A new unenlagiine (Theropoda, Dromaeosauridae) from the Upper Cretaceous of Brazil
Abstract
The record of unenlagiines in Brazil, except for one dorsal vertebra, is still under debate based on isolated teeth. Here, we describe Ypupiara lopai gen. et sp. nov., the first dromaeosaurid species from Brazil, from the Maastrichtian of the Bauru Group, Paraná Basin. The specimen consists of a partial right maxilla (with three teeth in loci) and a right dentary. Ypupiara is characterized by a restricted number of neurovascular foramina on the lateral surface of the maxilla, a rectangular and anteroposteriorly expanded interdental plate, and a labiolingual compression of the teeth, which have a labiolingual diameter more than 3/5 of the rostrocaudal diameter. Our phylogenetic analysis recovers Ypupiara as an unenlagiine based on the fluted teeth and places it as the sister taxon of Austroraptor due to the ratio of the labiolingual and mesiodistal diameters of the teeth being more than 3/5. We also erect the new group Unenlagiinia, which includes Unenlagiinae and Halszkaraptorinae. Ypupiara nests within Unenlagiinia based on the widely spaced teeth and the lack of mesial and distal carinae in the maxillary teeth. The morphology of the teeth is similar to Buitreraptor, although the proportions are markedly distinct. The teeth and comparisons with other Unenlagiinia also support fish being part of the diet for Ypupiara. We also performed a parsimony analysis of endemicity, which suggested that the presence of unenlagiines in Brazilian Upper Cretaceous outcrops is explained by dispersion or extinction events. The new species provides new information on the evolution of Gondwanan dromaeosaurids, and its preserved teeth provide new data to enable the assignment of isolated dromaeosaurid teeth from the Bauru Group.
Dromaeosauridae are present in all continents during the Mesozoic (Turner et al. 2012; Oms et al. 2016; Cau et al. 2017; Brownstein 2019). In Gondwanan landmasses, the Unenlagiinae lineage constitutes a diversification of dromaeosaurids, comprising five species recovered from Argentinean localities (Gianechini & Apesteguía 2011). These dromaeosaurids are diagnosed by numerous teeth, which lack denticles and both carinae, and which have longitudinal grooves on the crown (Gianechini et al. 2011). Among all unenlagiines, only two species (Buitreraptor gonzalezorum Makovicky et al., 2005 and Austroraptor cabazai Novas et al., 2009) have cranial elements, including maxillary and dentary teeth, which limits the study of dental traits in the group (Gianechini et al. 2011).
The presence of Unenlagiinae specimens in Brazil is restricted to a single dorsal vertebra from the Campanian–Maastrichtian sequences of the Adamantina Formation (Bauru Group; Candeiro et al. 2012). Some isolated teeth from the same formation were tentatively assigned to dromaeosaurids (Franco-Rosas 2002; Tavares et al. 2014), but none has been assigned to Unenlagiinae so far(except for a putative tooth reported by Ghilardi & Fernandes 2011). Although the dental material was tentatively attributed to dromaeosaurids, other bone material also indicates the presence of small maniraptorans in Upper Cretaceous sequences of the Bauru Group: it consists of an isolated maniraptoran scapula (Machado et al. 2008) and ungual (Novas et al. 2005), and several bone fragments tentatively assigned to Deinonychosauria (Delcourt & Grillo 2014).
This study presents the first evidence of unenlagiines in the Maastrichtian Marília Formation (Bauru Group, Brazil) and the second confirmed evidence of this clade in Brazil (as well as the first cranial remains referred to the Bauru Group in the country). The specimen (DGM 921-R) consists of a partial maxilla, with associated teeth and a posterior portion of a dentary. This material has features in common with Unenlagiinae, especially with B. gonzalezorum, and will enable the assignment of small isolated teeth from the Bauru Group that potentially belong to dromaeosaurid theropods.
Institutional abbreviations
AMNH, American Museum of Natural History, New York, USA; DGM, Museu de Ciências, CPRM, Rio de Janeiro, Brazil; MCP-D, Institute of Paleontology & Geology, Mongolian Academy of Sciences (formerly IGM), Ulaanbaatar, Mongolia; MPMA, Museu de Paleontologia de Marília, Marília, Brazil (formerly MPM); NMMNH, New Mexico Museum of Natural History & Science, Albuquerque, New Mexico, USA; RTMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada.
Geological setting
The Bauru Group (sensu Soares et al. 1980) is a second order sequence of the Paraná Basin deposited over the basaltic Serra Geral Formation (Milani et al. 1998, 2007). The origin of the Bauru Group is related to the tectonism during the rupture of the Gondwana supercontinent (Fernandes & Coimbra 2000). The Bauru Group consists of two distinct intervals (Pinheiro et al. 2018), markedly separated by age and depositional system. The first interval is formed by aeolian to fluvial–aeolian sandstones of the Aptian, and the second by alluvial and fluvial conglomerates, sandstones and mudstones dating from the Santonian to the Maastrichtian (Dias-Brito et al. 2001; Pinheiro et al. 2018). Although there are several stratigraphic proposals for the Bauru Group, here we follow the main lithostratigraphic proposal of Soares et al. (1980) with updates by Pinheiro et al. (2018), with eight recognized formations.
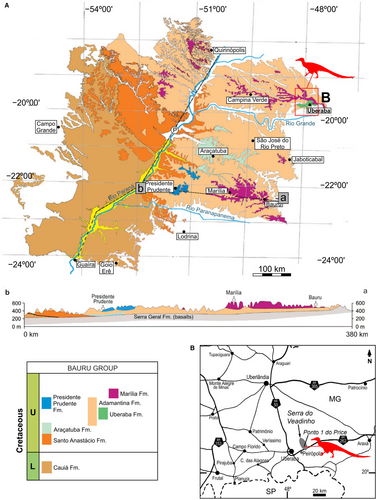
The specimen DGM 921-R studied here was recovered at Peirópolis, a rural district of Uberaba municipality, state of Mina Gerais, where the Uberaba and Marília formations crop out (Fig. 1). Due to the cataloguing data left by L. I. Price, we know that the specimen was collected in what became known as ‘Ponto 1 do Price’ or ‘Caiera quarry’. This quarry is near the Serra do Veadinho hill region and is well-known due to the finds of several vertebrate fossils in excellent preservation (e.g. Kellner & Campos 1999; Campos et al. 2005; Bandeira et al. 2018). In this area there are extensive exposures of the Marília Formation, the sedimentary rocks of which consist of fine to medium-grained sandstones intercalated with conglomeratic levels (Fúlfaro & Barcelos 1991; Martinelli et al. 2019). The rocks are formed by tabular beds of pale brown medium to coarse-grained sandstone as well as pebbly sandstone, alternating with beds of red fine-grained sandstone and successive white carbonate layers (Martinelli et al. 2019). The depositional system is usually interpreted as alluvial fans reworked by a braided fluvial system, with lacustrine limestones (Barcelos & Suguio 1987; Brusatte et al. 2017; Martinelli et al. 2019).
Material and method
Specimens
The specimen DGM 921-R consists of a right partial maxillary preantorbital body with teeth in loci (Figs 2, 3) and a right posterior region of a dentary (Fig. 4). As recorded in the catalogue book at Museu de Ciências da Terra (MCT) in Rio de Janeiro, both elements are considered to belong to a single individual, having been found closely associated in the same locality and horizon. The studied material had originally been housed at the MCT, currently part of the Serviço Geológico do Brasil–CPRM, and was on loan to the Museu Nacional-Universidade Federal do Rio de Janeiro (MN-UFRJ) for study. After the great fire that destroyed the main building of the MN-UFRJ in September 2018 (e.g. Kellner et al. 2019a), however, the specimen was not recovered and is here considered as lost.
Nomenclatural acts
The fossil material designated as the name-bearing type of a new genus and species is considered lost. Despite being uncommon and previously unpermitted, designation of lost specimens as name-bearing types is currently permitted by the ICZN under specific circumstances and is not unprecedented.
Our act is in accordance with Declaration 45 of the ICZN (2017), which allows the designation of a lost specimen as a name-bearing type if it had been, originally, adequately collected and housed in a collection and only then lost; and if the reasons for accidental specimen loss are properly justified. This was the case, for example, of the sauropod Maraapunisaurus fragillimus, whose name-bearing type specimen is the famous holotype of Amphicoelias fragillimus, described by Cope (1878) and subsequently lost. Recently, reassessment of the available data on this specimen led Carpenter (2018) to name it as the new rebbachisaurid genus Maraapunisaurus, invoking Declaration 45 of the ICZN (2017). Similarly, Apesteguía (2007) named the rebbachisaurid Nopcsaspondylus alarconensis when the holotype specimen was already lost, subsequent to its first description (without naming) by Nopcsa (1902). It is important to note that Declaration 45 of the ICZN (2017) does not permit the naming of new taxa based on photographs of specimens that have never been safely stored in a public scientific collection. In addition, the specimen described here (DGM 921-R) was also under study at the time of the MN-UFRJ fire (Holgado et al. 2018), and the manuscript was about to be submitted when the accident occurred.
Anatomical nomenclature and measurements
We follow Hendrickx & Mateus (2014) for general anatomical maxillary nomenclature and measurements, and Hendrickx et al. (2015) for dental nomenclature and measurements. The measurements of Buitreraptor gonzalezorum and Austroraptor cabazai were done using ImageJ (Schneider et al. 2012), from images available in the literature (Gianechini et al. 2011; Gianechini & Apesteguía 2011; Gianechini et al. 2017).
Linear morphometric abbreviations
AL, apical length; CA, crown angle; CBL, crown base length; CBR, crown base ratio (CBW/CBL); CBW, crown base width; CH, crown height; CHR, crown height ratio (CH/CBL); DDL, distal denticle length; LAF, number of flutes on the labial surface of a crown; LIF, number of flutes on the lingual surface of a crown; MCL, mid-crown length; MCR, mid-crown ratio (MCW/MCL); MCW, mid-crown width; MDL, mesial denticle length; MSL, mesial serrated carina length.
Heuristic phylogenetic tree search
The phylogenetic analysis was performed based on the data matrix from Cau et al. (2017), with the inclusion of the specimen DGM 921-R, a new character, and a few codification changes (Brum et al. 2021). The phylogenetic analysis was performed using TNT version 1.5 (Goloboff & Catalano 2016). The memory available for the analysis was 1000 MB, which enabled the program to hold 600 000 trees. A search for the minimum-length trees (MLTs) was conducted using the TNT algorithms Sectorial Search, Ratchet (parameters: 25 substitutions made, or 99% swapping completed, six up-weighted probabilities, six down-weighted probabilities, and total number of iterations = 10) and Tree-fusing in a driven search (initial addseq = 15, find the minimum tree length 50 times), with random seed equal to 0, and without collapsing the trees after the search. The aim of the first analysis was to recover the island with the most MLTs. Subsequently, the recovered island was used as the starting point for a traditional search (tree bisection and reconnection (TBR) swapping algorithm; starting with trees held in the RAM) and without collapsing the trees after the search. The aim of this second analysis was to recover the maximum number of MLTs from the island recovered in the first analysis. The MLTs were collapsed using ‘condense 1; collapse;’ and the script ‘STATS.RUN’ was used to retrieve the retention and consistency indexes (RI and CI, respectively). A strict consensus was then generated for the recovered MLTs. It is important to note that as a consequence of the size of the matrix and memory limitation of the computer, the generated consensus is not the most complete (i.e. it was generated based on all MLTs available for the best recovered island) for this data matrix, but it is still more exhaustive than previous analyses. All characters were assigned equal weights and no additive characters were considered.
Tooth assignment
We conducted analyses of the teeth of DGM 921-R to obtain more details on the tooth morphotypes in theropods and to evaluate the morphological and morphometric details. Such analyses followed the protocols commonly used in the assignment of isolated teeth (e.g. Delcourt & Grillo 2018; Young et al. 2019; Delcourt et al. 2020; Hendrickx et al. 2020).
Heuristic search for tooth assignment
Dendrograms based exclusively on dental features have been used traditionally to facilitate discussions of the teeth, and they use similar parameters to those in phylogenetic dendrograms. However, we do not consider them phylogenetic dendrograms, but morphological similarity dendrograms. We partially followed the system described by Young et al. (2019) and Hendrickx et al. (2020) for teeth assignment. The specimen DGM 921-R was scored and included in the tooth-based matrix provided by Hendrickx et al. (2020), which contained 148 characters and 108 taxa (Brum et al. 2021). We modified the scores of B. gonzalezorum from the absence of flutes on the lateral surface of the lateral teeth to flutes on the lateral teeth present on labial or both labial and lingual surfaces (char. 111:2) and the average number of flutes on the crown from inapplicable to varying from 1 to 7 (char. 112:0). This modification was based on the descriptions provided by Gianechini pers. comm. (2020) and Gianechini et al. (2011). The heuristic search was performed using TNT version 1.5 (Goloboff & Catalano 2016). The memory available for the analysis was 1000 MB, which enabled the program to hold 800 000 trees. We performed two heuristic searches and altered some of the parameters from those described by Hendrickx et al. (2020).
In the first search we followed Hendrickx et al. (2020), with the combination of TBR (branch swapping with random seed = 0 and 5000 replicates, and 10 trees saved per replica) and the TNT algorithms Sectorial Searches, Ratchet (perturbation phase stopped after 20 substitutions), and Tree-fusing (five rounds), until 100 hits of the same MLT were achieved. We conducted a final round with the MLTs obtained with TBR branch swapping, with the command ‘xmult = hits 100 rss fuse 5 ratchet 20’ followed by the ‘bb’ command. This approach uses the constraint described and provided by Hendrickx et al. (2020) with the command ‘force+[ ]’ (Brum et al. 2021). The second analysis did not use the command ‘force+[ ]’. The first step used the same parameters in TBR as those in the first analysis. The MLTs were then reanalysed with TBR using the parameter ‘stop when maxtrees hit’.
Linear morphometric analyses
We performed the multivariate analysis using the dataset of Young et al. (2019) and we added data on avian-toothed taxa provided by Dumont et al. (2016) (datasets in Brum et al. 2021). The analyses were carried out using PAST version 4.02 (Hammer et al. 2001). As per the morphometric protocols (Young et al. 2019; Hendrickx et al. 2020), we used 12 morphometric measurements: CBL, CBW, CH, AL, MCL, MCW, MSL, LAF + 1, LIF + 1, CA, MDL and DDL.
The first analysis consisted of linear discriminant analysis (LDA) of the whole dataset, which involved 1369 measured teeth (from 79 non-avian taxa (1335 teeth) and 2 avian taxa (3 three teeth), including 3 teeth of DGM 921-R, 26 indeterminate avian teeth and 2 dromaeosaurid teeth from the Bauru Group from Tavares et al. 2014), at the ‘clade’ level (Brum et al. 2021). The second LDA, as well as the principal component analysis (PCA), involved only the small theropods (CH < 20 mm; Young et al. 2019; Hendrickx et al. 2020) and was carried out at the ‘taxa (genus)’ level (669 measurements including the three teeth of DGM 921-R; Brum et al. 2021).
The second analysis consisted of two distinct cluster analyses at the ‘taxa (genus)’ level (following Young et al. 2019 and Hendrickx et al. 2020), and considered only taxa with small crowns (CH < 20 mm; Brum et al. 2021). The first was a classical hierarchal clustering analysis and the second was a joining clustering analysis. The parameters used in both analyses were Euclidean distances for the similarity index, a paired group algorithm, and rooting the tree at the final branch.
Parsimony analysis of endemicity
To discuss the biogeographic relationships between the geological formations and their fauna, we carried out a parsimony analysis of endemicity (PAE; Rosen 1988; Morrone 1994), based on the cladistic analysis of presence and absence data matrices of supraspecies taxa (Morrone 2009; Morales-Guerrero et al. 2017), which allow the identification of biogeographical assemblages and their hypothetical relationships. These biotic components are graphically represented as areas of endemism (Morrone 2009).
We considered only Upper Cretaceous formations to establish the study areas in this work. The poorly known and/or poorly sampled taxa were grouped into the most inclusive taxonomic unit (e.g. Podocnemididae, Squamata). We considered only tetrapod vertebrates for analysis, and excluded the teeth and ichnological data. In addition, solely osteological records confirmed in the literature were chosen for the analysis (e.g. we excluded the dubious assignment of the turtle record to the Santo Anastácio Formation; Menegazzo et al. 2015; Romano 2016). We also considered only the formations that crop out (i.e. subsurface formations such as Santo Anastácio were not considered). The data matrix (composed of one hypothetical outgroup area, 14 formations and 25 taxon characters; see Brum et al. 2021) was edited using Mesquite version 3.6, and the PAEs were conducted using TNT version 1.5 (Goloboff & Catalano 2016).
Systematic palaeontology
DINOSAURIA Owen, 1842
THEROPODA Marsh, 1881
COELUROSAURIA Huene, 1914
MANIRAPTORA Gauthier, 1986
DROMAEOSAURIDAE Matthew & Brown, 1922
UNENLAGIINIA nov.
Included clades
Unenlagiinae (sensu Bonaparte 1999) and Halszkaraptorinae (sensu Cau et al. 2017).
Branch-based definition
All taxa closer to Unenlagia comahuensis Novas & Puerta, 1997 and Halszkaraptor escuilliei Cau et al., 2017 than to Microraptor zhaoianus (Sloan, 1999) or Dromaeosaurus albertensis Matthew & Brown, 1922.
Synapomorphies
Skull longer than 3-fold the occipital height; posterodorsal process of the postorbital longer than the anterodorsal process of the postorbital (homoplastic with the avialans Longipteryx and Gobivenator + Byronosaurus); mesial and distal carinae absent in maxillary teeth (homoplastic with the avialans Archaeopteryx, Eosinopteryx, Serikornis and Aurornis); anteroposterior diameter of the ischium at mid-shaft more than 1/5 of the proximodistal length (homoplastic with the avialans Archaeopteryx, Rahonavis, Zenyualong and Tianyuraptor); and anteroposterior length of the medial ridge in the preacetabular process of the ilium subequal to the length of the postacetabular process.
Ambiguous synapomorphies
More than 75 cheek teeth with relatively small alveoli; presence of the sixth sacral vertebra (homoplastic with some therizinosaurs); transition point in the tail extending across more than three caudal vertebrae (homoplastic with Aurornis and Mei); and flexor tubercle of the pedal unguals III and IV poorly developed (recovered in some MLTs to Paraves).
UNENLAGIINAE Bonaparte, 1999
Genus YPUPIARA nov.
Figures 2-4
LSID
urn:lsid:zoobank.org:act:00069916-4671-4266-A048-6DDF1AB14F00
Type species
Ypupiara lopai sp. nov., type by monotypy.
Derivation of name
‘Ypupiara’, which means ‘the one who lives in the water’, an allusion to a Tupian myth about an aquatic creature. This also alludes to the inferred fish diet of Ypupiara.
Diagnosis
As for the type and only species.
Ypupiara lopai sp. nov.
LSID
urn:lsid:zoobank.org:act:04F236A0-2BD7-41A7-8013-16BE2BF6F56C
Derivation of name
The specific epithet ‘lopai’ is in honour of Alberto Lopa (of the former Divisão de Geologia e Mineralogia, Departamento Nacional de Produção Mineral–DNPM of Peirópolis). He is responsible, together with Llewellyn I. Price, for starting the systematic palaeontological exploration of the region of Peirópolis, Minas Gerais state, and for recovering most of the material studied by Price. In addition, Alberto Lopa also recovered the fossil material studied here.
Holotype
DGM 921-R, consisting of a partial preantorbital portion of a right maxillary, with three teeth in loci, and a partial posterior portion of a right dentary.
Locality and horizon
The specimen was found at the Caiera site, also known as Ponto 1 do Price, in Serra do Veadinho, close to Peirópolis, a rural district of Uberaba, on the road BR 262 (Kellner et al. 2005). In this locality, the Marília Formation crops out (Maastrichtian; Dias-Brito et al. 2001). As noted earlier, the maxilla and dentary were found closely associated and in the same horizon. The specimen DGM 921-R also shows some signs of superficial damage, but without the loss of the bone surface that has occurred in other theropods (Bandeira et al. 2018). The bone surface preservation is also consistent with the maxilla and dentary belonging to the same horizon. Interestingly, a fish jaw (also presumed lost), was found associated with DGM 921-R.
Differential diagnosis
The new species described here can be distinguished from other unenlagiines by the following four characters: preantorbital body of the maxilla with a restricted number of maxillary neurovascular foramina (only one; distinct from the numerous foramina observed in dromaeosaurids such as Velociraptor and Halszkaraptor); maxillary interdental plate rectangular and anteroposteriorly expanded, with the ratio of the interalveolar and alveolar widths close to 1 (shared with Buitreraptor gonzalezorum, but distinct from other dromaeosaurids); maxillary teeth with a CBR of between 0.6 and 1 (shared with A. cabazai (CBR near to 1) and distinct from B. gonzalezorum (CBR < 0.5)); and teeth of the preantorbital body of the maxilla widely spaced.
Description
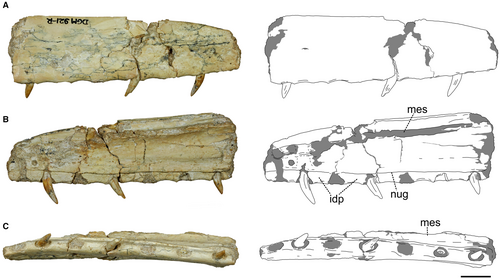
Maxilla
The fragmentary preantorbital body of a right maxilla (Fig. 2; Table 1; see also Brum et al. 2021) exhibits seven tooth alveoli along its extension, with three teeth preserved in situ and one fragmentary root partially covered by rock matrix. The dorsal margin of the bone is straight, lacking any signs of the ascending ramus. The angle formed between the dorsal and ventral margins of the maxilla is acute (c. 21°). The lateral surface of the maxilla is flat and lacks any sign of the ventral limit of the antorbital fossa. The angle between the dorsal and ventral margins, as well as the lack of any antorbital fossa delimitation, indicates that this fragment is a maxillary preantorbital body. In ventral view, the alveolar margins are misaligned, forming a slight concavity. The lateral surface is devoid of numerous neurovascular grooves, which is typical of this region in most theropods, and presents only one neurovascular groove in the specimen. The medial surface reveals a fragment of the mesial (palatal) shelf, close to the dorsal margin. In the medial view, the interdental plates are fused with the lateral margin of the maxilla and are anteroposteriorly wide, assuming a rectangular conformation and having a wide space between tooth alveoli.
| Maxilla | |
| Total width* | 79.08 |
| Anterior height | 15.13 |
| Posterior height | 23.25 |
| MTD | 8.15 |
| MTV | 5.01 |
| Dentary | |
| Total width* | 58.5 |
| Total height* | 13.55 |
| MT | 7.71 |
- MT, mean thickness; MTD, mean thickness of dorsal margin; MTV, mean thickness of ventral margin.
- * Measurement of an incomplete fragment.
Dentition
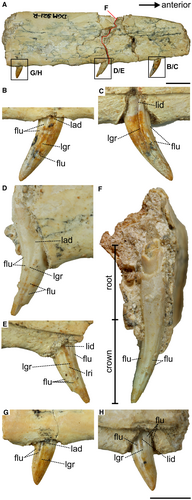
The maxillary teeth have a well-preserved surface, with enamel structures intact. They also lack any apparent taphonomic deformation. Going from anterior to posterior, the second tooth is partially broken at the apex (Fig. 3; see also Brum et al. 2021). The crowns of the teeth are small and ziphodont (CH < 10 mm and MCW/CH < 0.6, respectively; Table 2) (sensu Hendrickx et al. 2015). The crowns are distally curved, especially at the apex. The lingual surface is convex, and accentuated at the mid-point of the basoapical length of the crown. The teeth lack both mesial and distal carinae. Both labial and lingual surfaces exhibit flutes, but the number of these structures varies along teeth and surfaces. The most anterior tooth has three flutes on the labial surface and two flutes on the lingual surface, while the second tooth has four flutes on each surface and the most posterior teeth have two and four flutes, respectively. Both surfaces of the crown in all teeth also have a centrally positioned longitudinal groove from the most basal region of the crown to the apex, which confers a figure of 8 shape to the cross-section of the teeth. The enamel texture is smooth. The root is elongated and deeply implanted in the maxilla, which is a typical thecodont condition.
| Tooth | CBL | CBW | CH | AL | CBR | CHR | MCL | MCW | MCR | MSL | LAF+1 | LIF+1 | CA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.858 | 2.022 | 7.388 | 8.67 | 0.707 | 2.585 | 2.271 | 1.434 | 0.631 | – | 4 | 3 | 63.108 |
| 2 | 2.739 | 2.719 | – | – | 0.993 | – | 2.311 | 1.93 | 0.835 | – | 5 | 5 | – |
| 3 | 2.41 | – | 4.741 | 4.846 | – | 1.967 | 1.967 | – | – | – | 3 | 5 | 37.909 |
- Tooth number: 1, most anterior; 2, middle; 3, most posterior.
- AL, apical length; CA, crown angle (in degrees); CBL, crown base length; CBR, crown base ratio (CBW/CBL); CBW, crown base width; CH, crown height; CHR, crown height ratio (CH/CBL); DDL, distal denticle length; LAF, number of flutes on the labial surface of a crown; LIF, number of flutes on the lingual surface of a crown; MCL, mid-crown length; MCR, mid-crown ratio (MCW/MCL); MCW, mid-crown width; MSL, mesial serrated carina length.
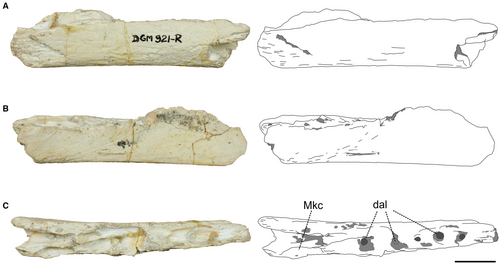
Dentary
The specimen fragment consists of a posterior portion of a right dentary (Fig. 4; Table 1; see also Brum et al. 2021). Most of the ventral portion of the bone is preserved, lacking the dorsal margin. Both medial and lateral surfaces are flat. The ventral margin is marked by a straight keel along its extension, which gives a triangular shape to the cross-section of the dentary. In dorsal view, the broken region has at least five alveoli and no signs of interdental plates between them. In this view, the posteriormost region of this fragment exhibits a medial ramification, which constricts the alveolar margin and marks the border of the Meckelian cavity, found in the posterior portions of the dentary.
Comparisons
The specimen has a thecodont ziphodont dentition, which is found in theropods, sphenacodontids, basal archosaurs and crurotarsians (see Hendrickx et al. 2015). However, the occurrence of unserrated ziphodont thecondont teeth is exclusive to some small theropods (e.g. unenlagiines, compsognathids). The mesial shelf perpendicular to the maxilla, as well as the dorsoventrally high maxilla, also indicate that DGM 921-R belonged to a theropod. The medial surface of the maxilla DGM 921-R is vertically oriented, implying the presence of a dorsally recessed palate relative to the horizontal plane of the tooth line, as commonly found in theropods (e.g. Tsuihiji et al. 2014), and in contrast to crocodilians and pterosaurs, in which the palate is level with the tooth line (e.g. Witton 2013; Pol et al. 2014). Therefore, based on these dentition characteristics, our cladistics, and morphometric analyses (see further), we propose that these bones belong to a small-sized theropod and unenlagiine. We compared the preserved portion of the maxilla and dentition of DGM 921-R with A. cabazai and B. gonzalezorum, the only unenlagiine specimens to have preserved cranial remains, and with other small-sized theropods (Fig. 5). The proportions of both the maxilla and dentary (e.g. the height and thickness) are consistent with what is found in unenlagiines.
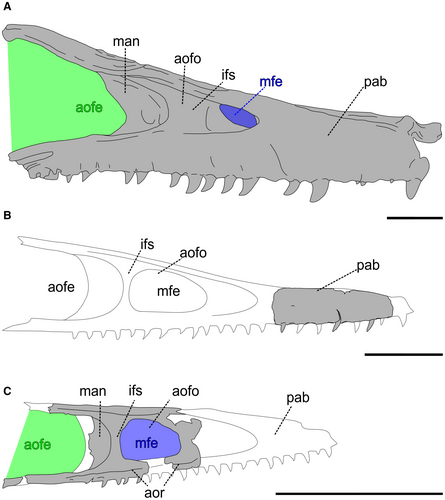
The maxilla in the studied specimen is dorsoventrally low. Although the maxillary preantorbital body is not preserved in B. gonzalezorum, the low anterior margin of the antorbital fossa suggests that it is also dorsoventrally low, unlike that observed in A. cabazai. Although Gianechini et al. (2017) reported wide spacing between the teeth in the middle of the maxillary tooth row, which is not preserved in DGM 921-R, we could assume that it was present in DGM 921-R because of the wide spacing observed in the preantorbital body fragment of DGM 921-R. In both DGM 921-R and B. gonzalezorum, the spacing between alveoli/width of alveoli ratio is ≥1, which also occurs in Archaeopteryx and Anchiornis, but not in A. cabazai (Table 3). The lateral surface of this part of the maxilla is flat, which is also found in A. cabazai. The lateral surface of the maxillary preantorbital body in DGM 921-R lacks numerous neurovascular grooves, a lack that is also commonly observed in dromaeosaurids (e.g. Lü et al. 2010; Evans et al. 2013). Such a feature is hard to observe in the lateral surface of A. cabazai due to the poor preservation of the bone surface.
| Ypupiara lopai (DGM 921-R) | |
| MAW | 5.39 |
| MIaW | 6.34 |
| MIaW/MAW | 1.18 |
| Buitreraptor gonzalezorum | |
| MAW | 1.06 |
| MIaW | 1.11 |
| MIaW/MAW | 1.05 |
| Austroraptor cabazai | |
| MAW | 10.16 |
| MIaW | 5.81 |
| MIaW/MAW | 0.57 |
- MAW, mean alveolar width; MIaW, mean interalveolar width.
Based on the diagnosis provided by Gianechini et al. (2017), focusing on the cranial elements of B. gonzalezorum, DGM 921-R and B. gonzalezorum are similar in their small teeth, which lack carinae and denticles and the constriction between the crown and root, and which have crowns exhibiting parallel longitudinal striae (Gianechini et al. 2011). However, DGM 921-R differs from B. gonzalezorum in that the teeth of DGM are less compressed (DGM 921-R CBR between 0.7 and 1, B. gonzalezorum CBR <0.5). Small teeth also occur in A. cabazai, and this is considered a homoplastic condition that is present in alverezsaurids, ornithomimosaurs, troodontids, and basal avialians such as Archaeopteryx and Jeholornis. The absence of denticles in the teeth is synapomorphic for Unenlagiinia, while the presence of two or more longitudinal grooves is synapomorphic for Unenlagiinae (Fig. 3; Gianechini et al. 2011). The absence of carinae in the teeth is also shared by A. cabazai, as is the fluted condition (Novas et al. 2009; Gianechini et al. 2011). However, the ziphodont condition resembles that observed in B. gonzalezorum rather than in A. cabazai, which presents a conidont condition.
The absence of denticles on both mesial and distal edges is also seen in specimens attributed to the Laurasian wastebasket genus Paronychodon, such as AMNH 27127B, NMMNH P-30218, NMMNH P-30233, RTMP 2000.19.2 and RTMP 88.211.66 (Sankey et al. 2002; Hwang 2005; Williamson & Brusatte 2014). Notwithstanding, AMNH 27127B and RTMP 2000.19.2 differ from DGM 921-R and unenlagiines in exhibiting a constriction between the crown and root, typical of troodontid teeth (Makovicky et al. 2003; Hwang 2005). Furthermore, NMMNH P-30218 and NMMNH P-30233 differ from unenlagiines in exhibiting both mesial and distal carinae (Williamson & Brusatte 2014). Finally, even though RTMP 88.211.66 presents lateral longitudinal grooves on the crown, it differs from unenlagiines in tooth morphology, density and location (Sankey et al. 2002; Gianechini & Apesteguía 2011).
The dentary of DGM 921-R shows a constriction of the alveolar margin, with a marked Meckelian cavity. The development of the Meckelian groove is similar to that observed in the referred dentary material of Rahonavis ostromi Forster et al., 1998 (see Forster et al. 2020) and the derived paravian Archaeopteryx and Anchiornis (Pei et al. 2017), in which the groove runs conspicuously along the length of the medial surface of the dentary. It differs from that observed in A. cabazai by being wide and by beginning in the middle portion of the dentary. In DGM 921-R the Meckelian groove is more constricted. Therefore, the constriction of the alveolar margin with the Meckelian cavity indicates that it is the posterior portion of a dentary, posterior to the antorbital fenestra. The lateral surface of the dentary lacks any sign of a longitudinal groove or a nutrient groove, the lack of which is also observed in B. gonzalezorum and A. cabazai, and which has been previously recovered as synapomorphic to Troodontidae (Gianechini et al. 2017). However, the dorsal half of the dentary is not preserved and we are therefore unable to assert that this feature is lacking. The ventral margin is thin and straight, and forms a triangular cross-section, as in the paravians R. ostromi, Archaeopteryx and Anchiornis. Although the posterior portion of B. gonzalezorum is not preserved, the preserved regions indicate that the ventral margin is straight. The dentary of DGM 921-R lacks any signs of interdental plates. This feature was recovered as synapomorphic for the Maniraptoriformes, but these structures are present in A. cabazai (Gianechini et al. 2017). Among the unenlagiines, the absence of dentary interdental plates resembles the condition observed in B. gonzalezorum (Gianechini et al. 2017).
Phylogenetic analysis
Our search produced >600 000 MLTs, reaching the maximum capacity of the computer, with 6455 steps each, a CI of 0.231 and an RI of 0.606 (Fig. 6; Brum et al. 2021). The clade Unenlagiinae was supported by the following synapomorphies: character state 72(0 > 1): shape of the lateral margin of the frontal in dorsal view describing an angular (sharp-cornered) transition between the anterior half and the postorbital process (homoplastic in MLTs with Eudromaeosauria); character state 157(0 > 1): much larger mesiodistal diameter relative to adjacent alveoli of the dentary interdental septa, and teeth widely spaced (homoplastic with Archaeopteryx and Eosinopteryx, Serikornis + Aurornis); character state 210(0 > 1): post-axial centra in cervical vertebrae with a ventral sulcus delimited by ventrolaterally directed ridges; and character state 1774(0 > 1): longitudinal grooves and ridges on the lateral sides of the teeth, giving rise to a fluted appearance. We note that character 72(1) was slightly modified (see Brum et al. 2021). Originally, the statement for character 72(1) was ‘shape of the lateral margin of the frontal in dorsal view describing an abrupt transition between the anterior half and the postorbital process’; and here, we have substituted ‘abrupt’ with ‘angular (sharp-cornered) transition’. This change in statement did not alter in any way the original coding, which was not modified. This change was introduced because the usage of ‘abrupt’ can be ambiguous with regard to unenlagiines. The lack of an abrupt or angular transition is clear in taxa such as Bambiraptor or Halszkaraptor (Burnham et al. 2000; Cau et al. 2017), in which the transition is gradual. In the unenlagiines Buitreraptor and Austroraptor, there is an angular transition (Novas et al. 2009; Gianechini et al. 2017), as there is in the eudromaeosaurians Velociraptor and Dromaeosaurus (Sues 1977; Currie 1995). However, even though the transition in Velociraptor and Dromaeosaurus is abrupt (90°), the angle is more obtuse in unenlagiines, and could therefore be regarded as ‘non-abrupt’, hence our adjustment of the wording of the character statement. Future revisions of this data matrix (which is beyond the scope of the present work) may involve the use of different states between unenlagiines and eudromaeosaurians.
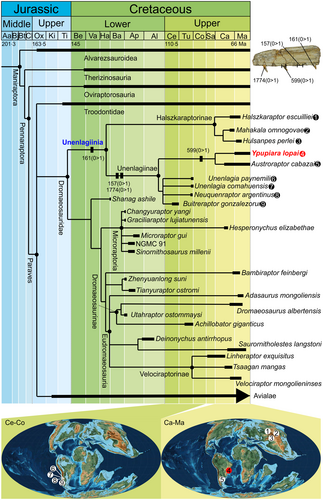
The taxon Y. lopai was classified as a sister-group of A. cabazai (Fig. 6) based on one synapomorphy: character state 599(0 > 1): labiolingual diameter relative to mesiodistal diameter at mid-crown in maxillary teeth more than 3/5 (homoplastic with Avialae and Similicaudipteryx, the last recovered only in some trees).
Finally, the clade Unenlagiinia (Unenlagiinae + Halszkaraptorinae) was supported by the following five synapomorphies in all trees: character state 89(0 > 1): posterodorsal process of the postorbital longer than the anterodorsal process of the postorbital (recovered as homoplastic with Velociraptor in some trees); character state 161(0 > 1): mesial and distal carinae absent in maxillary teeth (homoplastic with Xixiasaurus and Ornithomimosauria); character state 419(1 > 0): anteroposterior diameter of the ischium at mid-shaft more than 1/5 of the proximodistal length (homoplastic with Archaeopteryx, Rahonavis, and the node Zhenyualong + Tianyuraptor); character state 1049(0 > 1): skull longer than 3-fold the occipital height (homoplastic with Longipteryx and Gobivenator + Byronosaurus, the last recovered in some trees); character state 1142(0 > 1): anteroposterior length of the medial ridge in the preacetabular process of the ilium subequal to the length of the postacetabular process; and the following four synapomorphies in some trees (homoplastic with Velociraptor in some trees): character state 34(0 > 1): more than 75 cheek teeth with relatively small alveoli (recovered as plesiomorphic to Maniraptoriformes); character state 1505(1 > 0): flexor tubercle of the pedal unguals III and IV poorly developed (recovered in some trees as plesiomorphic to Paraves and in Kol); character state 1575(1 > 0): transition point in the tail extending across more than three caudal vertebrae (homoplastic with Aurornis and, in some trees, with Mei); and character state 1709(0 > 1): presence of the sixth sacral vertebra (homoplastic in some trees with some therizinosaurs).
Tooth assignment
Heuristic search
The heuristic search resulted in MLTs with tree length = 1072, CI = 0.246 and RI = 0.588. Although we obtained distinct topological resolutions, in both the TBR + TNT algorithms and the TBR heuristic searches Ypupiara was recovered as a sister-group of Buitreraptor. The assignment of Ypupiara + Buitreraptor was also stable (Fig. 7; for full topologies, see Brum et al. 2021). The TBR + TNT algorithms analysis resulted in 33 520 MLTs, while the TBR analysis resulted in 360 MLTs. No autapomorphies were recognized for Ypupiara. The TBR + TNT algorithms analysis was marked by poor resolution, and resulted in a polytomy with 61 theropod taxa and 8 groups (including the group Ypupiara + Buitreraptor). The TBR synapomorphies resulted in a well-resolved topology.
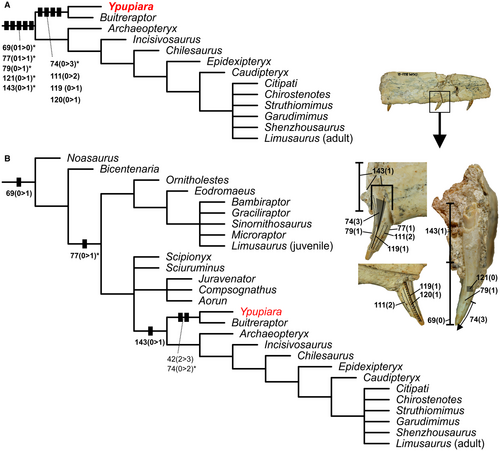
The characters that group Ypupiara + Buitreraptor in the TBR + TNT algorithms analysis are as follows (* indicates that they were recovered only in some trees; characters are based on Hendrickx et al. 2020): mesiodistal curvature of the labial surface of the crown at 1/3 of the central region of the crown surface, with the labial depression restricted to the crown base (char. 74(0 > 3*)); teeth with flutes present on the labial surface or on both the labial and lingual surfaces (homoplastic with Sinornithosaurus and spinosaurids; char. 111(0 > 2)); single longitudinal groove centrally positioned on the labial and/or lingual surface of the crown (also occurs as a polymorphism in Byronosaurus; char. 119(0 > 1)); single ridge centrally positioned (differing from flutes) on the lingual surface of the crown (also occurs as a polymorphism in Byronosaurus; char. 120(0 > 1)). The synapomorphies that group Ypupiara + Buitreraptor within the major group are: CH < 10 mm (also present in Eoraptor and in the group formed by Jianchangosaurus, Erlikosaurus, Falcarius, Eshanosaurus, Zanabazar, Byronosaurus, Sinusonasus, Almas, MCPD 1001128, Mononykus, Shuvuuia and Nqwebasaurus; char. 69 (01 > 0*)); absence of mesial carina (also occurs in Orkoraptor, Halszkaraptor and in some trees in the group formed by Zanabazar, Byronosaurus, Sinusonasus, Almas, MCPD 1001128, Mononykus, Shuvuuia and Nqwebasaurus; char. 77(01 > 1*)); absence of distal carina (also occurs in Halszkaraptor and in some trees in the group Suchomimus and Baryonyx; char. 79(0 > 1*)); texture of the enamel surface smooth or irregular (present in some trees in the group Majungasaurus, Rugops and Kryptops, as in the Eoraptor + some maniraptorans; char. 121(01 > 0*)); lateral view of the root showing convex margins, with a root significantly larger than crown base (also present in Dilong, Troodon, Jianchangosaurus + Erlikosaurus + Falcarius + Eshanosaurus and Almas + MCPD 1001128; char. 143(0 > 1*)).
The characters that grouped Ypupiara + Buitreraptor in the TBR analysis were: CHR < 2 in mesial teeth (char. 42(2 > 3) is not scored in Ypupiara); and the mesiodistal curvature of the labial surface of the crown at 1/3 of the central region of the crown surface, with the labial depression restricted to the crown base (char. 74(0 > 2*)). This analysis recovered less support for the group Ypupiara + Buitreraptor and one synapomorphy is not scored for Ypupiara: that character 74(0 > 2) is scored as 74:3 for both Ypupiara and Buitreraptor. Therefore, we consider that the result recovered in the first analysis better represents what is observed in both genera. Regarding the plesiomorphic characters that grouped both taxa in the major recovered group (Fig. 7), they consist of characters 69(1 > 0); 77(0 > 1*); and 143(0 > 1). We note that the characters recovered for this group were considered as more derived in the TBR + TNT algorithms analysis.
Linear morphometric analyses
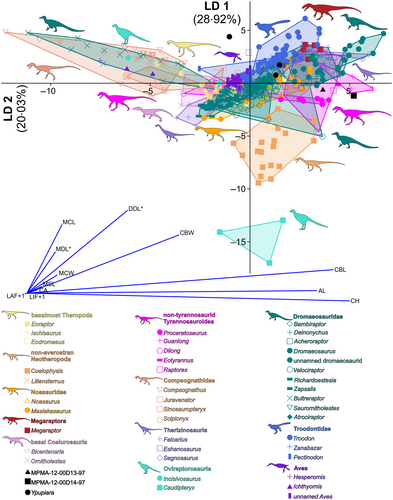
The first LDA conducted on the whole dataset showed that the teeth of Ypupiara, as well as those of the Bauru dromaeosaurid specimens MPMA-12-00D13-97 and MPMA-12-00D14-97, occupy the morphospace of small-sized theropods (LD1 (first linear discriminant axis), 51.93%; LD2, 18.21%; see Brum et al. 2021). In the LDA classification the Ypupiara teeth are classified as Therizinosauria and Spinosauridae, while the Bauru specimens occupy the Neovenatoridae morphospace (Brum et al. 2021). However, the morphospaces of these specimens overlap, therefore we performed a second LDA of taxa with small crowns (CH < 20 mm). Although both Ypupiara and the Bauru specimens occupied morphospaces in which we observe troodontids, dromaeosaurids, therizinosaurs, Coelophysis, Megaraptor and non-tyrannosaurid Tyrannosauroidea, the Ypupiara and MPMA specimens are distinct from each other (LD1, 28.92%; LD2, 20.03%; Fig. 8). The teeth of Ypupiara are in a region of overlap of the morphospaces of dromaeosaurids (except Buitreraptor), troodontids, noasaurids and non-tyrannosaurid Tyrannosauroidea. Regarding the MPMA specimens, they were located in the morphospace of non-tyrannosaurid Tyrannosauroidea. The classification assigns the teeth of Ypupiara to Masiakasaurus, while MPMA-12-00D13-97 is assigned to Liliensternus and MPMA-12-00D14-97 to Gualong (success rate of 60.78%). The PCA (see Brum et al. 2021) shows that the main measurements that influence the variance observed in the genus morphospaces are CBL, CBW, CH and AL.
The classical hierarchical and the joining clustering analyses produced similar morphoclades for the placement of the teeth of the Ypupiara and MPMA specimens (Brum et al. 2021). Two teeth of Ypupiara occupy a morphoclade that is mostly composed of troodontids, but which contains some dromaeosaurids. One tooth of Ypupiara, which is the most anterior, was different to the others and occupies the morphoclade shared with Eoraptor, Dilong and the dromaeosaurid Saurornitholestes. Regarding the MPMA specimens, they occupy a different morphoclade to that of Ypupiara, with both MPMA specimens in a morphoclade that mostly includes dromaeosaurids, Megaraptor, non-tyrannosaurid Tyrannosauroidea, Masiakasaurus and basal theropods.
Results of the parsimony analysis of endemicity
An exhaustive search was able to be performed, due to the small taxa–character data matrix, and the final cladogram is a consensus of nine trees found with 60 steps (Fig. 9). As already established in the literature, when taxa are coded as zero this implies that such taxa are absent and excludes dispersal a priori (Morrone 2014).
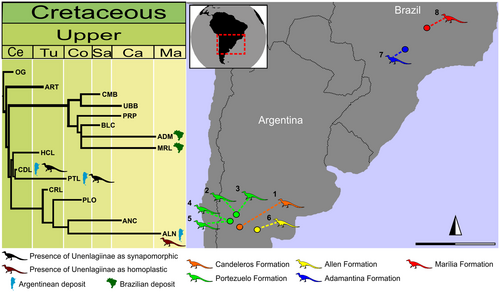
Then the cladogram divides into two major branches, with the Argentine formations in one and the Brazilian formations in the other (Fig. 9). The exception is the Bajo de la Carpa Formation (Santonian), which forms a polytomy with the Adamantina (Coniacian to late Maastrichtian, sensu Castro et al. 2018) and Marília (Maastrichtian) formations.
In the two resulting MLTs, the relationships in the clade (Marília + Adamantina + Bajo de la Carpa formations) are sustained by the presence of the advanced notosuchian, non-Unenlagiinae maniraptorans and avialans. The presence of Unenlagiinae taxa is recovered as a synapomorphy in the clade (Candeleros + Portezuelo formations), and as an autapomorphy (homoplasy) in the Allen Formation. Despite this, the Unenlagiinae presence in the Marília and Adamantina formations was not detected as an apomorphy (see the output file in Brum et al. 2021).
Discussion
Unenlagiinae affinities
The specimen DGM 921-R shares with the Unenlagiinae the secondary loss of both teeth carinae, as well as the fluted teeth and the presence of two or more longitudinal grooves on tooth surfaces. The dentition of Ypupiara lopai shows mixed features with unenlagiines. Among unenlagiines, Y. lopai is more similar to Buitreraptor gonzalezorum, due to the wide space between the maxillary tooth alveoli, the ziphodont tooth condition and the lack of interdental plates in the dentary, which are present in Austroraptor cabazai. Although not conidont, Y. lopai was grouped as the sister taxon of A. cabazai based on the similar CBR ratio. Therefore, the morphology is similar to that observed in B. gonzalezorum, but the proportion resembles A. cabazai. This unique combination of features, in addition to the maxillary preantorbital body with the restricted number of neurovascular foramina and the rectangular interdental plate, indicate that the new specimen represents a new species of Unenlagiinae, probably closely related to the contemporaneous A. cabazai.
According to the heuristic search analysis, the teeth of Y. lopai are markedly similar to those of B. gonzalezorum due to the mesiodistal curvature of the teeth, the occurrence of flutes and the longitudinal central groove with ridges. They share with other small theropods the small crown (CH < 10 mm), the lack of carinae, a smooth enamel surface and roots significantly larger than the crown base. Although they are morphologically similar, they occupy distinct morphospaces and morphoclades in multivariate analysis, with Y. lopai falling in the overlap between dromaeosaurids and troodontids. We interpret the overlap of Y. lopai with Spinosauridae and Masiakasaurus in the LDA analyses as being due to the occurrence of flutes in the teeth crown, which are present in B. gonzalezorum, but which were not included in the dataset (see further in Brum et al. 2021). Regarding the overlap of Y. lopai with dromaeosaurids and troodontids, as well as the marked separation of the morphoclades of Y. lopai and B. gonzalezorum, this could be related to the occurrence of different ecological niches, as suggested by the differences in the teeth of B. gonzalezorum and A. cabazai. We also highlight the marked variation between the proportions of B. gonzalezorum and the morphospaces occupied by most dromaeosaurids, troodontids and Y. lopai. We also note that the low number of flutes in the teeth of B. gonzalezorum could also contribute to the observed difference to Y. lopai, although most of the variation relates to the dental crown proportions. Instead, we can first conclude that Y. lopai is a dromaeosaurid based on both a heuristic search and multivariate analyses of the teeth. We can also conclude, as a result of the teeth data, that Y. lopai is an unenlagiine based on phylogeny and the morphological similarity to B. gonzalezorum.
The record of deinonychosaurs from Brazil
The presence of small maniraptorans and small deinonychosaurs was previously reported in Brazil as a result of teeth and isolated bone remains (Franco-Rosas 2002; Novas et al. 2005; Machado et al. 2008; Ghilardi & Fernandes 2011; Delcourt & Grillo 2014; Tavares et al. 2014). However, only one dorsal vertebra from the Campanian–Maastrichtian of the Adamantina Formation has been tentatively assigned to Unenlagiinae (Candeiro et al. 2012). As expected for the theropods of the Bauru Group (Bandeira et al. 2018), DGM 921-R is incomplete, although it is the third specimen from the Bauru Group to be found with some associated material. This pattern of poor preservation of theropod fossils indicates a preservation bias in comparison with baurusuchids (for a detailed discussion, see Bandeira et al. 2018).
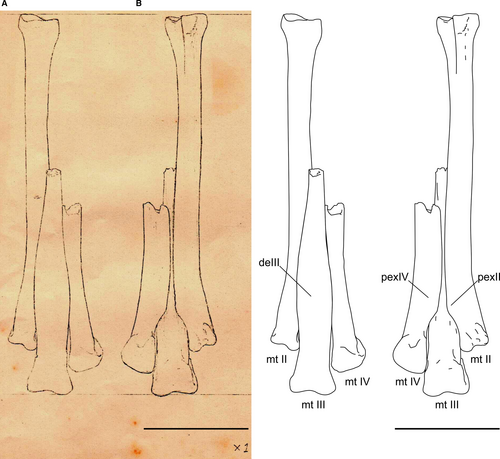
A record of a partial right metatarsus also exists from the Marília Formation in the Ponto 1 do Price site. This specimen disappeared after Price’s death and is only recognized by an unfinished plate (Fig. 10), indicated as ‘Lopasaurus’. The plate showed an articulated metatarsus, with a complete metatarsal II and distal portions of metatarsals III and IV (Fig. 10). Based on this illustration, the material could be assigned to Dromaeosauridae based on the ginglymoid distal end of metatarsals II and III (Turner et al. 2012). We could assign this illustrated specimen to Unenlagiinae by the following shared similarities with B. gonzalezorum, Neuquenraptor argentinus Novas & Pol, 2005 and Pamparaptor micros Porfiri et al., 2011 in the distal portion of the metatarsus: the anterior surface of metatarsal III transversally expanded; the expansion of the posterolateral region of metatarsal II; and the expansion of the posteromedial region of metatarsal IV. In posterior view, the expansions observed in metatarsals II and IV overlap metatarsal III. However, such missing material was not associated with Y. lopai in the geological horizon. This illustration indicates the potential diversity of the maniraptorans in the Bauru Group. Further fieldwork in Peirópolis could provide new data on this theropod group.
The dromaeosaurid tooth record from the Bauru Group is still scarce, with most of them exhibiting carinae, as well as denticles (Franco-Rosas 2002; Tavares et al. 2014), although a small number of reports note that the dromaeosaurid teeth lack carinae (Ghilardi & Fernandes 2011). Regarding the MPMA specimens from Tavares et al. (2014), and reanalysed here with Y. lopai, their assignment based only on morphometry is still unclear due to the overlap and the lack of some morphometric data (e.g. the number of denticles per mm in each carina). The morphology is also distinct from Y. lopai. However, we could not rule out that the MPMA specimens are not dromaeosaurid. Further studies should focus on updating the teeth assignments based on recent approaches, as observed for the abelisaurids in the Bauru Group (e.g. Delcourt & Grillo 2018; Delcourt et al. 2020). However, the MPMA specimens (which may be dromaeosaurids) and Y. lopai, together with noasaurids, indicate a hitherto hidden diversity of small-sized theropods in the Bauru Group, which reinforces the niche-partitioning hypothesis of Bandeira et al. (2018).
The presence of unenlagiine remains could indicate that: (1) unenlagiines were the part of the maniraptoran diversity of the Bauru Group; and (2) the unenlagiines and dromaeosaurids of the Bauru Group exhibited a diversity of forms during the Campanian–Maastrichtian. The recovery of Y. lopai, with cranial bones and associated teeth, could prove to be an important comparative key for the assignment of isolated teeth from the Bauru Group and to clarify aspects of the distribution of the group.
Comments on Unenlagiinia
Our analysis provides support for the Unenlagiinia, with the Halszkaraptorinae as a sister-group within the Unenlagiinia (as independently found by Brownstein 2019, Hartman et al. 2019 and Cau 2020) instead of falling at the base of the Dromaeosauridae (as in Cau et al. 2017). Five unambiguous synapomorphies support Unenlagiinia (see Systematic Palaeontology, above). Furthermore, another four ambiguous synapomorphies were recovered: more than 75 cheek teeth with relatively small alveoli (also recovered by Cau 2020); presence of the sixth sacral vertebra (also recovered by Brownstein 2019 and Cau 2020); transition point in the tail extending across more than three caudal vertebrae; and flexor tubercle of the pedal unguals III and IV poorly developed.
In this way, it is possible that up to nine synapomorphies could support the Unenlagiinia. Even though the understanding of the phylogenetic relationships and evolutionary history of deinonychosaurs has been thoroughly investigated recently (e.g. Cau et al. 2017; Hartman et al. 2019; Brownstein 2019; Cau 2020; Currie & Evans 2020), further work is needed. Of particular note are the results presented by Hartman et al. (2019), which indicate a large Unenlagiinae clade involving the Laurasian taxa Ornithodesmus, Pyroraptor and Dakotaraptor; and a clade ‘Unenlagiidae’ (Unenlagiinae + Halszkaraptorinae) falling outside of the Dromaeosauridae (Hartman et al. 2019). In the present work, we preferred to use a dataset modified from Cau et al. (2017) because it is has a more comprehensive character list. We note that both Pyroraptor and Dakotaraptor have serrated teeth (Allain & Taquet 2000; DePalma et al. 2015), which would exclude these taxa from the Unenlagiinae and the Unenlagiinia as a whole. Dakotaraptor was originally recovered as a eudromaeosaurian (DePalma et al. 2015), and this result was independently replicated recently by Currie & Evans (2020). Ornithodesmus is an elusive taxon represented exclusively by a sacrum, which deserves further investigation.
Finally, it is interesting to note that the current ecological interpretations of unenlagiines and halszkaraptorines are consistent with their close relationship and the monophyly of Unenlagiinia as herein defined. Unenlagiines have been interpreted as piscivores (Gianechini et al. 2011; Cau et al. 2017; Cau 2020 contra Brownstein 2019), which is here corroborated (see below). Piscivory implies the foraging for fishes in aquatic environments, irrespective of whether this involves swimming or standing on foot (e.g. Souza et al. in press). Halszkaraptorines, in turn, have been interpreted as semi-aquatic dromaeosaurids with some level of swimming abilities (Cau et al. 2017; Cau 2020; but see Brownstein 2019). If both interpretations are correct, then the most parsimonious conclusion is that some degree of preference for foraging in aquatic environments was present at the base of the Unenlagiinia; thus characterizing their evolutionary history as intimately linked to some level of semi-aquatic habits.
Feeding mode of Unenlagiinia
The anatomy of fossil species is the key to understanding their behaviour and environmental interactions, and there are several difficulties involved in this kind of study (e.g. Kellner et al. 2019b). Here we discuss the anatomical evidence related to Unenlagiinia feeding behaviour. Buitreraptor gonzalezorum and A. cabazai exhibit tooth crowns that lack carinae, lack any serrations and bear longitudinal ridges and flutes. Furthermore, the tooth crowns are conical in Austroraptor and only slightly laterally compressed in Buitreraptor, rather than strongly compressed pattern that is most common among theropods (e.g. Hendrickx et al. 2015). The dentition of Austroraptor is thus conidont (sensu Hendrickx et al. 2015, approximately conical, recurved teeth without carinae or serrations), while that of Ypupiara can be considered as somewhat intermediate between conidont and ziphodont, which is more common throughout dromaeosaurids and theropods overall (e.g. Hendrickx et al. 2015).
The conidont condition of some unenlagiines and the oval to circular cross-section of the tooth crown is typical of piscivorous reptiles (Souza et al. in press). Regarding the grooves in the surface of the teeth, this feature is present in purported piscivorous tetrapods, which supports a piscivorous habit for unenlagiines (Gianechini et al. 2011). We highlight that, even though inferred piscivorous archosauromorphs have a plethora of bauplans (as disparate, for example, as gavialoid crocodylians, spinosaurid theropods and anhanguerid pterosaurs), two of the anatomical features that are common to most toothed piscivorous archosauromorphs are a conidont dentition (or at least a rounded cross-section of the crown), and an extensive tooth count, both of which are related to an inertial mode of feeding (i.e. using teeth to hold the prey and then swallow it whole), which is typical of, although not exclusive to, piscivorous reptiles (Souza et al. in press). We thus find it plausible that unenlagiines consumed fish at least as part of their diet, and potentially as the main source of food at least in Austroraptor due to its conidont dentition.
Still, Gianechini et al. (2020) recently proposed that unenlagiines were not mainly piscivorous (contra Gianechini et al. 2011). According to Gianechini et al. (2020), tooth morphology is not unequivocal evidence for piscivory; and other evidence must be considered (to which we fully agree; Souza et al. in press). Furthermore, through morphometric analyses and morphofunctional comparisons, Gianechini et al. (2020) showed that the hindlimbs of unenlagiines could have performed cursorially even better than other dromaeosaurids, being similar to ground birds that feed on terrestrial prey, such as Cariama, implying that unenlagiines too could have foraged on the ground for terrestrial prey. Based on the faunal record of La Buitrera, Gianechini et al. (2020) inferred that small tetrapods, such as sphenodonts, crocodyliforms and mammals, were the potential prey of Buitreraptor. However, Gianechini et al. (2020) did not include in their analyses ground birds that forage for fish (e.g. ardeids, ciconiids), which could be appropriate analogues for possible piscivorous unenlagiines such as we describe here. Furthermore, as mentioned above, although not unequivocal, tooth morphology is still strongly associated with piscivory.
Nonetheless, as observed by Gianechini et al. (2020), Buitreraptor could have used inertial feeding for small prey items other than fish, such as invertebrates and small terrestrial vertebrates. A somewhat generalist diet for Buitreraptor as suggested by Gianechini et al. (2020) seems plausible if this taxon comes from aeolian strata (Apesteguía et al. 2016), and not fluviolacustrine ones, as is the case for Austroraptor and Ypupiara. Furthermore, with all of this taken into account, we find it plausible that at least Austroraptor and Ypupiara would have consumed fish as a considerable part of their diet (Fig. 11), and that Buitreraptor would have been more of a generalist due to its drier environment, as well as its labiolingually compressed teeth. Although the morphology of the maxillary teeth in Ypupiara lopai is remarkably similar to that observed in B. gonzalezorum, the morphometric analyses indicate some differences in their proportions, and that they fell into separate morphospaces and morphoclades. Such a difference could be related to their distinct ecological traits and feeding habits.

Within dromaeosaurids, there are two other cases of proposed piscivorous habits. A specimen of Microraptor gui Xu et al., 2000 has fish remains in its abdominal cavity, and this was noted as the first definitive evidence of piscivory in a non-avian maniraptoran (Xing et al. 2013). In M. gui, serration on the mesial carinae is absent, which Xing et al. (2013) have interpreted as supporting evidence for a diet inclined towards piscivory. However, the dentition of M. gui still presents distal serrations and lateral compression. Furthermore, another specimen of M. gui had an enantiornithean bird in its gut (O’Connor et al. 2011), showing that this taxon could have in fact been a generalist. More recently, ecomorphological analyses of the Mongolian dromaeosaurid Halszkaraptor indicated a probable semi-aquatic lifestyle (Cau et al. 2017; Cau 2020). Its dentition is similar to that of unenlagiines, in that it lacks serrations and the anterior teeth are less compressed than ziphodont teeth (Cau et al. 2017). Considering that there is evidence of fish-eating elsewhere in the Dromaeosauridae, it seems not unreasonable to hypothesize that some dromaeosaurids consumed fish as a considerable part of their diet.
Distribution of the unenlagiines
In the PAE analysis, the synapomorphies in the generated consensus area cladogram are traditionally interpreted as vicariance events (e.g. Morrone 1994, 2014). In this case, the distribution of Unenlagiinae is a result of vicariance events only in the clade that involves the Candeleros and Portezuelo formations. The presence of unenlagiines in the formations studied here suggests dispersal events or extinction events (Morrone 2014), especially given the systematic affinities of Ypupiara.
The spatial reunion of populations of Unenlagiinae from the Bauru Group and the Neuquén Basin formations (as well as the other tetrapods analysed here) may have been stimulated after the Cenomanian–Turonian interval (known as the Cretaceous Thermal Maximum, CTM; Rothmann 2002; McInerney & Wing 2011). During this interval, the temperatures started to drop and many humid palaeoenvironments started to form (Rothmann 2002; McInerney & Wing 2011). The Bauru Group was surrounded by the Caiuá desert, which is primarily Aptian (sensu Pinheiro et al. 2018), and which separates the Bauru Group from the Neuquén Basin. The Caiuá palaeodesert, from which an interesting fauna of reptiles is beginning to emerge (e.g. Kellner et al. 2019b) may have acted as a geographical barrier, preventing the dispersion of some taxa between the Neuquén Basin and the Bauru Group. This barrier could have isolated the Unenlagiinae in the Neuquén Basin, until the Coniacian–Santonian. Later, with the posterior regression of the Caiuá desert and a change to a more humid palaeoenvironment (e.g. Pinheiro et al. 2018) in the Coniacian–Santonian, as suggested by the Adamantina Formation (Pinheiro et al. 2018; Castro et al. 2018), the establishment of palaeo-lakes, and subsequently of rivers, may have allowed these animals to disperse to these palaeoenvironments.
At the same time, the differentiation of the palaeoenvironment over time may have been a limiting factor for some taxa. The stratigraphic units of the Bauru Group congregate in general deposits of aeolian, lake and fluvial origin (with meandering and interlaced channels; Fernandes 2004), suggesting a warm seasonal climate. The arid conditions intensified towards the top of the Bauru Group (e.g. Marília Formation, sensu Bittencourt & Langer 2011), which may have been a limiting factor for dispersion throughout higher latitudes. Further evidence may facilitate the understanding of the faunal similarities and historical (evolutionary and biogeographical) relationship between the geological units discussed here.
Traditionally, the tetrapod taxa of the Bauru Group have been considered endemic (e.g. Bertini et al. 1993; Santucci & Bertini 2001), especially the small-bodied animals (Santucci & Bertini 2001). More recently this endemism has been contested (e.g. Bittencourt & Langer 2011) due to some tetrapod genera (e.g. titanosaurs, baurusuchids, peirosaurids, etc.) being recorded in Argentinian and Brazilian strata. We found a closer ecological relationship between the Bauru Group formations, indicating that the sharing of similar taxa in Argentina and Brazil may not reflect historically structured biotic assemblages.
The record of B. gonzalezorum (Cenomanian; Corbella et al. 2004) and that of A. cabazai (Maastrichtian) are, respectively, the earliest and latest records of unenlagiines (Fig. 8). Specimen DGM 921-R is the sister-group of A. cabazai, being coeval with this taxon. The similarity between Y. lopai and B. gonzalezorum indicates a more recent divergence from the others. The species U. comahuensis and U. paynemili are interpreted here as syntopic (e.g. Hartman et al. 2019), that is, a type of sympatry in which sister-taxa occur in the same habitat within the same region, because they could coexist in the same habitat without outcompeting each other. Deeper ecological affinities of Neuquenraptor and other taxa need to be investigated in the future.
Unenlagiines are also referred to as widespread through South America, with Argentinean records also accompanied by Brazilian and Bolivian records, albeit fragmentary (Gianechini & Apesteguía 2011). However, the incompleteness of the fossil material means that it is still not confirmed that the group was indeed widely distributed throughout South America.
Conclusion
Specimen DGM 921-R represents a new species, Ypupiara lopai gen. et sp. nov., that can be recognized as an unenlagiine and which exhibits a combination of features observed in both Buitreraptor gonzalezorum and Austroraptor cabazai. The dental features, however, share more morphological similarities with B. gonzalezorum. The recovery of such cranial material with associated teeth is important to diagnose and trace comparisons with the dromaeosaurid teeth from Bauru Group outcrops, and as an indicator of the diversity of these small theropods. Such an unenlagiine occurrence in the Campanian–Maastrichtian intervals of Brazil indicates that the lineage was present not only in the Neuquén Basin (Argentina), but also in the Upper Cretaceous of the Paraná Basin (Brazil).
Acknowledgements
We thank the Willi Hennig Society for making TNT freely available; Borja Holgado (Museu Nacional, Universidade Federal do Rio de Janeiro), Rafael Delcourt (Universidade Estadual de Campinas (Unicamp)) and Natan S. Brilhante (MN) for fruitful discussions and insights; and Orlando N. Grillo (MN) for photographing the specimen DGM 921-R. We also express our gratitude to an anonymous reviewer and Federico A. Gianechini (Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) – Universidad Nacional de San Luis), who revised early drafts of the manuscript, and Christophe Hendrickx (CONICET, Unidad Ejecutora Lillo) for providing the dataset for the statistical analyses. We thank PhyloPic for the theropod silhouettes used in Figures 1, 8 and 9 that were drawn by Matt Martyniuk, Scott Hartman, and Tasman Dixon. Guilherme Gehr is acknowledged for the palaeoart of Figure 11. This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP#2019/10231-6 to RVP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES-PROANTAR #88887.336584/2019-00 to ASB), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ #E-26/202.905/2018 to AWAK), and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #420687/2016-5 and #313461/2018-0 to AWAK). LGS is supported by a PhD scholarship from CAPES (#88882.183263/2018-01), the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM #062.00166/2020), and has received financial support from the Paleo and Geohistory exhibition at Museu da Amazônia (PRONAC 183808). We also thank the rescue team of the Museu Nacional-UFRJ, coordinated by Claudia Rodrigues Carvalho and Luciana Carvalho, for their work in recovering the scientific collections from the rubble after the fire of 2018, and particularly for their attempts to rescue the specimen described here.
Open Research
Data archiving statement
This published work and the nomenclatural acts it contains, have been registered in ZooBank: http://zoobank.org/References/1fb81fee-959b-4626-aacc-8652b641d8b7. The new clade name Unenlagiinia is registered in the RegNum database (https://www.phyloregnum.org/); registration number 694.
Data for this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.crjdfn32j.




