cemented) groups, respectively, of the Sabellida.
The fossil record allies with assignment of molluscs, annelids, and lophophorates to the clade Lophotrochozoa Halanych et al., 1995, but not with the proposal that chitinous chaetae are a homology within the clade (fide Thomas et al. 2020). Convergent evolution apparently led to the appearance of chaetae in annelids and brachiopods, with anterior chitinous capillary chaetae unknown in forms confidently recognized as molluscs.
Systematic palaeontology
Phylum ANNELIDA Lamarck, 1802
Class POLYCHAETA Grube, 1850
Remarks
Polychaeta and polychaetes are traditional, not phylogenetically defined taxonomic designations in this report.
Clade CANALIPALPATA Rouse & Fauchald, 1997
Order SABELLIDA? Levinsen, 1883
Remarks
See the polychaete assignment of Pseudopelagiella, above.
Family PSEUDOPELAGIELLIDAE fam. nov.
LSID
urn:lsid:zoobank.org:act:72A91751-3B32-45A5-B226-6ACF1F84B12C
Derivation of name
Derived from Pseudopelagiella gen. nov.
Diagnosis
Small univalved, calcareous, coiled, conch-dwelling polychaetes; shell unattached to substrate. Conch sinistral in Pseudopelagiella gen. nov., more or less subtriangular–triangular aperture, rapidly expanding whorls, surface smooth–transversely undulated–longitudinally ridged. Paleal chaetae fan arrays at aperture.
Description
See description of shells and fan arrays of the type genus Pseudopelagiella exigua by Thomas et al. (2020).
Remarks
Further work may demonstrate a range of shell forms that may be as diverse as in the Spirorbidae (see Rzhavsky 1994). The assignment (herein) of Costipelagiella (a synonym of so-called Pelagiella fide Kouchinsky et al. 2011, 2015) to the family means that taxa may have longitudinally ridged conchs. Landing & Bartowski (1996) and Landing et al. (2002) described Pelagiella primaeva (Billings) conchs with prosocyrt growth lines. This may mean that a polychaete-like oral notch is present in taxa of the family.
Included genera
The gastropod Pelagiella atlantoides is now the only remaining genus and species of the family Pelagiellidae Knight and order Pelagielliformes MacKinnon (see Parkhaev 2008). Other genera (e.g. Costipelagiella, Tannuspira Missarzhevsky, 1989) previously assigned in various reports to the Pelagiellidae are assigned to the Pseudopelagiellidae.
Demonstration that Pseudopelagiella exigua is a polychaete suggests that other Cambrian taxa may be referable to or allied with the Pseudopelagiellidae. These include taxa known from nearly bilaterally symmetrical to dextrally and sinistrally coiled conchs with presently unknown muscle scars (e.g. possibly some helcionelloids, coreospirids; igarkiellids; khairkhaniids).
Genus PSEUDOPELAGIELLA nov.
LSID
urn:lsid:zoobank.org:act:2E9D0522-08E5-43FF-8D13-ED1F68DA5969
Derivation of name
Pseudo (from Greek ψɛυδής, lying, false) implies a coincidental similarity in biological (Linnaean) nomenclature; it is a common prefix for trace fossil and trilobite genera (e.g. Häntzschel 1975; Whittington et al. 1997).
Type species
Pelagiella exigua Resser & Howell, 1938.
Type horizon and locality
See lectotype for Pseudopelagiella exigua below.
Diagnosis
See diagnosis for Pseudopelagiellidae above.
Description
See description of the Pseudopelagiellidae above. The type species seem to have an elevated exterior whorl margin and depressed spire (Thomas et al. 2020).
Remarks
If Pelagiella primaeva (Billings) from Taconian Quebec and New York is a synonym of Pseudopelagiella exigua (synonymy list below), then the species has concave to convex dorsal surfaces.
Included species and distribution
The type species and possible senior synonym Pelagiella primaeva are confidently referable to the genus. Reassignment of some of the so-called Pelagiella species to Pseudopelagiella may be appropriate. Many Pelagiella spp. show considerable morphologic variation even among associated specimens, and an unstable species-level taxonomy (Bengtson et al. 1990; Parkhaev 2001) has to be evaluated. The long stratigraphic range and wide palaeogeographic and climatic distribution of named Pelagiella species may reflect a number of supraspecific taxa. These taxa assigned to Pelagiella may be provisionally assigned to Pseudopelagiella, with suppression of Pelagiella for any but the gastropod type species of the genus.
Pseudopelagiella exigua (Resser & Howell, 1938)
Figure 2
| ? 1872 | Platyceras primaevum Billings, p. 230. |
| 1938 | Pelagiella exigua Resser & Howell, p. 180, pl. 2, figs 2, 3. |
| ? 1956 | Pelagiella primaeva (Billings); Lochman, p. 1130, pl. 1, figs 19–21 (with synonymy to 1956). |
| ? 1996 | Pelagiella primaevum (Billings); Landing & Bartowski, fig. 5.22. |
| ? 2002 | Pelagiella primaevum (Billings); Landing et al., p. 299, fig. 8.8, 8.10. |
| 2020 | Pelagiella exigua Resser & Howell; Thomas et al., p. 20, 21, figs 2, 4–7, 13. |
Lectotype
USNM 90777A (Resser & Howell 1938, pl. 2, fig. 2) designated by Thomas et al. (2020).
Cotypes, topotypes and referred material
See Thomas et al. (2020, p. 20).
Diagnosis
As for Pseudopelagiella by monotypy.
Description
See Thomas et al. (2020).
Acknowledgements
This report is dedicated to R. D. K. Thomas and colleagues for their Kinzers Lagerstätte work. A. Kouchinsky, G. Budd, and an anonymous reviewer made important comments. Support includes grant GE 549/22-1 of the Deutsche Forschungsgemeinschaft (DFG) (to GG); the State Research Program at Moscow State University, project No. AAAA-A16-116021660062-9 (to IAJ); and the Italian National Antarctic Program (PNRA), projects 2010-A1.10 and PNRA18_00263 (to SS).
Author contributions
All of the authors contributed to this report.
Open Research
Data archiving statement
This published work and the nomenclatural act it contains, have been registered in ZooBank: http://zoobank.org/References/BD0C46AA-29A0-4DC5-910D-F96C2185088B




