Earliest fossil record of the genus Tetradium (Rutaceae) in Asia: implications for its evolution and palaeoecology
Abstract
A new species, Tetradium nanningense sp. nov. (Rutaceae), is described on the basis of well-preserved mummified wood from the upper Oligocene Yongning Formation of Nanning Basin, Guangxi Province, South China. This species represents the most ancient fossil evidence of the genus Tetradium in Asia, the region of its modern distribution. Its occurrence in the late Oligocene is consistent with the diversification age of the modern Asian species within this genus as estimated by molecular dating: T. nanningense could be closely related to an ancestor of extant Tetradium species. The fossil record of Tetradium suggests that this genus migrated from North America to eastern Asia in the Oligocene. The presence of (semi-)ring-porous wood and helical thickenings on vessel walls in T. nanningense provides new evidence for the independent gains of these traits in the course of evolution among different plant groups from eastern and south-eastern Asia in the Oligocene. These wood features might have arisen in response to the increase in climate seasonality following the abrupt climate cooling across the Eocene–Oligocene boundary.
The genus Tetradium Loureiro (Rutaceae) consists of nine species of evergreen or deciduous shrubs or trees that today occur in temperate to tropical regions of East, South and South-East Asia (Hartley 1981; Zhang et al. 2008; Fig. 1). These species had long been placed in the genus Euodia J. R. & G. Forst. until Hartley (1981) re-established Tetradium as a separate genus in its present sense. Although Tetradium is still routinely confused with Euodia (spelled also as Evodia), these two genera are not closely related (Appelhans & Wen 2020). Instead, Hartley (1981) suggested the affinity of Tetradium to Phellodendron Rupr. and Zanthoxylum L., and this was recently confirmed by molecular phylogenetic analysis (Appelhans et al. 2018; Appelhans & Wen 2020).
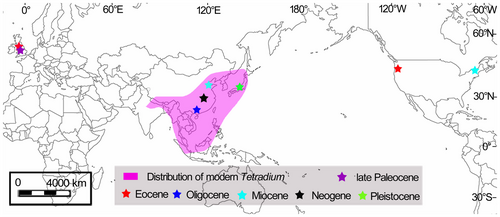
Although the modern species of Tetradium are restricted to Asia, the geographical ranges of this genus extended to other continents in its geological past and it has been found in Europe and North America (Fig. 1). The earliest reliable fossil records of Tetradium are seeds described as Euodia costata (Chandler) Tiffney from the late Paleocene to late Eocene of southern England (Tiffney 1981). In North America, the earliest fossil seeds of Euodia lignita Tiffney date from the early Miocene of Vermont, USA (Tiffney 1980, 1994). Both these fossils were subsequently attributed to Tetradium by Hartley (2001). Also, the fossil wood of Evodia gadijirian Gregory from the Eocene of Oregon, USA (Gregory 1976) shows certain similarity to the wood of Tetradium, but its description is not sufficiently detailed to enable a reliable attribution to this genus. In Asia, fossil leaves of Evodia miosinica Hu et Chaney, which are similar to those of modern Tetradium daniellii (Bennett) T. G. Hartley, have been reported from the Miocene of Shandong, China (Hu & Chaney 1940). Another fossil record from China is the wood of Euodia rutaecarpa (Juss.) Benth (the synonym of Tetradium ruticarpum (A. Juss.) T. G. Hartley) described from the Neogene deposits of Hubei (Chen et al. 1992; Qi et al. 2005). Finally, fossil seeds of Evodia glauca Miq. (the synonym of Tetradium glabrifolium (Champion ex Bentham) T. G. Hartley) have been reported from the early Pleistocene of Wakayama Prefecture, Japan (Momohara et al. 1990).
In this paper, a new fossil species of Tetradium, T. nanningense, is described from the upper Oligocene of Nanning Basin, Guangxi, South China, based on the discovery of well-preserved mummified fossil wood.
Material and method
Geological setting
The mummified wood was collected from the upper part of the Yongning Formation in the Nanning Basin located near the town of Santang, Xingning County, in the southwestern part of Guangxi, South China (22°52′50″N, 108°25′2″E; Fig. 2).
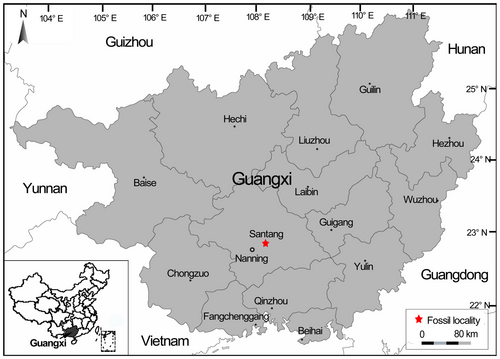
The Yongning Formation is the youngest formation in the Nanning Basin and is subdivided into lower, middle, and upper parts based on the dominant lithofacies (Zhao 1993). Numerous fossil woods, leaves, fruits, and seeds have been discovered (Quan et al. 2016; Han et al. 2018) in the upper parts of this formation, from which the materials investigated here were also collected. According to mammal fossils discovered from this part of the section, the geological age of the upper part of Yongning Formation is considered to be late Oligocene. These mammals include Anthracotherium changlingensis Zhao, Anthracokeryx kwangsiensis Qiu, and Heothema sp. (Zhao 1993), which all belong to the family Anthracotheriidae Gill. (Suiformes Jaeckel, Artiodactyla Owen) and which are believed to be related to modern Hippopotamus amphibious Linn., but which have been extinct since the Pleistocene, as well as late Oligocene Tragulidae (Ruminantia, Artiodactyla) fossils discovered in the uppermost part of this formation by Quang et al. (2016).
Anatomical examination of fossil wood
The fossil wood described here is preserved by mummification, which is rare and which preserves the wood in an almost unaltered state from when it was alive. This preservation might be due to rapid burial in hot or dry environments, which maintains the cells and tissue systems intact; it is not mineralized and in some cases can still be cut and burned like extant wood (Taylor et al. 2015). First, the sample was boiled in water for 12–24 h and soaked in a mixture of ethanol and glycerol (2:1) for 2 days to soften the tissues for sectioning with a microtome (RM2245; Leica) at a thickness of 15–40 μm. The sections were then examined with a light microscope (Axio Scope A1; Zeiss). Samples for scanning electron microscopy were cut into thin slices, dehydrated in alcohol, sputter-coated with gold, and examined with a JSM-6330F scanning electron microscope at 10 kV. Wood anatomical measurements and anatomical terminology used for the descriptions in this paper follow the recommendations of the International Association of Wood Anatomists (IAWA) List of Microscopic Features for Hardwood Identification (IAWA Committee 1989). The taxonomic position of the fossil material was determined by careful comparative work with similar modern and fossil wood specimens. This comparative work is based on reference materials, particularly the computerized InsideWood database (InsideWood 2020), in which we can search for similar modern and fossil wood by entering an IAWA feature number followed by the coding letters such as p (present) and a (absent).
Anatomical examination of pith in extant species
Because the fossil sample from Nanning contains a well-preserved portion of pith, we also examined its structure to use the pith traits for the identification of the extinct plant. Unfortunately, the available information on pith structure in different plant taxa is much more limited than the wood anatomical data published to date. To obtain some data for comparison of pith traits between the fossil sample and its probable nearest relatives, we examined the pith structure in Tetradium glabrifolium (Champ. ex Benth.) T.G. Hartley as an extant species with the greatest similarity to the fossil from Nanning based on its wood anatomy.
The anatomical sample of T. glabrifolium was collected by L-LH and AAO on 31 July 2018 in Heishiding Nature Reserve, Guangdong, China (voucher A. Oskolski 221-18 deposited at the University of Johannesburg Herbarium (JRAU)). The stem pieces (4 cm in diameter) were fixed in 70% ethanol. Bark fragments were embedded in glycol methacrylate (GMA) according to a modification of the Feder & O’Brien (1968) method. Transverse and radial sections of pith and adjacent xylem, c. 1 µm thick, were cut using a Porter Blum MT-1 ultramicrotome, and then stained with the Schiff–toluidine blue method before being mounted in Entellan.
The fossil material is housed in the Museum of Biology, Sun Yat-sen University, Guangzhou, China.
Systematic palaeontology
RUTALES
RUTACEAE Juss., 1789
Genus TETRADIUM Loureiro, 1790
Tetradium nanningense sp. nov.
Figures 3, 4, 5A, B
Derivation of name
The specific epithet is named for the Nanning Basin.
Holotype
Repository
The Museum of Biology, Sun Yat-sen University, Guangzhou, China.
Diagnosis
Wood semi-ring-porous to ring-porous, with mainly solitary vessels. Perforation plates exclusively simple. Intervessel pits alternate, minute to small. Helical thickenings present throughout the narrow vessel elements. Libriform fibres non-septate, with simple to minutely bordered pits. Axial parenchyma scanty, paratracheal, vasicentric, and sometimes in marginal, or in seemingly marginal, bands in earlywood. Rays 1–4 (occasionally 6) seriate, heterocellular; procumbent body ray cells with 1–2 rows of upright and/or square marginal cells. Vessel-ray pits with distinct borders: similar to intervessel pits in size and shape throughout the ray cells. Prismatic crystals present in chambered fibres and axial parenchyma cells. Secretory idioblasts occur in pith.
Description
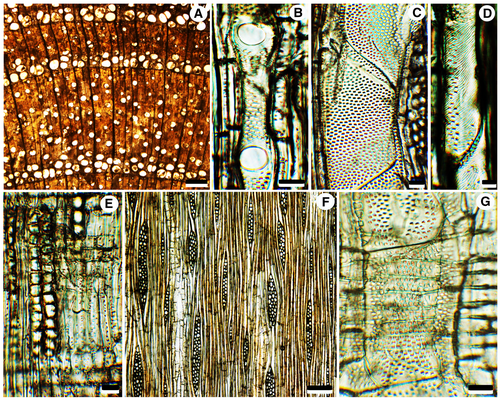
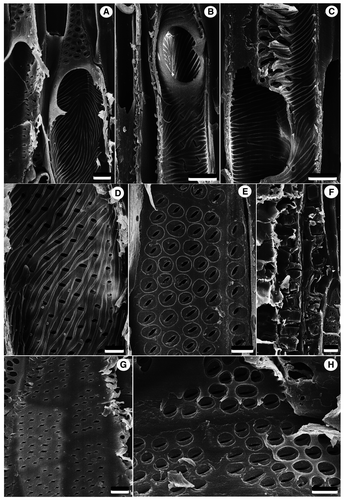
Wood semi-ring-porous to ring-porous, with distinct growth ring boundaries marked by differences in vessel size between earlywood and latewood and the marginal bands of axial parenchyma around the earlywood (Fig. 3A). Vessels are angular to round in outline, mainly solitary, sometimes 2–4 vessels in a group. Vessels in earlywood are wide, 88–236 μm (mean, 162 ± 5.8 μm) in tangential diameter and 68–240 μm (mean, 144 ± 6.4 μm) in radial diameter. Vessels in latewood are much narrower than the earlywood vessels, 51–128 μm (mean, 88 ± 4.6 μm) in tangential diameter and 37–121 μm (mean, 91 ± 3.9 μm) in radial diameter. Mean vessel frequency, 14 per mm2 (range, 12–17 per mm2). Vessel elements are 186–348 μm (mean, 265 ± 12.0 μm) in length. Perforation plates are exclusively simple, inclined (Figs 3B, 4A, B). Intervessel pits alternate, polygonal to oval, pit diameters are 2.5–7.2 μm (mean, 4.6 ± 0.30 μm) in horizontal size and 2.6–4.0 μm (mean, 3.2 ± 0.08 μm) in vertical size (Figs 3C, 4D, E), non-vestured. Helical thickenings are present throughout the narrow vessel elements (Figs 3D, 4A–D). Fibres are non-septate, oval, square, or polygonal in cross-section, 4.5–16 μm (mean, 10.5 ± 0.42 μm) in tangential diameter, walls thin to medium thick, 0.8–2.0 μm in wall thickness; with simple to minutely bordered pits of 0.9–2.3 μm (mean, 1.6 ± 0.13 μm) in diameter on radial walls. Prismatic crystals occur in chambered fibres. Axial parenchyma scanty, paratracheal, vasicentric, and sometimes forming marginal, or seemingly marginal, bands in earlywood. Prismatic crystals are abundant in chambered axial parenchyma cells (Figs 3E, 4F). Rays heterocellular, composed of procumbent body ray cells with 1–2 rows of upright and/or square marginal cells, 1–4 (occasionally 6) cells wide, mostly multiseriate rays, uniseriate rays are rare, ray frequency 3–5 per mm (Fig. 3F). Vessel-ray pits with distinct borders; similar to intervessel pits in size and shape throughout the ray cell, pit diameters are 1.8–3.2 × 1.3–2.8 μm in horizontal × vertical size (Figs 3G, 4G, H).
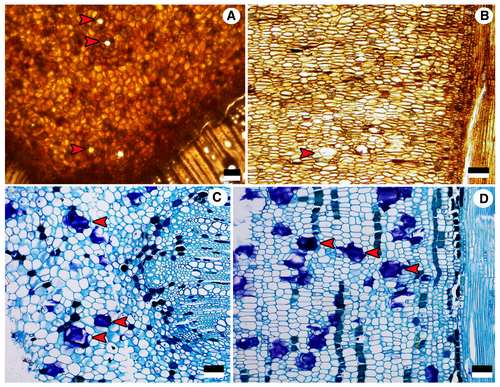
Pith consists of thin-walled vertically flattened polygonal parenchyma cells of 31–71 μm in horizontal size, and 14–40 μm in vertical size, without prominent intercellular spaces. Some parenchyma cells contain prismatic crystals or brown (tannin?) deposits. Isodiametric secretory idioblasts of c. 35–116 μm in diameter are scattered in the pith parenchyma (Fig. 5A, B).
Remarks
For comparative purposes, the following description of the pith anatomy of Tetradium glabrifolium is provided. Pith in the 4 cm stem consists of thin-walled isodiametric to vertically flattened polygonal parenchyma cells of 25–70 μm in horizontal size, and 15–50 μm in vertical size, without prominent intercellular spaces. Tanniniferous parenchyma cells are common, arranged into vertical files. Isodiametric secretory idioblasts of c. 40–120 μm in horizontal diameter are scattered in the pith parenchyma. No crystals found in pith parenchyma cells (Fig. 5C, D).
Stratigraphic horizon
Yongning Formation, upper Oligocene.
Locality
Nanning Basin, Guangxi, South China.
Discussion
Comparison with modern woods
The InsideWood (InsideWood 2020) search for the combination of ring-porous wood (3p), exclusively simple perforation plates (13p), minute to small alternate non-vestured intervessel pits (22p, 26a, 27a, 29a), helical thickenings on vessel walls (36p), distinctly bordered ray-vessel pits of the same size and shape as the intervessel pits (30p), non-septate libriform fibres (61p, 62a), non-storied arrangement of axial parenchyma and vessel elements (120a), the absence of included phloem (133a) and the presence of prismatic crystals in chambered axial parenchyma cells (142p) returns the following modern species: Melia azedarach L. and Melia sp. (Meliaceae), Condalia microphylla Cav. (Rhamnaceae), Tetradium glabrifolium (Champ. ex Benth.) T.G. Hartley (recorded in the InsideWood as Evodia meliaefolia (Hance) Benth.) and Cneorum tricoccon L. (Rutaceae). Among these species, Condalia microphylla (Schirarend 1991) and C. tricoccon (Carlquist 1988; Schweingruber 1990) differ from the fossil wood in having a very pronounced dendritic pattern of vessel arrangement, and the presence of crystals in ray cells. Two other species show much greater similarity to the Oligocene wood from Nanning, in as much as both M. azedarach and T. glabrifolium also share pith secretory idioblasts (their presence in the former species has been reported by Metcalfe & Chalk 1950). Melia azedarach is distinctive, however, from the fossil sample in having numerous groups of narrow latewood vessels arranged into a pronounced tangential or diagonal pattern, as well as in the absence of crystals in the fibres.
Thus, the fossil from Nanning shows the most similarity to T. glabrifolium, sharing with it a number of wood traits including the presence of helical thickenings in latewood vessels and the occurrence of prismatic crystals in the fibres (Cheng et al. 1985; Bhardwaj & Gupta 2009). Such an uncommon trait as the presence of secretory idioblasts in the pith of this species also confirms its affinity to the fossil wood. Tetradium glabrifolium, can be distinguished, however, from the present Nanning fossil wood by having wider (reportedly up to 11-seriate) rays as well as by the presence of vertical files of tanniniferous cells, and the lack of crystals in its pith.
As for other Tetradium species, T. daniellii (Benn.) T.G. Hartley has larger intervessel pits and wider (up to 10-seriate) rays than the Nanning fossil, as well as the latewood vessels arranged into tangential bands or a diagonal to radial pattern (Eom 2015). Tetradium ruticarpum (A. Juss.) T. G. Hartley (= Euodia rutaecarpa Benth.) has rays of nearly the same width (2–7-seriate) as the sample from Nanning, but this species differs from the Nanning fossil in having larger intervessel pits (Cheng et al. 1985; Eom 2015). Unlike the fossil wood, T. fraxinifolium (Hooker) T. G. Hartley (Euodia fraxinifolia (Hooker) Bentham) possesses no helical thickenings on vessel walls, and has druses in its axial parenchyma cells (Bhardwaj & Gupta 2009).
Apart from that, the fossil wood from Nanning differs from most Tetradium species examined by wood anatomists by having mostly solitary vessels in its latewood. This feature is also uncommon in other Rutaceae: most members of this family share mostly grouped vessels, often arranged into a diagonal or dendritic pattern (Cheng et al. 1985; Appelhans et al. 2012; Eom 2015; InsideWood 2020; FFPRI 2021). Exceptions also occur, however; for instance, the predominantly solitary vessels found in ring-porous wood of Zanthoxylum americanum Mill. (InsideWood 2020). Among species of Tetradium, the reported data on T. glabrifolium suggest considerable intraspecific variation of the incidence of solitary vessels (InsideWood 2020; FFPRI 2021). Although most published microphotos of the wood of this species show the vessels occurring mostly in small clusters, solitary vessels are very common in the latewood of the sample TWTw 17448 reported in the FFPRI Wood Database (FFPRI 2021). The latter condition is similar to that found in the fossil wood from Nanning.
In summary, our fossil wood sample shows the greatest similarity to the extant genus Tetradium (Rutaceae), but it cannot be convincingly placed into any of its modern species described to date by wood anatomists.
Comparison with other fossil woods
The search of the fossil woods recorded in InsideWood (InsideWood 2020) with the combination of semi-ring-porous wood (3p), exclusively simple perforation plates (13p, 14a), minute to small alternate intervessel pits (22p, 27a), helical thickenings on vessel walls (36p), distinctly bordered ray-vessel pits of the same size and shape as intervessel pits (30p), non-septate libriform fibres (61p, 62a), non-storied arrangement of axial parenchyma and vessel elements (120a) and rays (303a), and the presence of prismatic crystals in chambered axial parenchyma cells (142p) returns Gleditsioxylon riojana Martínez & Rodríguez-Brizuela (Fabaceae, Caesalpinoideae), Cedreloxylon cristalliferum Selmeier (Meliaceae), Pruninium sp. (Rosaceae), and Ulmus danielii Wheeler & Manchester (Ulmaceae). Gleditsioxylon riojiana from the Palaeogene of Argentina is, however, different from the fossil wood under study by having large groups of vessels, more abundant vasicentric axial parenchyma, and by possessing vestured pits (Martínez & Rodríguez-Brizuela 2011). Unlike Pruninium sp., described by Selmeier (1984) from the upper Miocene deposits of Bavaria, Germany, the Nanning wood sample shows appreciable differences in vessel diameters between earlywood and latewood, in the presence of paratracheal axial parenchyma, bordered pits on fibre walls, as well as the heterocellular rays. Ulmus danielii from the Eocene of Oregon, USA, differs from the fossil wood by having a prominent diagonal pattern of vessel arrangement (Wheeler & Manchester 2002).
Cedreloxylon cristalliferum described from the Miocene of Bavaria, Germany, (Selmeier 1987) shows the greatest similarity to the sample under study of the species returned by the InsideWood search. This species was also reported from other Miocene and late Eocene sediments in Europe (e.g. Gottwald 1992), as well as from the Miocene of Yamagata Prefecture, Japan (Choi et al. 2010), and from the Pliocene of Yunnan, China (Cheng et al. 2006). All these researchers suggest a relationship between C. cristalliferum and extant East Asian species of Toona (Endlicher) M. Roemer, which previously was included within Cedrela P. Browne (Smith 1960). Although the wood sample from Nanning shares many anatomical traits with Cedreloxylon cristalliferum, the Nanning fossil differs from it by the presence of crystaliferous axial parenchyma and by wider (up to 6-seriate) rays. Moreover, the fossil wood under study differs from most samples of C. cristalliferum (with the exception of the Miocene sample from Japan described by Choi et al. 2010) by having helical thickenings on the walls of narrow vessel elements.
In addition to the InsideWood records, we compared the sample from Nanning with other reports of fossil woods ascribed to Rutaceae. We found that the fossil sample under study shows a close similarity to Euodia rutaecarpa (Juss.) Benth from the Neogene deposits of Hubei Province, China (Chen et al. 1992; Qi et al. 2005). This fossil wood has been ascribed to the extant species that is widely recognized now under the name Tetradium ruticarpum (A. Juss.) T. G. Hartley (e.g. Zhang et al. 2008). Unlike the Nanning fossil, the Neogene wood from Hubei lacks helical thickenings on vessels, and it also has larger intervessel pits (5.5 μm in average diameter) and wider (up to 11-seriate) rays (although the modern woods of this species have up to 7-seriate rays; Cheng et al. 1985).
Therefore, the Oligocene wood from Nanning is very similar to the extant genus Tetradium (Rutaceae), as well as to the fossil wood ascribed to its modern species Tetradium ruticarpum (= Euodia rutaecarpa). At the same time, the Nanning fossil wood shows some differences from any extant and extinct species of Tetradium with known wood structure. On this basis we assign the wood sample from Nanning to this genus as a new species Tetradium nanningense sp. nov.
Diversification of the genus Tetradium
Although the modern species of Tetradium is now restricted to South Asia, East Asia and South-East Asia, it occurs as fossils on other continents, namely Europe and North America (see the review in the introduction, above; Fig. 1). The earliest fossils of this genus are known from the late Paleocene to late Eocene of southern England (Tiffney 1981). The fossil wood of T. nanningense discovered from the late Oligocene of South China is the earliest fossil record of Tetradium reported from Asia; subsequently this genus occurred in North America in the early Miocene (Tiffney 1980). It should be mentioned that there is also a fossil wood, Evodia gadijirian Gregory, reported from the Eocene of Oregon, USA (Gregory 1976) (although the description of this species is not detailed enough for its reliable attribution to Tetradium). Based on these fossil records, we speculate that the North Atlantic land bridge and the Beringian Corridor in Paleocene–Eocene times probably played a key role in the migration of this genus from Europe to North America and then to eastern Asia; the same migration route had been suggested by Appelhans et al. (2018) for the genus of Zanthoxylum L. (Rutaceae), which is closely related to Tetradium.
As suggest by molecular dating (Appelhans et al. 2018), the lineages of extant Tetradium species, restricted to Asia, started to diversify between the late Oligocene and mid-Miocene (19.7 Ma; 12.9–29.4 Ma); this estimation is consistent with the age of the fossil wood under study. Thus, T. nanningense from the late Oligocene of South China, might be closely related to an ancestor of extant Tetradium species. Unfortunately, the reported fossil records of this genus, up to now, are rather sparse; additional fossil findings and further study of this genus are needed to confirm these speculations on its phytogeographic history.
Character evolution and palaeoecology of Tetradium
Semi-ring-porosity and the occurrence of helical thickenings are two conspicuous features of T. nanningense that have also been found in other species from the Yongning Formation in the Nanning Basin. Ring-porous and semi-ring-porous wood occurs also in Castanopsis nanningensis Huang, Jin, Quan et Oskolski (Huang et al. 2018a), whereas helical thickenings have been reported in Litseoxylon nanningensis Huang, Jin, Quan & Oskolski and Magnolia nanningensis Huang, Jin, Quan & Oskolski (Huang et al. 2018b, 2020).
Ring-porosity and semi-ring-porosity are generally lacking in fossil woods from the Paleocene of eastern and south-eastern Asia (InsideWood 2020, 2004 onwards; Wheeler 2011). The only known semi-ring-porous wood from this region in the Eocene is Lithocarpoxylon radiporosum Suzuki & Ohba from Japan (Suzuki & Ohba 1991). In the early Oligocene, ring-porosity and semi-ring porosity are found in seven species from Japan (Suzuki 1976, 1982, 1984; Terada & Suzuki 1998; Srivastava & Suzuki 2001), whereas in the Miocene these features occur in 25 morphotypes from Japan and Korea, and in at least four of them from East and south-west China (Cheng et al. 2012, 2018; Li et al. 2015). It is noteworthy that the spread of ring-porosity and semi-ring-porosity is nearly in conjunction with the distribution of helical thickenings in Asian trees (Huang et al. 2018b, 2020).
Thus, the fossil flora from the Yongning Formation in the Nanning Basin provides the oldest known record of angiosperm woods possessing both these traits in China, as well as in south-eastern Asia. (Semi-)ring-porosity is commonly considered as a hydraulic strategy of adaptive value in seasonal climates, given that the wide earlywood vessels offer little resistance to flow, so they will become embolized fairly early in the growing season, while sap transport continues via the much more numerous but more transport-resistant narrow latewood vessels (Baas & Wheeler 2011). By assessing the wood porosity of 1176 species from temperate countries and 3886 from tropical countries, as well as their foliage characteristics, Boura & De Franceschi (2007) found that (semi-)ring-porous woods are confined to deciduous species of seasonal temperate climates (mostly northern hemisphere) and some highly monsoonal forest species in the tropics. Helical thickenings are also believed to have a hydraulic function, which might reduce in some way the danger of cavitation, aid in the refilling of vessels, or increase vessel wall strength (Carlquist 2001). Thus, both (semi-)ring-porosity and helical thickenings are considered as adaptive traits in regions that experience seasonal water stress created by drought or freezing (Carlquist 2001; Wheeler et al. 2007; Baas & Wheeler 2011). The presence of these two traits and the resulting hydraulic strategy, as a response to a seasonal climate, seems also to occur in the wood of Cneorm tricoccon L. (Spathelioideae, Rutaceae) (Appelhans et al. 2012). The wood of Tetradium nanningense provides new evidence for the appearance of these traits in different plant groups from this region in the late Oligocene (Huang et al. 2020). It might have been a response to the increase in climate seasonality following the abrupt climate cooling near the Eocene–Oligocene boundary (Mosbrugger et al. 2005; Liu et al. 2009; Buerki et al. 2013; Zhang et al. 2016).
Conclusion
In this paper, a new fossil species of Tetradium, T. nanningense, is described from the upper Oligocene of Nanning Basin, Guangxi, South China, based on the discovery of well-preserved mummified fossil wood. It is the earliest fossil record of this genus in Asia, the region of its modern distribution. Its occurrence in the late Oligocene deposits of South China is perfectly consistent with the diversification age of the modern Asian species within Tetradium as estimated by molecular dating, indicating that it could be ancestral to Tetradium in Asia. The presence of traits in the fossil material indicative of drought or freezing, provides new evidence for their appearance in different plant groups from the late Oligocene of this region, indicating a response to the increase in climate seasonality following the abrupt climate cooling near the Eocene–Oligocene boundary.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 41820104002, 42072020); China Postdoctoral Science Foundation (grant no. 2020M683027), the Russian Foundation for Basic research (grant no. 16-04-00725), the National Research Foundation of South Africa (incentive grant no. 109531), State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences) (no. 193118), and the Fundamental Research Funds for the Central Universities. We thank the University of Johannesburg and the Komarov Botanical Institute (institutional research project no. AAAA-A19-119030190018-1) for financial support for AAO. We thank the graduate students majoring in plant science at Sun Yat-sen University who participated in the field collection of the fossils. We are grateful to Prof. Robert A. Spicer (The Open University, UK) for improvements to the English in this paper. Dr Pieter Baas and an anonymous referee commented on an earlier draft of this manuscript.




