Morphological–phylogenetic analysis of the late Cenozoic Chlamydini von Teppner (Bivalvia, Pectinidae) of southern South America
Abstract
The tribe Chlamydini was highly diversified in the marine Neogene of southern South America, reaching its maximum taxonomic diversity during the Miocene. However, the evolutionary relationships of South American taxa remain uncertain. This is the first phylogenetic analysis based on a large morphological matrix on Pectinidae, which focuses on South American taxa and species related to Chlamys s.s. The phylogenetic analysis is based on a matrix composed of 145 shell-characters scored for 48 species, and multiple searches were conducted using equal and implied weighting. Two new monophyletic clades are defined, Multiplicata and Pauciplicata. The first includes Dietotenhosen, Ckaraosippur, Zygochlamys, Moirechlamys (South America), the North-West Pacific Azumapecten, and the North-East Pacific Chlamys hastata. Pauciplicata is represented by Chokekenia (Patagonia, Argentina), Laevichlamys (tropical Atlantic and Indo-Pacific), Semipallium (Indo-Pacific), Swiftopecten (South America and North Pacific), and Jorgechlamys + Reticulochlamys (Patagonia, Argentina). All of these genera are monophyletic except for the paraphyletic Jorgechlamys. The oldest documented occurrence of the tribe is Semipallium foulcheri from the lower Oligocene, a derived taxon that pushes the divergence time of basal genera to the Eocene–Oligocene boundary, generating ghost lineages in all clades, except Jorgechlamys + Reticulochlamys. Pauciplicata and Multiplicata diverge in the early history of the tribe, at the Eocene–Oligocene boundary. Future analyses are necessary to gain a better understanding of the taxonomic arrangement of this poorly understood tribe. Further insights into the relationships of its deepest nodes may well resolve many ghost lineages.
The taxonomy of Pectinidae Rafinesque, 78 is unstable because of a lack of well-defined diagnostic traits (Serb 88), phenotypic plasticity in response to environmental conditions (Orensanz et al. 70; Waller 0105, Wilbur & Gaffney 110; Culver et al. 15; del Río et al. 20; Trovant et al. 0104) and morphological convergence of shell shape (Stanley 99; Waller 0105; Alejandrino et al. 1; Serb et al. 89; Sherratt et al. 93; Serb et al. 90). Pectinidae includes in the order of 400 (Brand 10), 275 (MolluscaBase 0201) or 250 (Raines & Poppe 79) recognized extant species, with the subfamily Chlamydinae von Teppner, 0103 and particularly the tribe Chlamydini von Teppner, 0103, taxonomically the largest groups. Chlamydinae has around 98 extant species (MolluscaBase 0202) from which 75 or 73 belong in Chlamydini (Alejandrino et al. 1; MolluscaBase 0203; respectively). The subfamily embraces different morphologies ranging from chlamydoid shells such as those of Chlamys Röding, 82 and Talochlamys Iredale, 50 to the fan-shaped shells of the byssally-attaching, nestling and facultative boring Pedum Bruguière, 12, and the highly variable shell shape of Crassadoma Bernard, 5 that adapts due to its cementing life habit. Until now, the most complete phylogenetic studies of Chlamydinae include 37% and 47% of the extant species (Alejandrino et al. 1; Sherratt et al. 93; respectively), but as the internal phylogenetic relationships of this group are unclear, its evolutionary history is currently poorly understood. The tribe Chlamydini is probably a waste-basket taxon that clusters phylogenetically unrelated lineages, and is therefore probably a non-monophyletic group (Alejandrino et al. 1; Sherratt et al. 93). Moreover, Chlamydinae is resolved as a paraphyletic group according to Alejandrino et al. (1). The internal relationships of this group therefore need to be reviewed.
Phylogenetic background of pectinids
Previous morphological analyses
In recent decades, the taxonomy and systematics of scallops have been revolutionized by the innovative morphological phylogenetic analyses based on extant and fossil species developed by T. Waller, who proposed a taxonomic scheme that is currently followed by most taxonomists, with few modifications. Based on microsculpture traits and hinge teeth type, Waller (0105, 0106) subdivided pectinids into four subfamilies: Camptonectinae Habe, 41, Palliolinae Korobkov in Eberzin, 55, Chlamydinae and Pectininae Rafinesque, 78. Waller (0106) also proposed that Chlamydinae is represented by the tribes Chlamydini, Mimachlamydini Waller, 0106, Aequipectinini Nordsieck, 68, and Crassadomini Waller, 0106, according to several synapomorphies, but he failed to provide a morphological matrix to support his results. Later, Waller (0107) published a matrix for the superfamily Pectinoidea Rafinesque, 78, that includes 14 morphological characters with controversial definitions, one of which refers to the ctenolium (char. 10) as having three states: presence, primary absence, and secondary absence. This contradicts the proposal of de Pinna (21), who stated that secondary homologies are those that resist phylogenetic analysis; however, Waller (0107) established his character states before carrying out a phylogenetic analysis and therefore this homology was not properly tested.
Relevant recent molecular analyses
Molecular phylogenetic studies such as those of Puslednik & Serb (76), Serb et al. (89), Alejandrino et al. (1), Sherratt et al. (93) and Serb et al. (90) were based on a large taxonomic sample of the Pectinidae and adequately proved the monophyly of this family through the addition of many taxa into the outgroup. The first three analyses included 46, 52 and 81 species, respectively, and the studies of Sherratt et al. (93) and Serb et al. (90) considered 143 species. Puslednik & Serb (76) compared their results with the tribes scheme of Waller (0106); the other analyses did not.
The phylogenetic status and position of the subfamily Chlamydinae remain unclear according to current molecular evidence, which provides contradictory results. As new more comprehensive analyses are performed, with an increasing sampling of taxa, the status of Chlamydinae is continuously changing. Alejandrino et al. (1) suggested that Chlamydinae is a paraphyletic and basal group. In contrast, Sherratt et al. (93) found that Chlamydinae is a monophyletic lineage well-distinguished from other Pectinidae, and diverging early during the evolutionary history of the group. The monophyly and arrangement of the Chlamydinae tribes have been barely debated in previous works (Puslednik & Serb 76). The most comprehensive studies on Pectinidae comprise large-scale phylogenies with under-sampled genera and do not discuss the composition of tribes or subfamilies (Alejandrino et al. 1 (81 species); Sherratt et al. 93 (143 species); Serb et al. 90 (143 species)). The phylogenetic status of the tribe Chlamydini is also unresolved. Alejandrino et al. (1) distinguished three clades in the subfamily Chlamydinae that are not directly equivalent to the tribes defined by Waller (0106). Although the traditional taxonomic definitions of Chlamydinae and Chlamydini of Waller (0106) seem to describe paraphyletic groups, the clade containing Chlamys s.s. (Chlamydini) is monophyletic, and is the sister group of a lineage constituted by Azumapecten Habe, 41, Laevichlamys Waller, 0106 s.l. and Crassadoma. In contrast, Sherratt et al. (93, fig. S1) found a monophyletic Chlamydinae with four major lineages, including a monophyletic Chlamydini (but differing from that of Waller 0106) and composed of a clade containing Chlamys and Swiftopecten Hertlein, 43. The latter clade is related to a group that clusters Azumapecten, Scaeochlamys Iredale, 50, Crassadoma gigantea (Gray, 40), and some Laevichlamys (polyphyletic) and could also be considered as Chlamydini (Sherratt et al. 93, fig. S1).
The tribe composition of Chlamydinae as erected by Waller (0105, 0106) was subsequently modified following the results of molecular phylogenies, with the tribe Aequipectinini separated from Chlamydinae and placed within Pectininae (Alejandrino et al. 1; Serb et al. 89; Sherratt et al. 93; Serb et al. 90). This new consensus was accepted by Waller (0107, 0108). Modern molecular evidence also reveals that the taxonomic arrangement proposed by Waller (0106) needs to be reviewed and it is likely that several undescribed lineages could be recognized in future.
Taxonomic status of the tribe Chlamydini
Dijkstra & Beu (28) challenged the validity of Chlamydini (and consequently of Chlamydinae) replacing it with Pedini Bronn, 11, while stating that the correct name for the subfamily should be Pedinae Bronn, 11. These authors claimed that the name Pedini takes priority based on the Waller's (0106) association of Pedum with other Chlamydini, and on the phylogenetic tree obtained by Dufour et al. (29), in which Pedum falls into the tribe Chlamydini, being closely related to Chlamys islandica (Müller, 67) (type species of Chlamys). However, that assertion deserves further discussion since other molecular phylogenies do not support it (Alejandrino et al. 1; Sherratt et al. 93).
Initially, Waller (0106) pointed out that Pedum probably evolved from Laevichlamys squamosa (Gmelin, 35) and stated that Pedum belonged in the tribe Chlamydini. Accordingly, the subfamily name Peduminae Habe, 41 (emended to Pedinae) would be a junior synonym of Chlamydinae. Subsequently, Matsumoto & Hayami (62) recovered a similar relationship, with Pedum showing a close affinity and basal to L. squamosa. Later, Carter et al. (13) considered Pedini to be a distinctive tribe, separated from Chlamydini and containing only Pedum.
Comprehensive molecular studies are still insufficient to elucidate the internal relationships of Chlamydini and show Pedum separated from Chlamys s.s. (Alejandrino et al. 1; Sherratt et al. 93). Alejandrino et al. (1) found Pedum to be nested in a clade containing the type species of Mimachlamydini (Mimachlamys asperrima), whereas, according to Sherratt et al. (93), Mimachlamydini is basal to Pedum. In sum, according to the phylogenetic study of Alejandrino et al. (1), Pedum would be included in Mimachlamydini rather than Chlamydini but following Sherratt et al. (93), Pedum and Chlamys belong in separate and distantly related clades. Therefore, Pedini and Chlamydini cannot be synonymized as proposed by modern molecular phylogenies.
Settling a nomenclatural act based upon a small phylogenetic study is unsuitable, especially when more comprehensive phylogenetic studies are available (Alejandrino et al. 1; Sherratt et al. 93). The phylogenetic analysis presented by Dufour et al. (29) includes five Chlamydinae species that are recovered in a polytomy in which Pedum is related to Chlamys as well as to Mimachlamys Iredale, 50, a genus belonging in a different tribe. Therefore, the study of Dufour et al. (29) does not solve the relationships among the tribes of Chlamydinae. According to the results achieved by Alejandrino et al. (1), the tribe Chlamydini is unrelated to Pedum, whereas according to Sherratt et al. (93), Chlamys and Pedum belong in clades distantly related but contained in the same lineage. Not only do molecular phylogenies present such conflicting results but most of the systematic and phylogenetic works carried out over almost a century have used Chlamydinae and Chlamydini. We therefore endorse nomenclatural stability (Art. 23.9.3, ICZN 47).
The tribe Chlamydini in South America
The tribe Chlamydini constitutes one of the most highly diversified groups in Cenozoic strata of southern South America where it is represented by the endemic genera Moirechlamys Santelli & del Río, 84, Pixiechlamys Santelli & del Río, 84, Chokekenia Santelli & del Río, 84 (Argentina), Zygochlamys Ihering, 49, Reticulochlamys del Río, 19, Jorgechlamys del Río 19, Ckaraosippur Santelli & del Río, 85 (Chile and Argentina) and Dietotenhosen Santelli & del Río, 85 (Chile, Argentina, and Peru). Another member of this group is the genus Swiftopecten from Asian and North American coasts of the North Pacific (Yoshimura 111), which has also been found in Miocene strata of Argentina (del Río 18, 19; Santelli & del Río 84, b) and Chile (MBS, pers. obs. 2015).
According to Santelli & del Río (84, b) this tribe occurred from the late Oligocene to the early middle Pliocene or early Pleistocene, when it became extinct in the southern South American region. This statement was based on the assumption that the Recent Psychrochlamys patagonica (King, 54) (= Ps. amandi (Hertlein, 42) = Zygochlamys phalara (Roth, 83); synonymized by Jonkers 53) and Ps. delicatula (Hutton, 46) do not belong to the chlamydini genus Zygochlamys (Santelli & del Río 84, p. 137) as believed by Beu (8), who had previously proposed that Ps. patagonica evolved from Zygochlamys geminata Tavera Jerez, 100 (non Z. geminata (G. B. Sowerby I, 97); Beu 7). That proposal was strengthened by the results of the two most comprehensive phylogenetic studies based on molecular data sets, Alejandrino et al. (1) and Sherratt et al. (93), which found that Psychrochlamys Jonkers, 53 is clearly unrelated to Chlamydini. In the first work, Psychrochlamys is unrelated to Chlamydinae, whereas according to Sherratt et al. (93), the clade containing Ps. patagonica and Ps. delicatula is the sister group or even a member of this subfamily, although certainly not a member of Chlamydini. Therefore, the evolutionary relationships of this genus still remain unknown. As already discussed by Santelli & del Río (84), Psychrochlamys is a non-Chlamydini genus because of the presence of orbicular shells with a wide umbonal angle, very short and symmetrical auricles, having a very shallow byssal notch, minute and few ctenolium teeth, and lacking the byssal sinus. Other distinguising traits are the absence of shagreen microsculpture, typical of Chlamydini, and the presence of coarse commarginal lamellae on the entire disc, which are thinner and developed as patches in Chlamydini (Santelli & del Río 84).
The main goal of this contribution is to establish phylogenetic hypotheses for the Cenozoic and extant species of Argentina, Chile and Peru, which have historically been referred to the tribe Chlamydini (Beu 7; Morra 66; Waller 0105; del Río 17; Beu 8; Jonkers 53; del Río 19, Santelli & del Río 84, b). Moreover, we aim to test whether the genera recently described by Santelli & del Río (84, b) are monophyletic or not, and to explore their relationships with the Recent Psychrochlamys, and analyse its placement within Chlamydini. Based on current phylogenetic evidence, Chlamydini (sensu Waller 0106) seems to be a waste-basket taxon. Our phylogenetic analysis is taxonomically restricted to those Chlamydini genera that have been closely related to Chlamys s.s. (the eponymous taxon of Chlamydini) according to morphological and molecular evidence, such as Swiftopecten and Azumapecten. Also, this analysis includes South American taxa which have been recently revised or identified. It is performed avoiding the inclusion of taxonomically uncertain species or those that are morphologically dissimilar to Chlamys, in order to recognize the hypothetical relationships among true Chlamydini and southern South American groups. Other taxa traditionally mentioned as Chlamydini from the Indo-Pacific and South Pacific oceans, and the Caribbean Sea (Semipallium Jousseaume in Lamy, 57, Laevichlamys and Talochlamys) are also considered in this study to test their affinities with southern American taxa.
Because large morphological matrices on pectinids have never been published, a secondary goal of this contribution is to provide a morphological matrix with broad sampling for use in future phylogenetic analyses of this group.
Material and method
The present contribution is focused on 17 species of Chlamydini from the late Cenozoic of Argentina, Chile and Peru, and the analysis comprises 48 species of pectinids in total. Two South American species of Psychrochlamys are also included. Most of those taxa were studied from specimens in hand and some using high-quality images following comparisons with detailed systematic descriptions.
Institutional abbreviations
The studied material is listed in the Santelli et al. (86, appendix S1) and is housed at the following repositories: AMS, Australian Museum, Sydney, Australia; BAS (ROW.BAS), British Antarctic Survey, Cambridge, UK; CPBA, Cátedra de Paleontología of the Universidad de Buenos Aires, Buenos Aires City, Argentina; EASPU (Ortmann collection, see del Río 19), Purdue University, West Lafayette, Indiana, USA; GNS (GNS WM, GNS TM), Institute of Geological & Nuclear Science, Lower Hutt, New Zealand; MACN-In, División Invertebrados, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires City, Argentina; MACN-Pi and CIRGEO-PI, División Paleoinvertebrados, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires City, Argentina; MGGC, Giovanni Capellini Geological Museum, Bologna, Italy; MLP, Facultad de Ciencias Naturales y Museo de la Universidad Nacional de La Plata, Buenos Aires Province, Argentina; MNHN (MNHN IM, MNHN.F.), Muséum national d'Histoire naturelle, Paris, France; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; NHMUK (NHM, NHML), Natural History Museum, London, UK; NMR, Natural History Museum of Rotterdam, Rotterdam, The Netherlands; NMV, Museum Victoria, Melbourne, Australia; PMBP 2004, 2004 Panglao Marine Biodiversity Project (PMBP) to Panglao, Philippines (see Dijkstra 27); PRI, Cenozoic Marine Mollusks, Paleontological Research Institution, Ithaca, New York, USA; RMNH.MOL, Rijksmuseum van Natuurlijke Historie, Leiden, The Netherlands; SAM (F), South Australian Museum, Adelaide, Australia; SGO.PI, Colección Paleoinvertebrados, Museo de Historia Natural, Santiago, Chile; SNSB-BSPG, fossile Wirbellose collection, Bayerische Staatssammlung für Paläontologie und Geologie, Münich, Germany; UNISTRA, Collection de Paléontologie, Université de Strasbourg, France; USNM MO, Mollusk Collection, National Museum of Natural History, Smithsonian Institution, Washington DC, USA; WAM, Western Australian Museum, Perth, Australia; ZMA.MOLL, Zoölogisch Museum Amsterdam, The Netherlands.
Phylogenetic analysis
Definition and character sampling
General morphological traits of pectinids are summarized in Fig. 1. Terminology follows Waller (0105, 0106). The matrix comprises 146 morphological characters corresponding to 132 discrete and 14 continuous traits of the external and internal shell morphology (Santelli et al. 86, appendix S2, S3).
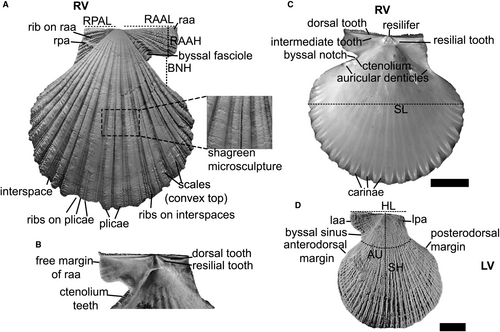
Discrete characters (chars 14–145) are grouped into categories according to the shell structure or trait: general shell-outline and shell shape (chars 14–32), shell convexity (chars 33–36), byssal notch (chars 37–39), ctenolium (chars 40–41), auricular denticles (chars 42–43), auricles shape (chars 44–57), sculpture on auricles (chars 58–65), microsculpture on auricles (chars 66–72), microsculpture on disc (chars 73–90), growth lines (chars 91–92), radial plicae (chars 93–109), radial ribs (chars 110–123), interspaces (chars 124–127), hinge (chars 128–132), internal sculpture (chars 133–134), scales (chars 135–142), growth ledges (chars 143–144) and nodes (char. 145) (Santelli et al. 86, appendix S3). Microsculpture and macrosculpture features on pectinids have traditionally been considered to be useful tools in systematic and phylogenetic studies (Waller 0105, 0106, 0107, 0108), and herein correspond to 51% of the discrete characters. Some of these characters or related structures are shown in detail in Fig. 2, including linear measurements and angles of morphological features used to define continuous characters.

The continuous characters refer to values of average shell height, umbonal angle, number of radial ribs on left and right auricles (ranges), number of plicae on each valve (ranges), number of ctenolium teeth (ranges), and ratios as hinge length/shell length (Santelli et al. 86, appendix S2). Ratios of continuous characters were logarithmized following Mongiardino Koch et al. (64).
Included taxa
Taxon sampling of Chlamydinae was designed considering the phylogenetic results of Waller (0105, 0106) and Alejandrino et al. (1), and taxa of each tribe ever placed in Chlamydinae have been incorporated into the study.
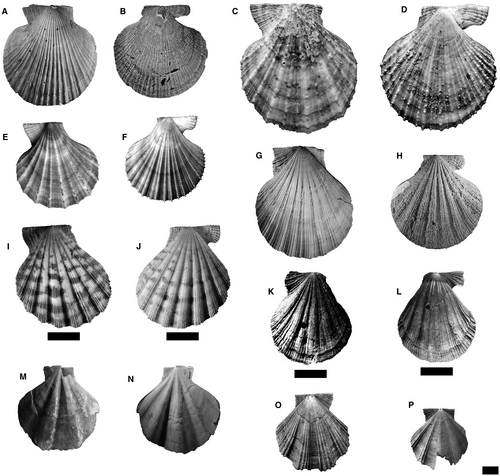
The phylogenetic analysis was performed using 48 species-level terminals, including the type genera of the tribes and their type-species along with co-generic species. The outgroup consists of 17 species, including representatives of the tribes: Pectinini Rafinesque, 78 (subfamily Pectininae) (Pecten maximus (Linnaeus, 59), P. jacobaeus (Linnaeus, 59)); Amusiini Ridewood, 81 (subfamily Pectininae) (Euvola ziczac (Linnaeus, 59), Leopecten oblongus (Philippi, 74), Leopecten pyramidesius (Ihering, 49)); Aequipectinini (subfamily Pectininae) (Aequipecten opercularis (Linnaeus, 59), A. tehuelchus (d'Orbigny, 1842), A. paranensis (d'Orbigny, 1842)); Adamussiini Habe, 41 (subfamily Palliolinae) (Adamussium colbecki (Smith, 95)); Crassadomini (subfamily Chlamydinae) (Caribachlamys sentis (Reeve, 1853)); Mimachlamydini (subfamily Chlamydinae) (Mimachlamys asperrima (Lamarck, 56), M. crassicostata (G. B. Sowerby II, 96), M. varia (Linnaeus, 59)); and Fortipectinini Masuda, 61 (subfamily Chlamydinae) (Mizuhopecten yessoensis (Jay, 51)). The outgroup also includes Psychrochlamys patagonica, Ps. delicatula and Ps. moerickei (Hertlein, 43) (subfamily Chlamydinae), species historically related to the extinct Chlamydini from southern South America. Members of the studied genera are illustrated in Figures 2-4. It is commonly accepted that representatives of Chlamydinae are closely related to the tribe Chlamydini; therefore, they are the most useful taxa to establish the relationships within the ingroup. The root was fixed on P. maximus.
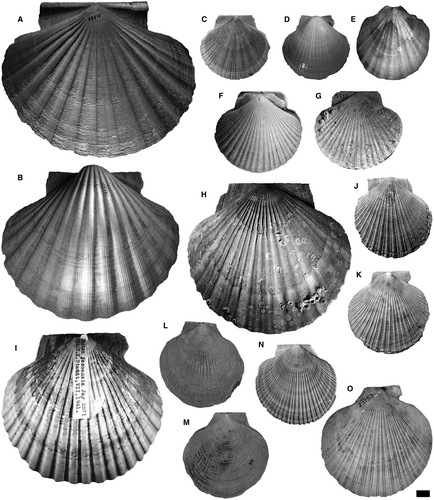
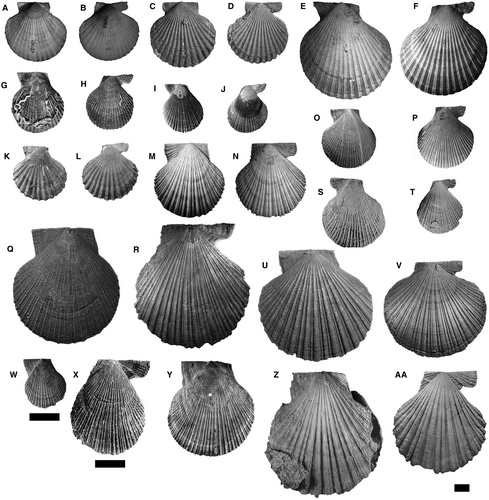
The ingroup comprises 31 species traditionally assigned to Chlamydini (Santelli et al. 86, appendix S1), including all late Cenozoic Argentinean and south-eastern Pacific Ocean taxa. This temporal and geographical category included: Zygochlamys geminata (G. B. Sowerby I, 97) (type species), Z. jorgensis Ihering, 49, Z. sebastiani Morra, 66; Pixiechlamys quemadensis (Ihering, 48); Chokekenia nicolasi (Morra, 66); Moirechlamys actinodes (G. B. Sowerby I, 97) (type species), M. aurorae (Feruglio, 34); Dietotenhosen hupeanus (Philippi, 73) (type species), D. remondi (Philippi, 73); Ckaraosippur calderensis (Möricke, 65) (type species), C. camachoi Santelli & del Río 85; Jorgechlamys juliana (Ihering, 49) (type species), J. centralis (G. B. Sowerby I, 97); Reticulochlamys proximus (Ihering, 49) (type species), R. borjasensis del Río, 19, R. zinsmeisteri del Río, 19, and Swiftopecten iheringii del Río, 18. The north-eastern Pacific type species of Swiftopecten (S. swiftii (Bernardi, 6) was also included as well as three representatives of Chlamys s.s.: Ch. islandica (type species), Ch. rubida (Hinds, 1845) and Ch. hastata (G. B. Sowerby II, 96). The ingroup also contains some Australian and New Zealand taxa: Semipallium flavicans (Linnaeus, 59) (type species), Se. hallae (Cotton, 14), Se. foulcheri (Tenison-Woods, 0102), Talochlamys pulleineana (Tate, 0101) (type species), T. dichroa (Suter, 98), T. badioriva Beu & Darragh, 9, and T. laticostata Beu & Darragh, 9. Finally, this group also comprises the north-western Pacific species Azumapecten farreri (Jones & Preston, 52) (type species) and two species of Laevichlamys, the Caribbean L. multisquamata (Dunker, 30) (type species), and the Indo-Pacific L. squamosa. Talochlamys is here considered to be part of the ingroup because taxonomists have traditionally referred it to Chlamydini and molecular phylogenetic studies have suggested incongruent relationships (Alejandrino et al. 1; Sherratt et al. 93). Alejandrino et al. (1) showed that Talochlamys is unrelated to the clade Chlamys s.s., while Sherratt et al. (93) suggested that Talochlamys is polyphyletic and that one of its clades with several Talochlamys spp. is the sister group of Chlamys s.s. The taxonomic composition and affinities of Talochlamys are poorly understood because T. pulleineana (its type species) was omitted by the two mentioned studies. All genera studied herein are illustrated in Figures 2, 4, 5, except for Laevichlamys.
Matrix scoring
The matrix (Santelli et al. 86, appendix S4) was scored in Winclada 1.61, the character states were recorded as polymorphic when different states of a single character are present in different specimens of a single taxon, using the notation: (state A, state B, state C). Unknown characters states were scored as missing data (?), such as those of the unknown right valve of Ckaraisippur camachoi, and microsculpture characters of pre-radial and early radial stages of most fossil taxa in which the umbonal area is frequently broken or abraded. Microsculpture traits on auricles of T. pulleineana (studied using Beu & Darragh 9, fig. 29) were also scored as unknown. Inapplicable characters states are those that correspond to subsidiary characters of some structures absent in some taxa, such as the byssal notch, ctenolium, ribs on right posterior auricles, shagreen and commarginal microsculpture, plicae, radial ribs, bifurcation of radial ribs or plicae, and ribs and scales on plicae and they were scored with a hyphen (-). Few characters correspond to juvenile stages, and most of them were defined on well-preserved adult shells.
Phylogenetic searches, branch supports, and tree calibration
The software TNT v1.5 (Goloboff & Catalano 37) was used to conduct heuristic tree searches because the high number of herein included taxa. Space was configured for 20 000 trees in memory and the searches were conducted applying maximum parsimony criterion, starting from 100 replicates of Wagner trees with random addition sequence (RAS) of taxa, which is followed by the Tree Bisection and Reconnection (TBR) branch-swapping algorithm saving 10 trees per replication. To minimize the effect of the homoplasy, characters were weighted (as suggested by Goloboff et al. 39; Goloboff et al. 38), which allows obtaining more reliable and stable results when a complex matrix that includes a high number of homoplastic characters is analysed.
Search strategies involve two weighting arrangements: equal weights and implied weighting (Goloboff 36), the latter using concavity (k) values from 1 to 100 in increments of 1, and both strategies under traditional searches.
Clades recovered in different search strategies were illustrated using sensitive grids (Wheeler 0109; Pérez 72). To measure branch support, the resampling method was used by means of frequency differences (GC), and the Jackknife index (Farris et al. 32) was computed with p = 0.14 (equivalent to removing 10% of the characters) (Goloboff et al. 39) for 1000 pseudo-replicates. The consistency (CI) and retention (RI) indices were computed (Farris 31). Common synapomorphies of the selected topology (k = 48–68) are described and listed below in the results section.
Phylogenetic time-scaling
One of the most frequent topologies (implied weighting, k = 48–68, recovered for 20 values of concavity) was selected to be temporally calibrated using R (R Core Team 77). The packages paleotree (Bapst 3), ape (Paradis et al. 71; Popescu et al. 75) and strap (Bell & Lloyd 4) were loaded to read the matrix and tree topology, and implement different functions in R. The functions timePaleoPhy and geoscalePhylo were applied by means of the minimum branch length (mbl) method of calibration with an mbl of 0.1 myr, in which a minimum branch duration is setting a priori. The mbl method guarantees preservation of the temporal structure of events (Laurin 58). The stratigraphic ranges of each analysed taxon are specified in Santelli et al. (86) and were considered to be the lower (FAD, first appearance datum) and upper (LAD, last appearance datum) bounds of the geological stages.
Phylogenetic nomenclature
New phylogenetic definitions are proposed using stem-based definitions according to the principles of phylogenetic nomenclature (de Queiroz & Gauthier 23, 24, Sereno 91). These definitions were based on unambiguous synapomorphies, although supporting ambiguous synapomorphies are also indicated for both clades (see Systematic Palaeontology, below).
Results
The tribe Chlamydini is the most diverse group of Pectinidae and its evolutionary history is poorly understood; it is therefore likely that some taxa traditionally referred to the tribe are unrelated. However, it must be specified that our results are restricted to the hypothetical relationships among the scallops of southern South America. Two most parsimonious trees (MPTs) were obtained in the search under equal weights with a tree length of 1888 steps (CI = 0.29; RI = 0.65) and their strict consensus is figured in Santelli et al. (86, appendix S6). Searches applying implied weighting showed eight different tree topologies from k = 1 to k = 100, and these correspond to the following ranges of concavity: k = 1–2, k = 3–4, k = 5–7, k = 8–23, k = 24–39, k = 40–47, k = 48–68, k = 69–100. The two more frequent topologies correspond to a k value of 48–68 (Fig. 6), with a fit of 14.58877 (CI = 0.27; RI = 0.61) and k = 69–100 (Santelli et al. 86, appendix S6) with a fit of 45.54514 (CI = 0.27; RI = 0.60). We illustrate and shall discuss in detail the first topology because it also has the highest support values for all clades under study. The relationships obtained under the k = 48–68 weighting scheme are contrasted with those of other topologies under different concavity (k) values; their main differences are described in this section and examined further in the Discussion, below. Jackknife supports are shown for the topology under k = 40–48 (Fig. 6). Figure 6 corresponds to the sole topology obtained under k = 48–68, and shows sensitive grids outlined on the nodes of each clade of interest, representing whether that clade is recovered or unrecovered for each of the strategy of search implemented (equal or implied weighting) and for each k value (Fig. 6). Alternative tree topologies and Jackknife supports are detailed in Santelli et al. (86, appendix S6).
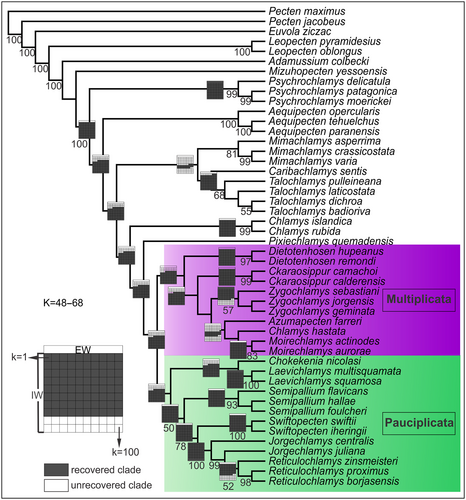
The ingroup is represented by Chlamydini taxa (Chlamys, Semipallium, Azumapecten, Laevichlamys, Zygochlamys, Reticulochlamys, Swiftopecten, among others) and is recovered as a monophyletic group in all trees (Fig. 6; Santelli et al. 86, appendix S6). Talochlamys is found outside Chlamydini except in the search under equal weights, where Mimachlamys and Caribachlamys Waller, 0106 (members of other tribes) are also recovered within Chlamydini. The sister clade of the ingroup changes through different values of k under implied weighting (see Santelli et al. 86, appendix S6).
The most remarkable result is that South American Chlamydini are separated into two major clades under k = 1–7 and k = 24–100 (Fig. 6), which are herein named as Multiplicata and Pauciplicata. The definition of both suprageneric clades follows the proposal of de Queiroz (22), Sereno (92), and Maxwell et al. (63), and these clades can be distinguished by substantial morphological differences. The name Multiplicata refers to the presence of shells with numerous plicae, with 11–36 narrow plicae and sculptured with scaly ribs. This group includes Dietotenhosen (Chile–Peru, Miocene–Pleistocene), Ckaraosippur (Chile–Argentina, middle Miocene – Pliocene), Zygochlamys (Chile–Argentina, late Oligocene – middle Miocene) and Moirechlamys (Argentina, late Miocene – early Pliocene), Azumapecten (Pleistocene–Recent, Japanese and East China seas), and Chlamys hastata (late Pliocene – Recent, north-east Pacific 60°N–32°N, and the north-west Pacific, Kamchatka). The Pauciplicata clade is characterized by taxa sculptured with four to nine wide plicae that bear scaly or non-scaly ribs. This group is represented by Chokekenia (early Miocene, Argentina), Laevichlamys (Recent, North and tropical Atlantic Ocean and Indo-Pacific Ocean), Semipallium (Eocene–Recent, Indo-West Pacific), Swiftopecten (Miocene–Recent, southern South America and North Pacific), Jorgechlamys and Reticulochlamys (early Miocene, Patagonia). The taxonomic composition of both major clades (Multiplicata and Pauciplicata) is consistent in topologies with k values from 24 to 100. The studied genera are monophyletic, except for Jorgechlamys in all searches and Zygochlamys for k values of 69–100. The topology under the k = 8–23 weighting scheme shows Multiplicata as a paraphyletic group while Pauciplicata is recovered as a derived and monophyletic clade nested within the first, with Dietotenhosen as the most basal genus of the ingroup (Santelli et al. 86, appendix S6). Under this tree topology, all Chlamydini genera are monophyletic.
We find that monotypic taxa show more unstable phylogenetic positions than non-monotypic genera, particularly the Patagonian Chokekenia (frequently recovered within Pauciplicata) and Pixiechlamys. Chokekenia is placed outside of Pauciplicata under k = 1–7 searches and it takes different phylogenetic positions, being related to Zygochlamys and Moirechlamys (k = 1–2) or related to Pi. quemadensis (k = 3–23) (Santelli et al. 86, appendix S6). Nevertheless, Chokekenia belongs in Pauciplicata in five different tree topologies under k = 8–100 searches. This genus resolves as the sister group of Laevichlamys, and is related to the Patagonian Reticulochlamys and Jorgechlamys under k = 24–100 searches. However, Chokekenia + Pi. quemadensis are related to a group that contains Chlamys and Pauciplicata taxa (Semipallium, Swiftopecten, Reticulochlamys and Jorgechlamys), under k = 8–23 tree topology (Santelli et al. 86, appendix S6). Pixiechlamys (monotypic genus) is recognized as the sister group of Pauciplicata and Multiplicata in the four topologies for k = 24–100 searches. In contrast, the k = 3–23 weighting scheme resulted in Pi. quemadensis changing its phylogenetic position within these clades (Santelli et al. 86, appendix S6).
There are some ingroup taxa that fall outside Multiplicata and Pauciplicata, such as Chlamys and Ckaraosippur and they have also an uncertain phylogenetic position that changes for different k values (Santelli et al. 86, appendix S6). The first genus is resolved as the sister group of Pauciplicata (k = 1–23) or is recovered as the most basal Chlamydini (k = 24–100), and Ckaraosippur belongs in Multiplicata (k = 1–4, k = 24–100) or is found as the most basal taxa of the ingroup (k = 5–23) (Santelli et al. 86, appendix S6).
Remaining southern South America clades (non-monotypic) are more stable than the monotypic genera, although their relative positions within Multiplicata and Pauciplicata change with varying values of k, under implied weighting. Zygochlamys is monophyletic for equal weighting and almost 70% of the values of k (k = 1–68). However, it is paraphyletic and basal to the Ckaraosippur + Ch. hastata + Moirechlamys clade (k = 69–100), which comprises taxa younger than Zygochlamys (Santelli et al. 86, appendix S6).
Internal relationships of Multiplicata and Pauciplicata
Southern South American Chlamydini are grouped into two well-differentiated clades under k = 1–7 and k = 24–100 (as explained above). These clades show the same taxonomic composition when k = 24–100, although the internal relationships of their taxa change throughout the four different topologies obtained for this range of k values. The sister taxon of both clades is Pi. quemadensis, and Chlamys is the most basal Chlamydini in these topologies (k = 24–100). Within these trees, the second most frequent topology was resolved by the k = 48–68 weighting schemes (e.g. Fig. 6, including Jackknife supports) and has the highest support values for all clades. Moreover, this tree shows the same internal relationships for the two new clades as the topology recovered when k = 40–47.
The tree of the k = 48–68 weighting schemes indicates that Dietotenhosen is the most basal genus of Multiplicata, and this genus is related to the Ckaraosippur + Zygochlamys + Azumapecten + Chlamys hastata + Moirechlamys clade. Ckaraosippur is basal to Zygochlamys, and Zygochlamys is closely related to a group that contains two extant non-Patagonian taxa, Azumapecten and Chlamys hastata, and Moirechlamys, the most derived genus within Multiplicata (k = 40–68). Multiplicata also includes two extant, non-Patagonian taxa: Azumapecten farreri (Pleistocene–Recent, Japanese and East China seas) and Ch. hastata (late Pliocene – Recent, north-east Pacific 60°N–32°N, and north-west Pacific, Kamchatka) (k = 1–7, k = 24–100). The placement of Ch. hastata varies; it is within Multiplicata in most of found topologies (k = 1–7 and k = 24–100), being closely related to Zygochlamys for k = 1–7, whereas it is resolved as the sister group of Moirechlamys when k = 24–100. In contrast, Ch. hastata is basal to a large clade comprising mainly Pauciplicata taxa in the topology of the k = 8–23 weighting scheme (see Santelli et al. 86, appendix S6).
The topology obtained under the k = 48–68 weighting scheme shows that Pauciplicata clusters to a Chokekenia + Laevichlamys group that is in turn related to Semipallium, Swiftopecten and the clade of Reticulochlamys (the most derived terminal taxon) and the paraphyletic Jorgechlamys (Fig. 6).
Synapomorphies of the ingroup (k = 48–68)
There are ten synapomorphies that sustain the ingroup (the taxa herein recovered as Chlamydini taxa, i.e. the smallest clade containing both Chlamys s.s. and Swiftopecten, excluding Talochlamys spp. and Psychrochlamys spp.) among which stand out: the presence of shagreen microsculpture on disc and left auricles, which extends over the entire right valve and is restricted to the left umbonal area on the left valve; and the restriction of commarginal microsculpture to umbonal areas on both valves. Also, the pre-radial stage is very short, microsculpture in advanced pre-radial and early radial stages is dominated by antimarginal striae, and the number of radial ribs increases by bifurcation and intercalation.
Pauciplicata + Multiplicata is grouped by five synapomorphies: shallow byssal sinus; a straight postero-dorsal margin of disc; concave free margin of left anterior auricle; plicae commencing at the early radial stage; and right valve with primary and secondary radial ribs.
The Multiplicata clade has seven synapomorphies in common: opisthocline valves; disc with dorsal gape; left anterior auricles sculptured with 14–16 ribs; ctenolium comprising strong and large teeth; the number of radial plicae increasing by bifurcation and intercalation on the right valve; and by intercalation on the left valve.
The Pauciplicata group is defined by eight synapomorphies: right-convex shells; eight plicae on right valve; nine plicae on left valve; right anterior auricle with seven ribs; ribs on plicae of homogenous thickness; 4–7 ribs on right central plicae; 6–7 ribs on left central plicae; and 4–5 radial ribs on left central interspaces. It is represented by the Semipallium + Swiftopecten + Reticulochlamys + Jorgechlamys clade along with the Chokekenia + Laevichlamys clade.
A detailed description of the synapomorphies that support the clades of interest for implied weighting tree of k = 48–68 is provided in Santelli et al. (86). The synapomorphies of all included taxa are also listed in this appendix (Santelli et al. 86, appendix S7B).
Origin of the lineages within Chlamydini
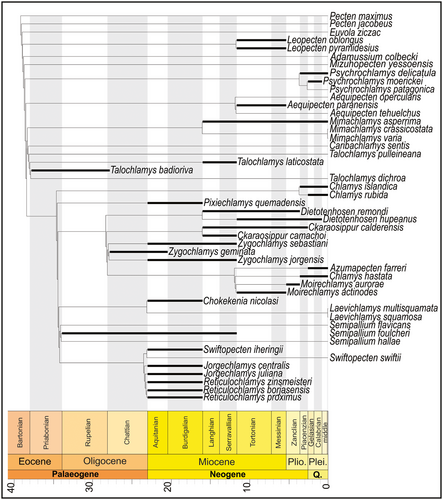
According to the time-calibrated phylogenetic tree, our results suggest that the divergence between both Multiplicata and Pauciplicata occurred before or in the late Eocene or early Oligocene (Fig. 7). This tree shows the existence of ghost lineages for every studied genus, the shortest one for Z. geminata, a species that occurs in the upper Oligocene. The sole clade without ghost lineages is Jorgechlamys + Reticulochlamys, a group that is represented in the lower Miocene of Patagonia. The stratigraphic occurrence of Semipallium (early Oligocene to Recent), a taxon that occupies a derived phylogenetic position, sustains an early diversification in the evolutionary history of the tribe Chlamydini.
Systematic palaeontology
CHLAMYDINI von Teppner, 0103MULTIPLICATA nov.
Definition
The most inclusive clade containing Dietotenhosen, Zygochlamys and Moirechlamys, but not Semipallium or Jorgechlamys, and their descendants (stem-based).
Synapomorphies
Disc with dorsal gape; left anterior auricles sculptured with more than 14 ribs; ctenolium with strong and large teeth; number of radial plicae increases by bifurcation and intercalation on right valves and by intercalation on left valves.
Taxa included
Zygochlamys Ihering, 49; Moirechlamys Santelli & del Río, 84; Dietotenhosen Santelli & del Río, 85; Ckaraosippur Santelli & del Río, 85; Azumapecten farreri (Jones & Preston, 52); Chlamys hastata (G. B. Sowerby II, 96).
Occurrence
Late Oligocene (Z. geminata) to Recent.
Remarks
This clade is resolved as paraphyletic and basal to Pauciplicata under k = 8–23. In the topologies recovered for low values of k (k = 1–7) the monotypic genera Pixiechlamys and Chokekenia are found within Multiplicata, and Ckaraosippur is placed as a basal group to Multiplicata and Pauciplicata when k = 5–23. This clade is also supported by an ambiguous synapomorphy, the presence of opisthocline shells, which is ambiguous because Zygochlamys is characterized by the presence of acline shells.
PAUCIPLICATA nov.
Definition
The most inclusive clade containing Semipallium, Jorgechlamys, Reticulochlamys and Swiftopecten, but not Zygochlamys or Dietotenhosen, and their descendants (stem-based).
Synapomorphies
Seven ribs on right anterior auricle; ribs on plicae of homogenous thickness.
Taxa included
Chokekenia Santelli & del Río 84; Laevichlamys Waller, 0106; Semipallium Jousseaume in Lamy, 57; Swiftopecten Hertlein, 43; Jorgechlamys del Río, 19; Reticulochlamys del Río, 19.
Occurrence
Early Oligocene (Se. foulcheri) to Recent.
Remarks
This clade is recovered in all implied-weighting searches. The Patagonian monotypic Chokekenia is nested within this clade for k = 24–100 searches, whereas when k = 1–23 it is placed outside from Pauciplicata. This clade is also supported by ambiguous synapomorphies: right-convex shells; eight plicae on right valve; nine on the left one; 4–7 ribs on right central plicae; 6–7 ribs on left central plicae; and 4–5 radial ribs on left central interspaces. These are ambiguous because Se. foulcheri and Se. hallae are grouped, among other synapomorphies, by having equiconvex shells and 2–3 ribs covering plicae on the left valve, and due to Swiftopecten having left-convex shells. Eight plicae on the right valve; nine on the left one; 4–7 ribs on right central plicae; and 4–5 ribs on left central interspaces are also ambiguous synapomorphies because the clade Swiftopecten + Jorgechlamys + Reticulochlamys is recognized by having 6 and 5 plicae on the right and left valves, 7–14 ribs on right plicae and 6–13 ribs on left interspaces, respectively. Nested within Pauciplicata, R. borjasensis and R. proximus are grouped by the synapomorphy (among others) of 7–11 ribs on the central plicae of the left valve instead of the 4–7 ribs that characterize the Pauciplicata clade, thus forming an ambiguous synapomorphy for Pauciplicata.
Discussion
This phylogenetic study provides a morphological matrix and suggests possible evolutionary relationships for the species of southern South America, focusing on a high number of extinct Chlamydini taxa. The only morphological matrix previously published was that of Waller (0107), which comprised only 14 characters, and was developed for the superfamily Pectinoidea. Therefore, it has many plesiomorphic characters that are ineffective for resolving the phylogenetic relationships of Chlamydini. Our data matrix was completely created anew and coded herein. Several considerations arose from the results outlined above and will be discussed as follows.
Many of the southern South American Chlamydini species, such as Z. geminata, Z. jorgensis, M. actinodes, M. aurorae, Cho. nicolasi, Pi. quemadensis, C. calderensis and D. hupeanus, were historically considered to be related to or assigned to Zygochlamys or Chlamys s.s. However, as proposed by Santelli & del Río (84, b), they belong in six different genera that are herein recovered as monophyletic groups supporting our previous taxonomic findings. These are Zygochlamys (Z. geminata, Z. jorgensis and Z. sebastiani), Moirechlamys (M. actinodes and M. aurorae), Ckaraosippur (C. calderensis and C. camachoi), Dietotenhosen (D. hupeanus and D. remondi), Pixiechlamys (Pi. quemadensis) and Chokekenia (Cho. nicolasi). The latter two genera were described as monotypic taxa (Santelli & del Río 84), which is also sustained by our present results.
This study allows us to test the hypotheses proposed by Beu (7) and Jonkers (53). We reject the hypothesis of Beu (7) that Ps. patagonica and D. hupeanus (= Chlamys vidali (Philippi, 73)) as closely related species; we find that D. hupeanus is unrelated to Ps. patagonica and Ps. delicatula. However, our results suggest that D. hupeanus is clustered with Moirechlamys actinodes, as suggested by Beu (7). Following Waller (0105), Beu (8) referred both M. actinodes and Ps. delicatula to Zygochlamys. However, our topologies reveal that Zygochlamys is composed of three species, while M. actinodes and Ps. delicatula belong in Moirechlamys and Psychrochlamys respectively, and that Ps. delicatula is unrelated to Zygochlamys; this is consistent with the results of Santelli & del Río (84). Finally, we reject the hypothesis of Jonkers (53) that stated that D. remondi (= Chlamys coquimbensis (Möricke, 65)) evolved from Z. geminata, because our analysis shows that Zygochlamys is a separate and more derived group than Dietotenhosen in all searches. Moreover, Dietotenhosen and Zygochlamys present notable morphological differences (as detailed by Santelli & del Río 85).
Because there are no previous morphological phylogenetic analyses, our results can only be compared to comprehensive molecular studies of Pectinidae. Our phylogenetic analysis is restricted to shell morphological traits of a limited taxonomic sample of 35 species within Chlamydinae (27 Chlamydini species), whereas the analyses of Alejandrino et al. (1) and Sherratt et al. (93) are based on a limited source of molecular information, in terms of the number of gene sequences and species (41 and 53 species of Chlamydinae in Alejandrino et al. (1) and Sherratt et al. (93), respectively). The current diversity of the subfamily Chlamydinae is much larger, being around 98 species (MolluscaBase 0202; see Pedinae). Moreover, neither molecular study tests the phylogenetic placement of the type species of the polyphyletic genus Talochlamys (T. pulleineana), which is a key taxon for a better understanding of this genus. Therefore, the specific diversity of Chlamydinae is under-represented in all precedent phylogenetic analyses, which is exacerbated by the technical limitations for including fossil species of Chlamydinae related to extant species in molecular analysis. Nonetheless, there are very interesting aspects in our results that can be compared with those achieved by molecular studies, despite the limitations in both methodologies.
Some interesting differences arise when comparing our findings with the studies of Alejandrino et al. (1) and Sherratt et al. (93), undoubtedly arising from the different sources of information, applied methodologies and taxa sampled.
The phylogenetic position of Swiftopecten is partially duplicated in our topologies for low values of k (k = 1–23) and the molecular phylogeny of Sherratt et al. (93). We resolved S. swiftii and S. iheringii within the Pauciplicata clade and related to Chlamys s.s., with the latter being basal to Pauciplicata (k = 1–23), while Sherratt et al. (93) found S. swiftii to be the sister group of Chlamys. However, our trees show Swiftopecten and Chlamys to be distantly related for high values of k (k = 24–100), differing from Sherratt et al. (93).
The present study indicates that extant species of South America that have traditionally been assigned to Zygochlamys (Ps. patagonica, Ps. delicatula) are unrelated to its type species (Z. geminata) and, therefore, to Zygochlamys, but that they belong in the circumpolar genus Psychrochlamys described by Jonkers (53). The latter genus has an unstable phylogenetic position in different modern molecular studies (mentioned as Zygochlamys) that also differs from our results. However, recent molecular evidence (Alejandrino et al. 1; Sherratt et al. 93) and our results agree that Psychrochlamys is monophyletic and that it is separated from Chlamydini. Our results also show that Psychrochlamys is basal to Aequipecten (k = 24–100) (Pectininae according to: Waller 2006b; Alejandrino et al. 1; Sherratt et al. 93; MolluscaBase 2021d) and therefore presumably a non-Chlamydinae taxon. Moreover, as we previously stated in Santelli & del Río (84, b), Psychrochlamys is morphologically different from Chlamydini in having orbicular shell with symmetrical and short auricles, with the free margin of anterior auricles sloping posteriorly, shallow byssal notches; shell sculptured with coarse commarginal lamellae extended over the entire surface, and lacking the shagreen microsculpture typical of Chlamydini. Those remarkable morphological differences are supported by molecular phylogenies in which Psychrochlamys is more closely related to Pectininae and Aequipectinini than to Chlamydini (Alejandrino et al. 1). A different placement is provided by Sherratt et al. (93), who found that the group containing Psychrochlamys is related to the lineage of Chlamydinae and could even be considered part of this subfamily, but always outside of Chlamydini. To summarize, morphological and recent molecular phylogenetic analyses reject the proposal of Waller (0106) that Psychrochlamys is related to Chlamys s.s. (i.e. Chlamydini). Furthermore, species currently assigned to Psychrochlamys are unrelated to D. hupeanus (= Chlamys vidali) and M. actinodes, contra Beu (7). Moreover, it is herein demonstrated that there is no close relationship between Psychrochlamys and any of the fossil Patagonian species traditionally considered to be members of Zygochlamys by Waller (0105), Beu (8), Beu & Darragh (9) and Dijkstra & Beu (28), among others. Given that the type species of Zygochlamys and its co-generic taxa are extinct, molecular studies cannot test the phylogenetic position of Zygochlamys nor its relationship with Psychrochlamys. For that reason, morphological phylogenies are also important to study the evolutionary relationships of the present diversity. It is a methodology that allows us to assess the taxonomic assignments with better accuracy as it is frequently supported by a systematic analysis (del Río 18, 19; Santelli & del Río 84, b). For example, Ps. patagonica is pseudo-replicated in the analysis of Alejandrino et al. (1) and Sherratt et al. (93), because the synonymous Z. amandi (synonymized with Ps. patagonica by Jonkers (53, p. 46) and accepted by Schejter & Bremec (87)) is sequenced separately. On the other hand, our morphological analysis is biased by the study of hard-shell structures (Alvarez 2, p. 686).
Preliminary findings that refer to non-southern South American taxa are detailed in Santelli et al. (86, appendix S8). Future studies can address the relationships of these groups that are barely outlined in the present contribution.
On the divergence time of South American Chlamydini
The evolutionary relationships of the tribe are still far from being completely understood. Nevertheless, the evidence presented here indicates that the earliest record of the tribe is followed by its diversification. Moreover, the topologies obtained show that Chlamys s.s. is basal to Semipallium in all searches and, because Se. foulcheri lived during the early Oligocene and middle Miocene (Beu & Darragh 9), this gives rise to many ghost lineages in groups with younger first occurrences. Most of the extinct Chlamydini analysed herein are from the Neogene. Perhaps, these ghost lineages are an artefact of the limited taxonomic sampling of the tribe Chlamydini being mainly restricted to Neogene southern South American taxa, hence omitting many older taxa. Therefore, our analysis probably fails to properly reconstruct the deeper nodes of these phylogenetic relationships. Therefore, the evolutionary history of the Chlamydini, and the relations of its lineages, are also poorly understood. Our results indicate that Jorgechlamys + Reticulochlamys is the clade with the shortest ghost lineage, and Zygochlamys has a short ghost lineage, with the oldest accepted record of Z. geminata in the upper Oligocene. The derived position of Semipallium reveals that the diversification of the tribe Chlamydini occurred early in its evolutionary history because it undoubtedly represents the oldest records of the tribe. It must be pointed out that there are earlier alleged representatives of the tribe mentioned in the literature, including lower or middle Eocene records of Serripecten Marwick, 60, Mesopeplum Iredale, 50, and Talochlamys (Feldmann & Maxwell 33; Beu & Darragh 9; Darragh & Kendrick 16). However, since the phylogenetic relationships of the fossil taxa of the first two were not studied, we are unable to discuss their affinities, and the third taxon is disregarded because it does not belong in Chlamydini according to our study (or those of Alejandrino et al. (1) and Sherratt et al. (93)). Because Beu & Darragh (9) described similarities between Serripecten and Psychrochlamys (referred by them to Z. delicatula), it is probable that the first does not belong in Chlamydini.
Our topologies indicate an earlier time of origin than the early Miocene suggested by Sherratt et al. (93) who, through molecular clock techniques, estimated that the diversification of Chlamys s.s. and Ch. hastata happened at c. 20 Ma. Since our results show that Chlamys s.s. is basal to Semipallium and Zygochlamys, Chlamys might have originated earlier than the early Miocene, although its oldest documented occurrence is late Pliocene. More comprehensive future phylogenies will lead to a better understanding of the relationships of the tribe and will probably yield a more precise origin time for the Chlamydini, especially if older taxa are incorporated. The study of the origin of Chlamydini will also benefit from the inclusion of type taxa and type species in phylogenetic analysis, particularly those of genera with uncertain affinities.
Conclusion
The Cenozoic Chlamydini of southern South American are separated herein into two clades that are usually recovered as monophyletic groups, named Multiplicata and Pauciplicata in reference to the number of plicae. The low number of plicae is recognized within the set of the synapomorphies that define Pauciplicata. Multiplicata and Pauciplicata are strongly consistent groups, each having slight variations in the internal relationships of the taxa within the different tree topologies recovered here. Pauciplicata is recovered in all topologies under implied weighting (although some tree topologies include unstable taxa, such as Chlamys and Laevichlamys). All southern South American genera are monophyletic, except for Jorgechlamys that is paraphyletic and nested within Reticulochlamys; for that reason, Jorgechlamys should be synonymized with Reticulochlamys. Zygochlamys is paraphyletic under very high values of k (k = 69–100). The sister and basal group to both clades (Multiplicata and Pauciplicata) is Pixiechlamys quemadensis, with Chlamys s.s. basal to all of them, for k = 24–100. Multiplicata includes Dietotenhosen, Ckaraosippur, Zygochlamys, Moirechlamys, Azumapecten and Chlamys hastata; Pauciplicata is represented by Chokekenia, Laevichlamys, Semipallium, Swiftopecten, Jorgechlamys and Reticulochlamys.
This study has shown that Zygochlamys spp., Moirechlamys actinodes and Dietotenhosen hupeanus are closely related in the same clade (Multiplicata) and that they belong in endemic and monophyletic southern South American genera. However, the latter two species neither belong in Zygochlamys nor are related to Z. patagonica (now referred to Psychrochlamys), as proposed by Beu (7, 8). Therefore, the present evidence does not support Beu's (7) hypothesis of a Miocene origin for the lineage of Ps. patagonica in South America nor its subsequent dispersion by the Antarctic Circumpolar Current (ACC) during Miocene or Pleistocene times.
Ckaraosippur and Dietotenhosen are the youngest survivors of the Tribe Chlamydini in the southernmost tip of South America.
Our calibrated topology allows us to confirm one of the most ancient records of a true member of the tribe (Semipallium foulcheri). It shows that the diversification of Chlamydini occurred early in the evolutionary history of the group, with many clades already diversified and separated at the boundary of the late Eocene and early Oligocene, such as Pauciplicata and Multiplicata. The early record of Semipallium generates multiple ghost lineages for all analysed clades and our results could reflect the lack of related taxa that were not incorporated in the analysis. Surely, an increased taxonomic sampling on future phylogenetic studies of Chlamydini will resolve many of the ghost lineages.
The present study shows that Azumapecten and Swiftopecten are related to Chlamys s.s., which is consistent with the relationships obtained by previous modern molecular phylogenies (Alejandrino et al. 1; Sherratt et al. 93). However, we found that Semipallium is also related to those taxa, contrary to the affinities suggested by molecular evidence (Alejandrino et al. 1; Sherratt et al. 93). Finally, Ps. patagonica and Ps. delicatula do not belong in Zygochlamys according to our study, and Psychrochlamys is definitely a non-Chlamydini taxon. Moreover, we question its placement in Chlamydinae. This outcome reinforces the hypotheses of Santelli & del Río (84, b) who proposed that the tribe Chlamydini became extinct in the southern South American region in early Pleistocene times.
A more representative sampling of the subfamily Chlamydinae could shed light on the subfamily and tribe arrangements, and its internal phylogenetic relationships. Moreover, the inclusion of additional extinct taxa in morphological analyses, as well as type species in molecular studies could contribute to clarify the evolutionary history of Chlamydini. The phylogenetic position and taxonomic status of Chlamydinae and Chlamydini within Pectinidae differ among modern molecular studies (Puslednik & Serb 76; Alejandrino et al. 1; Sherratt et al. 93), encouraging researchers to enlarge the taxonomic sampling to improve our understanding of the main lineages within Pectinidae.
Acknowledgements
The authors acknowledge with thanks to those curators and researchers who allowed us access to the specimens under them care: M. Longobucco (MACN-Pi, Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires, Argentina), M. Tanuz (CPBA, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina), A. Riccardi (MLP, Facultad de Ciencias Naturales y Museo, La Plata, Argentina), D. Rubilar (SGO.PI, Museo Nacional de Historia Natural de Chile, Santiago de Chile), C. Fernandes da Costa, (MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil), and C. Sarti (MGGC, Universidad de Bologna, Italy). We also thank W. Werner (SNSB-BSPG, Bayerische Staatssammlung für Paläontologie und Geologie, Germany), M. Terezow (GNS WM, Institute of Geological and Nuclear Science, New Zealand), A. Salvador and H. Taylor, (NHMUK, Natural History Museum, London, UK), V. Héros (MNHN, Muséum national d’Histoire naturelle, Paris, France) and K. Janneau (UNISTRA, Université de Strasbourg, France), for providing us with excellent photographic material. We are particularly indebted with O. Lehmann (MACN, Buenos Aires, Argentina), for the development of the script for multiple implied weighting searching: ttuiw.run. CONICET is acknowledged to promote funds this research work and the use of TNT software is facilitated by the Willi Hennig Society. This paper has been supported by PICT–ANPCyT 57 (Agencia Nacional de Promoción Científica y Técnica). This contribution has been greatly benefited from the careful reading of the manuscript and helpful comments made by J. Serb (ISU, Iowa, USA) and an anonymous reviewer, and by the editor M. Hautmann which have highly improved this work. We also thank M. B. von Baczko (MACN, Buenos Aires, Argentina) for her valuable time and help in polishing the manuscript language.
Open Research
Data archiving statement
This published work has been registered in ZooBank: http://zoobank.org/References/122AE4E3-FF79-4AC6-9185-E3E102D6754B.
Appendices for this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.0zpc866vd.




