Palynology of a short sequence of the Lower Devonian Beartooth Butte Formation at Cottonwood Canyon (Wyoming): age, depositional environments and plant diversity
Abstract
The Beartooth Butte Formation hosts the most extensive Early Devonian macroflora of western North America. The age of the flora at Cottonwood Canyon (Wyoming) has been constrained to the Lochkovian–Pragian interval, based on fish biostratigraphy and unpublished palynological data. We present a detailed palynological analysis of the plant-bearing layers at Cottonwood Canyon. The palynomorphs consist of 32 spore, five cryptospore, two prasinophycean algae and an acritarch species. The stratigraphic ranges of these palynomorphs indicate a late Lochkovian or Pragian age, confirming previous age assignments. Analysis of samples from three different depositional environments of the plant-bearing sequence (layers with in situ lycophyte populations, flood layers that buried those populations and an organic matter accumulation zone within a flood layer) demonstrate distinct palynofacies. Comparisons between palynomorph and plant macrofossil diversity reveal some discrepancies. Whereas zosterophylls and lycophytes, most diverse and abundant among the macrofossils, have only one known corresponding spore type (assignable to zosterophylls) in the palynomorph assemblage, the trimerophytes, rare in the macrofossil assemblage, are represented by three spore types. Some of these discrepancies reflect taphonomic differences between the macrofossils and palynomorphs, while others could be due to the fact that the parent plants of most palynomorph types in the Cottonwood Canyon assemblage are unknown. These observations emphasize the need for concerted efforts to bring together the knowledge of macro- and microfloras within Early Devonian localities. Nevertheless, given the palaeophytogeographic significance of the Beartooth Butte Formation flora, its palynofossil and macrofossil assemblages, taken together, provide new data relevant to future discussions of Early Devonian biogeography.
The Beartooth Butte Formation of Wyoming hosts rich and diverse Early Devonian fossil assemblages. These include fauna (fishes, eurypterids, microconchids), as well as the only extensive Early Devonian flora explored in western North America to date (Dorf 1933, 1934; Hueber 1972; Tanner 1983; Matsunaga & Tomescu 2017). Because of its geographic and stratigraphic position, this flora represents a key component in the overall picture of Early Devonian vegetation and plant biogeography. The macroflora of the Beartooth Butte Formation consists of rhyniopsids, zosterophylls, trimerophytes, and basal lycopsids, as well as thalloid gametophytes of putative bryophytic affinities. The geology of the unit, as well as its vertebrate and invertebrate fauna, have been studied extensively (e.g. Bryant 1932; Sandberg 1961; Denison 1970; Elliot & Johnson 1997; Fiorillo 2000; Lamsdell & Legg 2010; Caruso & Tomescu 2012). However, for the macroflora the only extensive survey was undertaken in a doctoral dissertation by Tanner (1983). Tanner’s unpublished survey is complemented by only a handful of published studies focused on specific taxa (Hueber 1972; Tanner 1982; Steenbock & Tomescu 2013; Matsunaga & Tomescu 2016, 2017). Importantly, aside from a brief palynomorph species list provided by Tanner (1983; who was reporting results communicated to him by D. C. McGregor; Table 1), the Beartooth Butte Formation has seen no in-depth study or illustration of the palynoflora.
| Taxa identified in this study | Figure | Corresponding taxa reported by Tanner (1983) |
|---|---|---|
| Acritarch | ||
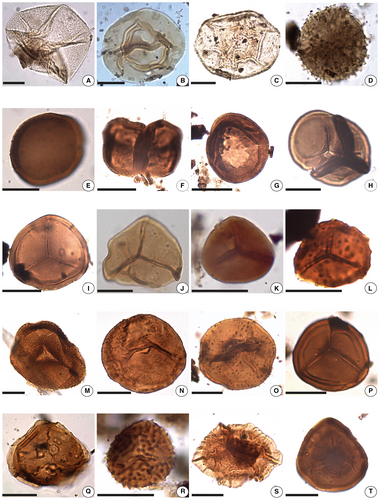
| Lophosphaeridium sp. | 4A | |
| Chlorophycean algae | ||
| Duvernaysphaera stellata Deunff, 1964 | 4B | |
| Leiosphaeridia sp. | 4C | |
| Cryptospores | ||
| Cymbohilates sp. cf. C. baqaensis Breuer et al., 2007 | 4D | |
| Cymbohilates sp. cf. C. comptulus Breuer et al., 2007 | 4E | |
| Dyadospora murusattenuata Morphon Strother & Traverse 1979 sensu Steemans et al., 1996 | 4F | |
| Gneudnaspora divellomedia var. divellomedia Breuer et al., 2007 | 4G | |
| Tetrahedraletes medinensis (Strother & Traverse) Wellman & Richardson, 1993 | 4H | |
| Trilete spores | ||
| Ambitisporites avitus Hoffmeister, 1959 | 4I | |
| Ambitisporites sp. cf. A. warringtonii (Richardson & Lister) Richardson et al., 2001 | 4J | |
| Ambitisporites warringtonii (Richardson & Lister) Richardson et al., 2001 | 4K | Retusotriletes warringtonii Richardson & Lister |
| Aneurospora sp. cf. A. tojoides var. A Steemans, 1989 | 4L | Aneurospora sp. |
| Apiculiretusispora brandtii Streel, 1964 | 4M | |
| Apiculiretusispora plicata (Allen) Streel, 1967 | 4N | Apiculiretusispora plicata |
| Apiculiretusispora spicula Richardson & Lister, 1969 | 4O | ?Apiculiretusispora spicula |
| Archaeozonotriletes chulus (Cramer) Richardson & Lister, 1969 | 4P | |
| Auroraspora sp. | 4Q | |
| Biornatispora dubia (McGregor) Steemans, 1989 | 4R | |
| Chelinospora sp. | 4S | |
| Concentricosisporites sagittarius (Rodríguez) Rodríguez González, 1983 | 4T | |
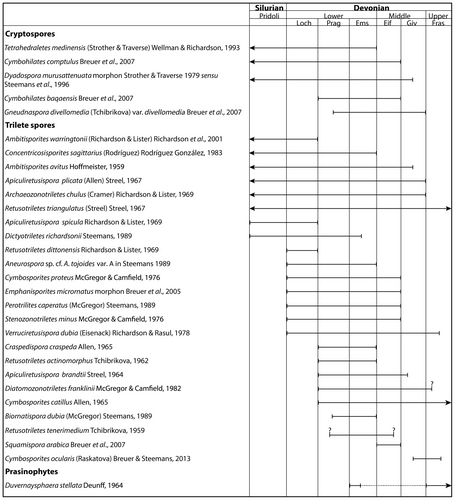
| Craspedispora craspeda Allen, 1965 | 5A | |
| Cymbosporites catillus Allen, 1965 | 5B | |
| Cymbosporites proteus McGregor & Camfield, 1976 | 5C | Cymbosporites proteus |
| Cymbosporites? senex McGregor & Camfield | ||
| Cymbosporites sp. cf. C. ocularis (Raskatova) Breuer & Steemans, 2013 | 5D | |
| Diatomozonotriletes sp. cf. D. franklinii McGregor & Camfield, 1982 | 5E | |
| Dictyotriletes richardsonii Steemans, 1989 | 5F | |
| Dictyotriletes sp. cf. D. richardsonii Steemans, 1989 | 5G | |
| Dictyotriletes sp. A | 5H | Dictyotriletes sp. |
| Emphanisporites micrornatus Morphon Breuer et al., 2005 | 5I | Emphanisporites micrornatus/Emphanisporites micrornatus? Richardson & Lister |
| cf. Emphanisporites rotatus McGregor | ||
| Perotrilites caperatus (McGregor) Steemans, 1989 | 5J | |
| Retusotriletes actinomorphus Tchibrikova, 1962 | 5K | Retusotriletes actinomorphus |
| Retusotriletes dittonensis Richardson & Lister, 1969 | 5L | |
| Retusotriletes simplex Naumova | ||
| Retusotriletes tenerimedium Tchibrikova, 1959 | 5M | |
| Retusotriletes triangulatus (Streel) Streel, 1967 | 5N | |
| Rhabdosporites sp. | 5O | |
| Squamispora arabica Breuer et al., 2007 | 5P | |
| Stenozonotriletes minus McGregor & Camfield, 1976 | 5Q | |
| Verruciretusispora dubia (Eisenack) Richardson & Rasul, 1978 | 5R | |
| Verruciretusispora sp. A | 5S | |
| Verrucosisporites sp. A | 5T | |
| Synorisporites sp. | ||
- The list is organized by major palynomorph groups, in alphabetical order (with illustration reference, where applicable) compared with the taxa reported by Tanner (1983) based on a personal communication to him by D. C. McGregor.
Here, we present an in-depth survey of the rich and well-preserved palynological assemblage recovered from the plant-bearing beds at the Cottonwood Canyon locality of the Beartooth Butte Formation in northern Wyoming. Although the taxonomy and stratigraphic distribution of the palynomorph species at Cottonwood Canyon provide the data for ascertaining the late Lochkovian to Pragian age of the plant-bearing layers in a palynostratigraphic context, the palynofacies components allow for more detailed characterization of the depositional environments. Given the palaeophytogeographic importance of this flora, the palynofossil and macrofossil assemblages, taken together, provide a more complete picture for inclusion in future discussions of the biogeography of the Early Devonian.
Geological setting
The Beartooth Butte Formation is a discontinuous Lower Devonian unit bounded by the Ordovician Bighorn Dolomite and the Upper Devonian Jefferson Dolomite (or Jefferson Dolomite) (Blackstone & McGrew 1954; Sandberg 1961). The unit consists primarily of dolomitized siltstone and shale with dolomitized sandstone interbeds (Sandberg 1961; Caruso & Tomescu 2012; Matsunaga & Tomescu 2017). The large-scale geometry of the Beartooth Butte Formation, its sedimentology and fossil content, along with isotopic data, suggest fresh- to brackish-water depositional environments of estuarine to fluvial nature (Dorf 1934; Denison 1956; Sandberg 1961; Fiorillo 2000; Matsunaga & Tomescu 2017).
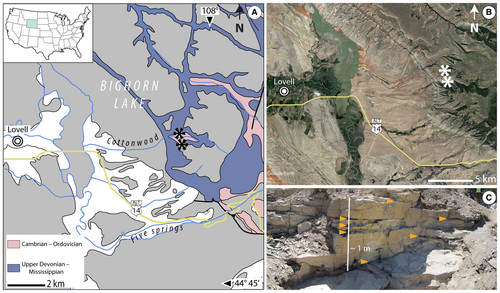
Aside from the type locality at Beartooth Butte (Park County, Wyoming), the unit is exposed at a few other known locations throughout the rugged landscape of northern Wyoming and southern Montana (Sandberg 1961). One of the best studied of these is the Cottonwood Canyon locality, in the Bighorn Mountains of northern Wyoming, Big Horn County (Fig. 1; Blackstone & McGrew 1954; Sandberg 1961, 1967). Analyses of the palynoflora at the two localities by D. C. McGregor (unpublished but summarized in Tanner’s 1983 dissertation) proposed a late Lochkovian to early Pragian age for the Beartooth Butte Formation at Cottonwood Canyon, and a mid-late Emsian age at the Beartooth Butte locality. These age assignments are broadly consistent with the fish biostratigraphy data published subsequently by Elliot & Ilyes (1996) and Elliot & Johnson (1997).
Material and method
Depositional environments and sampling
A total of 28 palynological samples were collected from different horizons of a c. 1-m-thick sequence of the Beartooth Butte Formation at Cottonwood Canyon (44°51′51″N, 108°02′46″W; Fig. 1A, B). The sampled horizons, rich in plant macrofossils, are part of a heterolithic sequence consisting of an alternation of two types of deposits (Fig. 1C). One type is represented by dark, finely laminated shales that preserve dense in situ populations of the early lycopsid Sengelia radicans (Figs 1C, 2C), which formed dense mats of interwoven stems with rhizomatous growth (Matsunaga & Tomescu 2017). The Sengelia shales alternate with massive beds of hard-cemented siltstones rich in transported plant material and other organic detritus, often highly fragmented (Figs 1C, 2D). The siltstone beds are penetrated by vertical in situ root-bearing axes from the Sengelia populations of the overlying dark shale layer. The heterolithic sequence has been interpreted as reflecting cycles of colonization by Sengelia of flood deposits at the water’s edge, and subsequent burial of Sengelia populations by periodic flood events (Matsunaga & Tomescu 2017).

Samples were processed at the Laboratory of Palaeopalynology (Palaeontology Section, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires, Argentina; MACN) following conventional methods with HCl and HF acid maceration and they were not oxidized. Extracted organic residues were sieved through a 10 µm mesh and the >10 µm fraction was mounted on standard microscope slides with acrylate (Noetinger et al. 2017). Productive samples (27) were analysed and imaged for identification of palynomorphs and quantification of palynofacies with a trinocular Leica DM2500 transmitted light microscope equipped with a Leica DFC290 camera. Measurements were carried out using ImageJ 1.49v open source software (Rueden et al. 2017). The position of illustrated specimens in the respective slides is given in England-Finder coordinates. All the material (slides, residues, rock samples) is deposited in the MACN collections under BA Pal accession numbers 6615–6641.
Palynofacies analysis
To explore the correlation between depositional environments and palynofacies, a subset of 11 samples differentiated between the two types of sediment were studied: Sengelia shales and flood deposits (Matsunaga & Tomescu 2017), hereinafter referred to as ‘Sengelia mat’ (3 samples) and ‘flood layer’ (8 samples), respectively. Aside from these two types of sediment, an especially thick flood layer hosted a thin (<1 cm thick) but conspicuous lens of dark material covering a c. 2 m2 surface area (Fig. 2A, B). This dark lens consisted of a concentrated accumulation of mostly fragmentary and amorphous organic matter, with a significant plant fraction. This lens rich in organics was sampled separately and is referred to as ‘organic lens’ (2 samples).
In each of the samples analysed for palynofacies two tallies were performed under transmitted light microscopy (Tyson 1995). To ensure the consistency of tallies between samples, we mounted 0.05 ml of organic aliquot extracted from each sample on slides, diluting it with the same amount of mounting medium. To account for the inherent variability in the distribution of palynofacies elements in the slides, we tallied all palynofacies elements (main axis >5 µm) observed in the field of view at 200× total magnification, in several randomly selected areas in each slide, until we reached a target minimum of 100 observations per sample and, when possible (in 10 out of 13 samples), more than 300 observations.
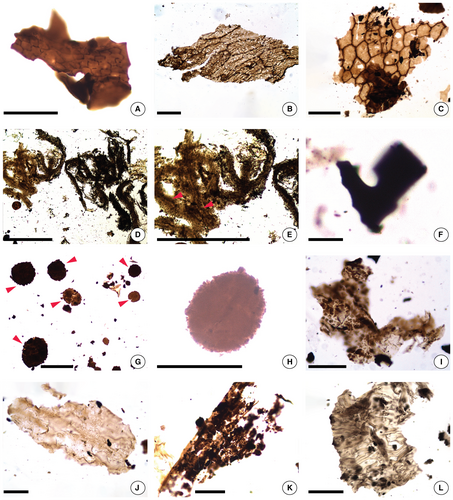
Preliminary test-tallies led us to distinguish the following categories of palynofacies elements for the analysis (Fig. 3): amorphous organic matter (AOM); wefts of fibrous structures (WFS); structured phytoclasts, translucent, sheet-like, showing cell outlines (SPCO); structured phytoclasts, mostly brown to dark brown, often with opaque areas and usually with sharp edges (SPSE); phytoclasts usually brown, with less well-defined surface structures or edges (PLD); phytoclasts with well-defined round outline (PDRO); opaque organic debris, black, mostly of small size (OOD); and spores (SPO). This categorization of palynofacies elements is simplified from the scheme developed by Mendonça Filho et al. (2012), and uses different designations and acronyms, except for WFS, which show more of a fibrous outline and better defined internal detail in our material, and for PDRO, which accounts for a distinct type of phytoclast not covered in that scheme.
Parent plants of dispersed spores
The Beartooth Butte Formation hosts a relatively diverse (Tanner 1983), albeit incompletely characterized, macroflora at Cottonwood Canyon. To explore correlations between this macroflora and the palynoflora, we queried the literature to assemble a list of parent plant species for those spores and cryptospores in our samples for which this type of data have been published.
Composition of the palynoflora
The Early Devonian Cottonwood Canyon palynoflora includes terrestrial elements (32 distinct trilete spore types and five cryptospores types), two types of prasinophycean algae, and an acritarch. Most of the palynomorphs are well preserved (Figs 4, 5).

Systematic palaeontology
Taxa left in open nomenclature are described below, organized by major groups and in alphabetical order. The complete list of recognized taxa, listed in alphabetical order for each major group of palynomorphs, is included in Table 1. The terminology follows the concepts outlined by Punt et al. (2007) relative to spores and cryptospores. In the descriptions, we list the lowest and highest measurements of the main axis of spores and cryptospores, as well as measurements for additional features (e.g. ornamentation, cingulum).
Cryptospores
Anteturma CRYPTOSPORITES (Richardson et al.) Richardson emend. Steemans, 2000
Genus CYMBOHILATES Richardson emend. Breuer et al., 2007
Type species
Cymbohilates horridus Richardson, 1996
Cymbohilates sp. cf. C. baqaensis Breuer et al., 2007
Figure 4D
Description
Cryptospore hilate proximally. Circular to subcircular amb. Distal and subequatorial exospore sculptured with closely packed bacula with concave tops. Elements 1.1–5.2 µm high and 1.0–2.3 µm wide at base and 1.3–2.2 µm wide at the top.
Dimensions (13 specimens)
Overall: 33.0–49.0 µm.
Comparisons
The specimens strongly resemble Cymbohilates baqaensis although the sculpture of the latter is lower. Cymbohilates baculatus Turnau et al., 2005 has fewer sculptural elements, which are also lower in height.
Cymbohilates sp. cf. C. comptulus Breuer et al., 2007
Figure 4E
Description
Cryptospore hilate proximally laevigate. Subcircular amb. Distal and subequatorial exospore sculptured with sparse coni 0.6–0.9 µm wide at base and 1.3–1.8 µm high.
Dimensions (1 specimen)
Overall: 43.2 µm.
Remarks
Cymbohilates comptulus has a diverse sculpture up to 1.5 µm high and the hilum has scars.
Trilete spores
Anteturma SPORITES Potonié, 1893
Turma TRILETES Reinsch, 1881 emend. Dettmann, 1963
Genus AMBITISPORITES Hoffmeister, 1959
Type species
Ambitisporites avitus Hoffmeister, 1959
Ambitisporites sp. cf. A. warringtonii (Richardson & Lister) Richardson et al., 2001
Figure 4J
Description
Cingulate spore, trilete. Amb subtriangular. Laesurae straight extended to the equator, covered by labra 1.7–1.9 µm high. Exine 1.5–1.7 µm thick, smooth.
Dimensions (1 specimen)
Overall: 45.0 µm.
Remarks
The single specimen encountered is bigger than the range proposed for the species (18.0–36.0 µm).
Genus AURORASPORA Hoffmeister et al., 1955
Type species
Auroraspora solisortus Hoffmeister et al., 1955
Auroraspora sp.
Figure 4Q
Description
Trilete pseudosaccate spore. Amb subtriangular with rounded angles. Exoexine 0.6 µm thick, laevigate with pleats. Intexine laevigate. Laesurae accompanied by labra 1 µm wide, extended to the margin of the inner body.
Dimensions (1 specimen)
Overall: 51 µm.
Comparisons
Auroraspora macromanifesta (Hacquebard) Richardson, 1960 is much larger than our specimen. Our specimen resembles Auroraspora minuta Richardson, 1965, but in the latter the central body is eccentrically placed.
Genus CHELINOSPORA Allen, 1965
Type species
Chelinospora concinna Allen, 1965
Chelinospora sp.
Figure 4S
Description
Patinate spore, trilete. Amb subcircular. Laesurae straight to sinuous, covered by labra 0.9–1.8 µm wide extending to the equator. Exine 2.0–6.1 µm thick at the equator and distally bearing convoluted muri 0.7–1.3 µm wide.
Dimensions (3 specimens)
Overall: 31.0–56.0 µm.
Comparisons
Chelinospora sp. resembles both C. hemispherica (Cramer & Diez) Richardson et al., 2001 and Chelinospora densa Breuer & Steemans, 2013. The former has narrower muri (<1 µm wide) than our specimens. Our specimens share more features with the latter species, defined in Gondwana, but their poor preservation prevents identification with that species. Chelinospora timanica (Naumova) Loboziak & Streel, 1989 has wider muri and verrucae than our specimens.
Genus CYMBOSPORITES Allen, 1965
Type species
Cymbosporites cyathus Allen, 1965
Cymbosporites sp. cf. C. ocularis (Raskatova) Breuer & Steemans, 2013
Figure 5D
Description
Patinate spore, trilete. Amb subtriangular with broad rounded angles. Laesurae straight extending to the equator. The exine seems to be bearing verrucae distally.
Dimensions (1 specimen)
Overall: 28.0 µm. Patina: 11.3–17.4 µm wide.
Remarks
The poor preservation of the specimen, which prevents the clear observation of the verrucae, and its smaller size preclude inclusion in the species C. ocularis.
Genus DIATOMOZONOTRILETES Naumova emend. Playford, 1962
Type species
Diatomozonotriletes saetosus (Hacquebard & Barss) Hughes & Playford, 1961
Diatomozonotriletes sp. cf. D. franklinii McGregor & Camfield, 1982
Figure 5E
Description
Trilete spore. Amb subtriangular with rounded angles and straight sides. Exine: 0.6–1.0 µm thick. Laesura straight, extended 5/6 the length of the radius and darkened along its edges. Along the equator the ornamentation is more concentrated in the middle of the interradial margins and is more scattered on the distal face. Sculpture elements are subcircular in plan view, 1.1–1.5 µm wide at the base and 0.6–1.0 µm high.
Dimensions (1 specimen)
Overall: 30.0 µm.
Remarks
The only specimen encountered does not show as dense an ornamentation as those illustrated by McGregor & Camfield (1982).
Genus DICTYOTRILETES Naumova ex Ishchenko, 1952
Type species
Dictyotriletes bireticulatus (lbrahim) Potonié & Kremp, 1955
Dictyotriletes sp. A
Figure 5H
Description
Trilete spore. Amb subtriangular with broad rounded angles. Laesurae straight, simple or accompanied by labra 0.9–1.0 µm wide, extended to the equator. Exine smooth on the proximal face. Distal face with a reticulum formed by muri 1.6–3.0 µm high and 0.2–0.8 µm wide; lumina mostly regular in shape, polygonal, each 1.4–2.8 µm in diameter and covering 1.6–4.0 µm2.
Dimensions (5 specimens)
Overall: 22–30 µm.
Comparisons
Dictyotriletes sp. A resembles D. richardsonii Steemans, 1989 but the latter has lower muri (0.5 µm both high and wide), as well as Dictyotriletes ?gorgoneus Cramer in McGregor (1973). Dictyotriletes emsiensis (Allen) McGregor, 1973 is bigger overall. Dictyotriletes subgranifer McGregor, 1973 is bigger and has sculptured contact areas on the proximal face. Dictyotriletes granulatus Steemans, 1989 has grana proximally.
Genus RHABDOSPORITES Richardson emend. Marshall & Allen, 1982
Type species
Rhabdosporites langii (Eisenack) Richardson, 1960
Rhabdosporites sp.
Figure 5O
Description
Trilete cavate spore. Amb subtriangular with rounded angles, almost oval. Exoexine 1 µm thick, irregularly granulate (even though the distribution of grana could be a preservation artefact), with elements less than 1 µm wide at base. Intexine laevigate, 2 µm thick. Laesurae accompanied by labra 1 µm wide, extending to the equator.
Dimensions (1 specimen)
Overall: 69 µm. Inner body 55 µm.
Comparisons and remarks
Rhabdosporites langii (Eisenack) Richardson, 1960 is bigger than our specimen and is uniformly sculptured. The comparison to the species R. minutus Tiwari & Schaarschmidt, 1975 is problematic because its type species was synonymized with Geminospora lemurata Balme emend. Playford, 1983, by Troth et al. (2011). Later on, R. minutus was included in the Apiculiretusispora brandtii morphon erected by Breuer & Steemans (2013), due to the type of sculpture and the variability in the separation of the exoexine in the homonymous species, which could reach a total detachment, as seen in R. minutus. Apiculiretusispora brandtii occurs in our samples, but the numerous specimens observed seem quite homogeneous in shape (exemplified by the one pictured in Fig. 4M) and they are notably different from Rhabdosporites sp.
Genus VERRUCIRETUSISPORA Owens, 1971
Type species
Verruciretusispora dubia (Eisenack) Richardson & Rasul, 1978
Verruciretusispora sp. A
Figure 5S
Description
Trilete spore. Amb distinctly circular with a pyramidal proximal face. Laesurae straight, extended 2/4 of the distance to the equatorial margin and ending at curvaturae. The trilete mark is surrounded by a darkened area. Contact faces laevigate to faintly granulated. Exine is 1.9–7.0 µm thick. The sculpture, irregularly distributed on the distal face, consists of verrucae that can anastomose to form rugulae and grana, very variable in size but up to 1.2 µm high and 5.4 µm wide at base.
Dimensions (9 specimens)
Overall: 70.3–137.0 µm.
Remarks
The density of ornamentation varies from one specimen to the other.
Comparisons
These specimens resemble Verruciretusispora labiosa Steemans et al., 2011, but the latter has labra accompanying the trilete mark and the ornamentation has a more regular distribution in the whole distal face. Verruciretusispora dubia (Eisenack) Richardson & Rasul, 1978 has wider verrucae (1–10 µm wide at base). Verruciretusispora ornata (Menéndez & Pöthe de Baldis) Pérez Leyton ex di Pasquo, 2005 has cylindrical processes of various sizes (1.1–4.4 µm high). Verruciretusispora pallida Owens, 1971 has sculptural elements that are topped by a small cone.
Genus VERRUCOSISPORITES lbrahim emend. Smith, 1971
Type species
Verrucosisporites verrucosus (Ibrahim) Ibrahim, 1933
Verrucosisporites sp. A
Figure 5T
Description
Trilete spore. Amb circular. Laesurae straight, simple, extended 3/4 of the distance to the equatorial margin. Contact faces laevigate to faintly granulate. Exine 1.6–4.4 µm thick. Distal face ornamented with closely packed verrucae that can anastomose to form rugulae, variable in size, 0.4–1.7 µm wide at base.
Dimensions (6 specimens)
Overall: 36.0–53.0 µm.
Comparisons
These specimens resemble Verrucosisporites nafudensis Breuer & Steemans, 2013, but the latter has a thinner wall and larger ornaments (0.5–3.0 µm).
Palynofacies and depositional environments
The tally results for each category of palynofacies elements (Fig. 3; Noetinger et al. 2021, table S1) were pooled by sample type (depositional environment) and normalized to eliminate the effect of the number of samples studied for each environment (i.e. divided by the number of samples (n) examined for each environment: Sengelia mat (n = 3), flood layer (n = 8), and organic lens (n = 2); Table 2). Overall, the palynofacies assemblages are overwhelmingly dominated by opaque organic debris (OOD), followed (in decreasing order) by SPSE structured phytoclasts, PLD phytoclasts, spores, and amorphous organic matter (AOM) (Table 2). The sheet-like phytoclasts with cell outlines (SPCO), fibrous wefts (WFS), and round phytoclasts (PDRO) occur in significantly smaller quantities.
| Palynofacies category | Sengelia mat (n = 3) | Flood layer (n = 8) | Organic lens (n = 2) |
|---|---|---|---|
| AOM | 0.0 | 18.6 | 20.5 |
| WFS | 0.0 | 4.6 | 7.5 |
| SPCO | 3.0 | 2.4 | 8.0 |
| SPSE | 54.3 | 35.6 | 19.0 |
| PLD | 43.7 | 35.0 | 23.0 |
| PDRO | 3.0 | 4.0 | 1.5 |
| OOD | 132.0 | 193.8 | 98.0 |
| SPO | 1.7 | 2.4 | 49.0 |
- Numbers are counts of palynofacies elements totalled for each depositional environment and normalized by number of samples (n) examined for each environment. Palynofacies categories: AOM, amorphous organic matter; OOD, opaque organic debris; PDRO, phytoclasts with round outlines; PLD, phytoclasts with less well-defined structure or edges; SPCO, structured phytoclasts with cell outlines; SPO, spores; SPSE, structured phytoclasts with sharp edges; WFS, wefts of fibrous structures.
The three depositional environments sampled exhibit significant differences in palynofacies (Table 2). The most conspicuous contrast is that between Sengelia mat samples, on one hand, and the rooting level and organic lens samples, on the other hand. Sengelia mat samples are dominated by SPSE and PLD phytoclasts, which are less frequent in the other two sample types, have low spore content, and lack entirely AOM and fibrous wefts (WFS), which are present in the other two depositional environments. In contrast, the organic lens samples have much higher spore content, the highest AOM and WFS content, as well as higher content of phytoclasts with cell outlines (SPCO) than the other two depositional environments. The palynofacies of the flood layers (rooting levels) is somewhat intermediate between that of the Sengelia mats and that of the organic lens, except for the higher content in opaque organic debris (OOD). Like the former, flood layers are richer in SPSE and PLD phytoclasts but contain fewer SPCO and fewer SPO than the organic lens. However, like the organic lens, flood layers contain AOM and WFS.
Parent plants of dispersed spores
Of the 37 different spore and cryptospore types included in the Cottonwood Canyon palynoflora, more than half (22 types) have no recognized parent plant, to date (Noetinger et al. 2021, table S2). Two other spore types (Dictyotriletes and Verrucosisporites; each represented by a form that does not fit in any previously recognized species of its respective genus) have been associated only with younger, Carboniferous plants representing both the lycophyte and the euphyllophyte clade, and potential producers in the Early Devonian have yet to be documented.
Of the remaining 13 types, whose affinities are better understood, two species of Apiculiretusispora (A. brandtii and A. plicata) have been associated with species in a total of six genera including rhyniophytes, trimerophytes and a barinophyte. Five other types in the genera Aneurospora, Cymbosporites, Dyadospora, Gneudnaspora and Rhabdosporites do not have recognized parent plants, but other species in their respective genera are known to have been produced by plants in at least seven genera of rhyniophytes, rhyniophytoids and barinophytes, but also aneurophyte progymnosperms and plants incertae sedis. Aside from these, it is most likely that Emphanisporites micrornatus was produced by a species of Horneophyton (Edwards & Richardson 2000) and spores assignable to genus Ambitisporites are associated with at least three Cooksonia species (Balme 1995; Edwards et al. 2014). Finally, four spore types in the assemblage (two species of Ambitisporites and two of Retusotriletes) have each been associated with one species in four genera that include rhyniophytes, zosterophylls and trimerophytes.
Discussion
Age and correlation of the Cottonwood Canyon flora
Previous palynological studies conducted by McGregor (unpublished results summarized by Tanner 1983) have suggested a late Lochkovian to early Pragian age for the Beartooth Butte Formation at Cottonwood Canyon. This assignment was based on the ‘size and level of structural complexity’ (Tanner 1983, p. 12) of a short list of palynomorph species. Our study yielded a much richer assemblage (Table 1) with well-defined global stratigraphic ranges (Fig. 6).
Few palynological studies addressing systematics and biostratigraphy have documented Early Devonian Laurentia. These studies come from eastern Laurentia: the Gaspé Peninsula of eastern Quebec (e.g. McGregor 1973, 1977) and the Moose River Basin, at the south-eastern end of Hudson Bay, in Ontario (McGregor & Camfield 1976). More recently, Wellman (2018) has described an Early Devonian spore assemblage from New Brunswick (Canada), also in eastern Laurentia. The Beartooth Butte Formation represents sediments accumulated in westernmost Laurentia and its palynomorph assemblage shares few species with the east-Laurentian microfloras (i.e. Tetrahedraletes medinensis, Apiculiretusispora brandtii, A. plicata, Archaeozonotriletes chulus, Biornatispora dubia (reported as Camptotriletes dubia), Cymbosporites proteus, Emphanisporites micrornatus, Perotrilites caperatus (reported as Camptozonotriletes caperatus), Retusotriletes actinomorphus, R. triangulatus, Stenozonotriletes minus, Verruciretusispora dubia). These species represent less than the 25% of the total species encountered in our assemblage and none of them is known exclusively from Laurentia. The relatively small amount of taxonomic overlap between the Beartooth Butte Formation assemblage and eastern Laurentian microfloras precludes extensive palynostratigraphic correlations across Laurentia and requires farther-reaching correlations. Furthermore, some of these shared species may have asynchronous occurrences (e.g. Biornatispora dubia) recorded since the Emsian in the Gaspé assemblages (McGregor 1973), but known from earlier occurrences in Gondwana (Breuer & Steemans 2013).
The common occurrence of Emphanisporites micrornatus, Cymbosporites proteus, Perotrilites caperatus and Stenozonotriletes minus throughout our samples is consistent with a Lochkovian–Emsian age (McGregor & Camfield 1976). Nonetheless, taking into consideration the age correction advocated by Stoakes (1978) for the Stooping River Formation in the Moose River Basin (see Troth et al. 2011), in which these palynomorph species occur, their stratigraphic range would reach the Eifelian. In the Old Red Sandstone Continent, the Emphanisporites micrornatus–Streelispora newportensis (MN) Assemblage Zone is dated to the Lochkovian (Richardson & McGregor 1986). However, the fact that in our assemblage we were able to document only two other species characteristic of this Assemblage Zone (i.e. Cymbosporites proteus and Apiculiretusispora plicata), and given the absence of Streelispora newportensis, the other eponymous species of this Assemblage Zone, this weakens the strength of an unequivocal Lochkovian age assignment for the Beartooth Butte Formation.
In the Ardennes and Rhenish regions of France and Germany, the earliest occurrence of Emphanisporites micrornatus var. micrornatus marks the beginning of Interval Zone M of the Oppel MN Zone, the age of which reaches the late Lochkovian (Streel et al. 1987; Steemans 1989). However, this Interval Zone is also marked by the disappearance of Dictyotriletes richardsonii, a species that occurs throughout our assemblage and which, farther south, in Gondwana, occurs in beds dated to the late Pragian to early Emsian (i.e. Grahn et al. 2013). In contrast, Perotrilites caperatus has its first appearance, along with other species not present in our assemblage, in the Si Phylozone within the Oppel MN Zone. This phylozone was dated to the late Lochkovian (Steemans 1989), therefore the presence of Perotrilites caperatus in our assemblage is another element supporting a late Lochkovian age.
The occurrence in our assemblage of some species originally defined in Gondwana (e.g. Duvernaysphaera stellata, Cymbohilates baqaensis, C. comptulus, Ambitisporites avitus, Squamispora arabica) allows for further comparisons with biostratigraphic schemes assembled for that continent. Cryptospore species, such as Cymbohilates comptulus, C. baqaensis, together with Gneudnaspora divellomedia var. divellomedia, are present in Gondwana in the Synorisporites papillensis – Cymbohilates baqaensis Assemblage Zone, which is dated to the late Pragian (Breuer & Steemans 2013). In the overlying Assemblage Zone (Latosporites ovalis – Dictyotriletes biornatus; Breuer & Steemans 2013), Apiculiretusispora brandtii becomes more common. Although the Latosporites ovalis – Dictyotriletes biornatus Zone is characterized by other species that are also present in our assemblage, such as Biornatispora dubia, Retusotriletes tenerimedium and cryptospores, our assemblage lacks not only the eponymous species, but also any other monolete spores typical of this zone. Together, these differences preclude correlation with the late Pragian to early Emsian Latosporites ovalis – Dictyotriletes biornatus Assemblage Zone.
In summary, the Cottonwood Canyon palynomorph assemblage does not fit unequivocally in any previously defined palynological zone. On one hand, the assemblage includes three species restricted to sediments no younger than the late Lochkovian (Ambitisporites warringtonii, Apiculiretusispora spicula and Retusotriletes dittonensis; Fig. 6). On the other hand, the assemblage also includes several species that have their earliest occurrences in the Pragian (e.g. Apiculiretusispora brandtii, Craspedispora craspeda, Cymbosporites catillus, Retusotriletes actinomorphus), as well as two species reported from the Middle Devonian (Fig. 6). However, one of these, Squamispora arabica, has only one known occurrence to date, in the Givetian of northwestern Gondwana (Saudi Arabia; Breuer et al. 2007). The other species with occurrences younger than the Early Devonian is Cymbosporites ocularis. However, our single Cymbosporites specimen cannot be unequivocally assigned to C. ocularis.
Thus, when considered together, the stratigraphic distributions of the different species that comprise our assemblage suggest a late Lochkovian to Pragian age for the plant-bearing layers of the Beartooth Butte Formation at Cottonwood Canyon, consistent with the earlier age assignment proposed by McGregor (in Tanner 1983).
Palynofacies and depositional environments
Because of their dispersed and often fragmentary state, palynofacies elements are often hard to pin down in terms of their nature and producers (taxonomic affinities), and the flow of information and interpretations related to them is often bi-directional, taking the form of reciprocal illumination. Thus, ideally, palynofacies inform us about depositional environments and the biota inhabiting or neighbouring these environments, in cases in which their origin or taxonomic affinities are known. Conversely, any information available on the depositional environment of sediments hosting a certain palynofacies assemblage can be used to understand more about the origin of at least some components of that assemblage. Our Cottonwood Canyon palynofacies analysis reflects this kind of reciprocal illumination. The relatively small number of samples included in the analysis precludes statistically supported conclusions and limits us to more general comparisons. Nevertheless, conspicuous palynofacies differences between the three depositional environments (Table 2) and correlations of the latter with the proportions of different palynofacies components allow us to draw an internally consistent system of hypothetical relationships between depositional environment and palynofacies composition.
The opaque organic debris (OOD; Fig. 3F) are the most abundant palynofacies component in all samples and, overall, they surpass in frequency by almost four orders of magnitude the next most abundant type of palynofacies elements, the structured phytoclasts with sharp edges (SPSE) (Table 2). Most OOD are significantly smaller than other palynofacies elements, which may reflect their brittle nature or more advanced taphonomic fragmentation, or both. These considerations, along with their black to very dark colour and their resemblance to small parts of SPSE elements (compare Fig. 3F and K), suggest that OOD are the result of reworking of relatively mature organic debris of plant and possibly animal origin: armoured fish plates, black and brittle, often several centimetres wide and c. 1–2 mm thick, and to a lesser extent fragments of eurypterid exoskeletons are relatively frequent in the rooting levels. Consistent with this interpretation, at a macroscopic level, the flood layers, where OOD are most abundant, are rich in highly fragmented centimetre- to submillimetre-size plant and animal detritus. Because of their probable complex taphonomic history and lack of taxonomically diagnostic structures, OOD are best left out of more nuanced interpretations of depositional environment. Thus, below, statements on the abundance of other palynofacies categories do not take into account the OOD.
The Sengelia mat layers provide useful clues on the nature of palynofacies components. They consist of grey shale that contains almost exclusively abundant interwoven Sengelia stems. Thus, it is not surprising that the palynofacies assemblage of these samples is relatively simple: high abundance of both SPSE (Fig. 3K, L) and PLD (Fig. 3I, J), accompanied only by some rare SPCO (Fig. 3A–C), PDRO (Fig. 3G, H), and SPO. The correlation between the high abundance of in situ Sengelia populations and the high frequencies of SPSE and PLD (highest among the three depositional environments) suggests that most SPSE and PLD are Sengelia phytoclasts. Indeed, most SPSE and PLD elements are plant-like and preserve sub-structures reminiscent of plant cellular patterns. The more obvious such sub-structures present in SPSE elements and in PLD phytoclasts may reflect the origin of these two palynofacies categories in different plant tissues, for example, more or less lignified, or with thicker versus thinner cell walls. The strikingly low spore content of the Sengelia mat layers, despite their dense content of plant fragments, is consistent with the extreme rarity of sporangia on the Sengelia plants, which have been interpreted for this reason as reproducing mostly clonally (Matsunaga & Tomescu 2017) in the Cottonwood Canyon populations. The lack of amorphous organic matter (AOM) in the Sengelia mat palynofacies may be explained by a narrow stratigraphic time interval represented by these layers, which precluded the accumulation and progressive decomposition of organic matter.
If SPSE and PLD represent primarily Sengelia fragments, it is not surprising that they are also abundant in the flood layers and the organic lens (where they are the most abundant debris categories after OOD). This is because Sengelia is the most abundant plant type, overall, in the Cottonwood Canyon assemblages. These plants were growing their rooting structures in the flood layers (rooting levels), and, in both the rooting levels and the organic lens, most of the identifiable plant fragments are assignable to Sengelia. Nevertheless, given the allochthonous nature of the majority of organic matter in the flood layers and in the organic lens, some of the SPSE and PLD contained therein probably represent other plant types.
The contrast between the organic lens and the Sengelia levels is informative from a palynofacies perspective. The thin sedimentary layer that is the organic lens consists of a concentrated accumulation of fragmented and amorphous organic matter (as a general category), which includes primarily, or a significant fraction of, plant material. Based on its stratigraphic position (in a rooting level that represents flood deposits), limited spatial extent (only a few square metres), and content (a concentration of the lightest sedimentary fraction, i.e. (mostly plant-derived) organic debris), this deposit is best interpreted as the product of calm sedimentation of organic debris in a local shallow depression, most likely a temporary puddle that trapped water carrying a rich load of suspended organic material. In flood areas, such local depressions hosting puddles are frequent; they are produced by the intersection between sharp changes in topography at the metre scale during the flood, on one hand, and short-term fluctuations in the influx of sediment-laden water and in its spatial distribution during a prolonged flood event, on the other. For the same reasons, such deposits can be re-inundated during the same major event by flood waters that bring additional sediment, sealing the rich organic lens. Consistent with the interpretation as an organic sediment concentration puddle, the palynofacies of the organic lens is dominated by spores (>20-fold more abundant than in the two other environments) and has the highest content of amorphous organic matter (AOM) and phytoclasts with cell outlines (SPCO), which represent almost exclusively fragments of plant cuticle. Additionally, the organic lens has the highest content of wefts of fibrous structures (WFS).
Two of the palynofacies categories are peculiar and enigmatic in nature: the wefts of fibrous structures (WFS; Fig. 3D, E) and the phytoclasts with a well-defined round outline (PDRO; Fig. 3G, H). One possible interpretation of the wefts of fibrous structures is as filamentous cyanobacterial (or other prokaryote) colonies. Multitrichomous cyanobacterial colonies preserved as carbonaceous organic material have been described from terrigenous sequences (Tomescu et al. 2006, 2009). In such cases, multiple filamentous colonies coexist within a common matrix of exudates formed by their combined extracellular polysaccharide sheaths. This common matrix of extracellular ‘slime’, if significant in volume, can both preserve the filaments (or traces thereof) and form the bulk of the carbonaceous fossil. Although detailed examination of the wefts of fibrous structures in the Cottonwood Canyon palynofacies is beyond the scope of this study, we note that some of the filamentous components of the wefts contain multiple relatively conspicuous dark lines (Fig. 3E), the widths of which fall within the thickness range of cyanobacterial filaments. If the wefts do indeed represent agglomerations of prokaryotic (cyanobacterial) colonies, their presence with highest frequencies in the organic lens palynofacies could be explained by the development of microbial mats in the puddle that concentrated the organic matter of the lens. Given that WFS are also present in the flood layers (but in a smaller quantity than in the organic lens), it is also possible that they represent fragments of microbial mats transported by the floods from environments favourable to their development located farther upstream in the basin. Conversely, one could speculate that the absence of WFS from the Sengelia mat palynofacies indicates an environment unfavourable to the development of microbial mats.
The phytoclasts with round outlines (PDRO; Fig. 3G, H) provide little information relevant about their affinities. In the palynological preparations they break off from larger clusters and, upon close examination, they exhibit a finely granular texture (Fig. 3H). One possible interpretation of these palynofacies elements is as fragments of larger colonies of coccoid prokaryotes held together by their extracellular investments. However, their infrequent occurrence in all of the samples precludes more in-depth study for the time being. If these phytoclasts do indeed represent fragments of prokaryote colonies or mats, their presence in the Sengelia mat palynofacies goes against the interpretation (presented above) of these layers as representing environments unfavourable to the development of microbial mats.
Palynoflora–macroflora relationships and Early Devonian plant diversity in westernmost Laurentia
Combined with the plant diversity reflected in the macroflora at Cottonwood Canyon, the palynoflora of this locality paints a more complete picture of Early Devonian plant diversity in western Euramerica. The 37 distinct types of spores and cryptospores identified indicate the presence at least 37 different plant types in the region surrounding the locality, during the Lochkovian–Pragian. A conservative assessment of the taxonomic affinities of the known or putative parent plants of those spore and cryptospore species for which this information is available, indicates that most of the major plant groups recognized in the Early Devonian were present: rhyniophytoids and rhyniophytes, zosterophylls, barinophytes and trimerophytes (Noetinger et al. 2021, table S2). The producers of the other 22 spore and cryptospore types of currently unknown affinity, when identified, will only broaden the plant diversity represented by the palynoflora.
Since Tanner’s (1983) description of the Cottonwood Canyon flora, these plant fossil assemblages have seen additional collecting and study. Nevertheless, aside from the detailed description and reconstruction of the lycophyte Sengelia (Matsunaga & Tomescu 2016, 2017), the macroflora awaits a thorough revision and formal publication. Irrespective of this, studies of the expanded Cottonwood Canyon fossil collections (unpub. data: Bippus & Tomescu; Bronson & Tomescu; Caruso et al.; Dorn et al.; Appendix S1) have assessed the morphological disparity in the assemblages. The most recent (unpublished) assessment (Bippus & Tomescu, unpub. data) has resulted in the identification of a minimum of ten (possibly up to 12) distinct plant morphotypes in the macroflora (Table 3).
| Plant macrofossil | Palynomorph |
|---|---|
| Rhyniophytoids & rhyniophytes | |
| Renalioid (1) | Gneudnaspora divellomedia v. divellomedia |
| Ambitisporites avitus | |
| Ambitisporites warringtonii | |
| Ambitisporites sp. | |
| Aneurospora sp. cf. A. tojoides A | |
| Apiculiretusispora plicata | |
| Emphanisporites micrornatus | |
| Zosterophylls | |
| Gosslingia americana (468) | Retusotriletes actinomorphus |
| Zosterophyllum-type (811) | |
| Zosterophyll with slender axes (1) | |
| Barinophytes | |
| ? | Apiculiretusispora brandtii |
| Cymbosporites sp. cf. C. ocularis | |
| Lycophytes | |
| Sengelia radicans (5457) | ? |
| Leclercqia-like (6) | |
| Large putative lycophyte (1) | |
| Trimerophytes | |
| Trimerophyton-type (2) | Apiculiretusispora brandtii |
| Pertica-like (1) | Apiculiretusispora plicata |
| Retusotriletes triangulatus | |
| Incertae sedis | |
| Thalloid gametophytes (139) | Dyadospora murusattenuata |
| Rhabdosporites minutus | |
- Note that no direct matches are implied.
One of the plant types is a Renalia-type plant (renalioid in Table 3). There are a minimum of three zosterophyll morphotypes: one probably corresponding to Tanner’s (1982) Gosslingia americana; one similar to Zosterophyllum, corresponding to what was described initially, in the absence of fertile structures, as Psilophyton wyomingense by Dorf (1933) and Tanner (1983); and one type with slender axes and widely spaced lateral sporangia. Three lycophyte types are present: Sengelia radicans; a Leclercqia-like type plant; and another putative lycophyte with stout axes. The trimerophytes are represented by two types: a Trimerophyton-like plant and a type with Pertica-like branching architecture (a stouter axis with thinner subordinate lateral appendages with a pseudo-whorled appearance). Finally, Riccia-like thalloid gametophytes, which may fall into up to three morphotypes, are also present; these fossils could represent bryophyte-grade plants but it is also possible that they are gametophytes of some of the tracheophytes present in the assemblages. These different plants have very uneven abundances: in the sample consisting of >6800 macrofossil specimens, Sengelia is the most abundant by several orders of magnitude (>5400 specimens); the only other types that occur in significant numbers are the Zosterophyllum-like plant (>800 specimens), the Gosslingia-type zosterophyll (>450 specimens), and the thalloid gametophytes (>130 specimens); the other six types are represented by single or very few specimens (Table 3).
Several striking discrepancies are apparent in comparing the palynoflora with the plant macroflora at Cottonwood Canyon. First, the diversity of the palynoflora is threefold greater than that of the macroflora. This is not surprising, given that spore and pollen spectra are known to represent more of the vegetation of a region than plant macro-remains, in modern environments, due to the small size and easy transport of palynomorphs and their high preservation potential in the fossil record. There is no reason why such broader regional representativity would not have characterized Early Devonian spores, therefore the palynomorph content must reflect both the local flora and plant types that were present not only outside the immediate vicinity of the locality, but also outside the source area of the allochthonous plant material present in the flood deposits. Second, if we consider only the spores with known parent plants, the unevenness in representation of macrofloral types is inverted in the palynoflora. Thus, the lycophytes and zosterophylls, which together comprise almost 98% of specimens in the macrofloral sample and include at least six plant types, have only one corresponding spore type (for zosterophylls, and none for the lycophytes) in the list of spores identified, with known parent plants. Therefore, several of the 22 spore types for which the parent plants are not known may have been produced by zosterophylls and lycophytes. Conversely, the trimerophytes, very rare in the macrofossil assemblage (three specimens out of >6800), have three corresponding spore types known to have been produced by this group (Table 3). Furthermore, although seven of the cryptospore and spore types at Cottonwood Canyon can be referred to the rhyniophyte–rhyniophytoid plexus, this plant group has no representation in the macroflora, with the possible exception of the renalioid specimen. Likewise, the barinophytes, not discovered as macro-remains at Cottonwood Canyon to date, are represented by at least two different spore types in the palynoflora.
Conclusion
A total of 37 spore and cryptospore taxa, two types of prasinophycean algae and an acritarch add to the diversity of the Early Devonian flora of the Beartooth Butte Formation at the Cottonwood Canyon locality (Wyoming). The stratigraphic ranges of the different palynomorph taxa indicate that the fossiliferous beds were deposited during the late Lochkovian to Pragian, an age suggested previously based on a much narrower set of palynomorphs and on fish biostratigraphy. This result is significant by eliminating any equivocation on the age of the only extensive Early Devonian flora known in western North America and, with that, on the age of one of the most thoroughly characterized basal lycophytes, Sengelia radicans. Analysis of a subset of samples revealed correlations between sedimentology and palynofacies that distinguish among depositional environments previously recognized based on local stratigraphy, lithology, and macrofossil content.
The taxonomic affinities recognized for some components of the palynological assemblage, together with the macrofossils, indicate that all the major plant groups recognized in the Early Devonian were present: rhyniophytoids and rhyniophytes, zosterophylls, barinophytes, lycophytes and trimerophytes. Assessment of taxonomic affinities also reveals significant discrepancies in taxonomic representation between the palynoflora and macroflora. Such discrepancies are commonplace in comparisons between palynomorph and macrofossil assemblages at one locality. On one hand, they are the natural result of differences in preservation potential and taphonomic history engendered by size differences. On the other hand, the conspicuous taxonomic disparities between the palynoflora and macroflora emphasize a need for concerted efforts to bring together the knowledge of the macro- and microflora at each fossil locality, with the aim of obtaining a more complete picture of the local and regional vegetation of the areas represented in the fossil assemblages.
Holding the key to the origin and initial radiation of vascular plants, which evolved to dominate terrestrial ecosystems, the late Silurian to Early Devonian interval is one of the most important times in the history of life and should be targeted in such efforts. Although we are far from having documented in detail the plant macrofossil record of this interval, several late Silurian to Early Devonian floras around the world have been sampled extensively and characterized in detail. Starting with these floras, our concerted efforts need to focus on characterizing the correlations between the palynoflora and the macroflora of each fossil assemblage, in order to establish a baseline understanding, with predictive power, for the level of representativity that palynofloras hold for macrofloras. This will be possible only in parallel with continued (and, hopefully, renewed) efforts of the palaeobotanical–palynological community toward: (1) discovering relationships between plant macrofossil taxa and spore taxa (studies of in situ spores); (2) resolving relationships between in situ spores and dispersed spore types; and (3) establishing a unique, active database for this type of data and implementing protocols to periodically update and revise it.
Acknowledgements
Samples were collected under US Bureau of Land Management permit PA10-WY-186 to AMFT; we thank Brent H. Breithaupt, Delissa L. Minnick, and Gretchen L. Hurley (BLM Wyoming) for assistance; Sebastián Mirabelli (MACN-CONICET) is acknowledged for processing the samples. This study was supported with funds from CONICET (grant number PIP 11220120100182CO) to SN. Fieldwork was supported variously by grants from the American Philosophical Society and from Humboldt State University (HSU) Sponsored Programs Foundation, HSU Office of Research, and HSU Emeriti and Retired Faculty Association to AMFT. The 2009–2014 field crews who made sampling possible included Jeffery Barrett, Hannah Barrett-Watson, Allison Bronson, Joseph Caruso, James Cornwell, Rachel Klassen, Kelly Matsunaga, Ashley Ortiz, Glenn Shelton, Christopher Steenbock, and Richard Tate. This study was designed during AMFT’s visit to Argentina, funded by a US National Science Foundation Catalyzing New International Collaborations grant (IIA-1322504 to AMFT). ACB’s work on plant macrofossil diversity at Cottonwood Canyon was supported by a US National Science Foundation Graduate Research Fellowship (1546593). We thank the two reviewers, John E. A. Marshall and Victoria J. García Muro, and editor Sally Thomas for valuable comments and suggestions.
Open Research
Data archiving statement
Data for this study, including tallies of palynofacies elements, a list of putative parent plants for palynomorph species and a list of links to online conference abstracts containing unpublished data referenced in this paper, are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.zcrjdfnb2




