A previously missing link in the evolution of dasytine soft-winged flower beetles from Cretaceous Charentese amber (Coleoptera, Melyridae)
Abstract
The soft-winged flower beetles are a diverse cosmopolitan family of cleroid beetles with an unresolved higher classification, the adults of which feed on nectar and pollen. The fossil record of Melyridae is sparse, with only two described fossils known from the Mesozoic. Here we describe the first representative of the family from the Mesozoic of Europe, from early Cenomanian Charentese (French) amber. Due to the opaque nature of French amber, X-ray microtomography was used to document fine morphological details of the fossil. Protodasytes cretaceus gen. et sp. nov. possesses a unique combination of characters within the subfamily Dasytinae, which is shared by the tribes Chaetomalachiini, Dasytini, and Listrini, namely the presence of a small antennal club, tarsomere 4 slightly shorter than 3 but not distinctly narrower, symmetrical claws, and a distinct pronotal and elytral fringe. This suggests that Dasytinae had begun to diversify by the Late Cretaceous, possibly in concert with the radiation of angiosperms. The fossil record of soft-winged flower beetles is critically reviewed, and a checklist of extinct species is provided. The fragmentary Cenozoic fossils Troglops punctatissima and Malachius vertummni are removed from the family Melyridae, and the record of Attalus lusitanicus from Eocene–Oligocene Bembridge Marls is regarded as unlikely. The potential role of Protodasytes gen. nov. and Cretaceous melyrids as pollinators of early angiosperms is discussed.
With more than 6000 described species placed in 300 genera and four subfamilies, the soft-winged flower beetles (Melyridae) are the most species-rich family of the diverse polyphagan superfamily Cleroidea (Mayor 45; Ślipiński et al. 61). The group more or less equivalent to modern Melyridae was historically treated as three to five separate families (e.g. Crowson 8, 9; Majer 40; Bocáková et al. 5), although modern molecular phylogenies indicate that it ought to be treated as a monophylum (e.g. Gimmel et al. 16). Due to their high diversity and convoluted taxonomic history, the higher classification of soft-winged flower beetles is still in its infancy (Mayor 45). The situation is complicated further by the scarcity of informative Mesozoic fossils that could contribute to the elucidation of the relationships among modern taxa. Only two Mesozoic melyrids have been described to date: the Middle Jurassic melyrine Sinomelyris praedecessor Kolibáč & Huang, 23 from near the Daohugou village in China, and the enigmatic dasytine Acanthocnemoides sukatshevae Zherikhin, 1002 from Albian Taimyr amber, which was formerly regarded as a member of Acanthocnemidae (Zherikhin 1002; Kolibáč & Huang 23; Kolibáč & Perkovsky 24). Melyrids do not become more abundant in the fossil record until the Cenozoic.
Dasytinae (treated as family Dasytidae in older works) is a group of mostly uniformly dark or metallically coloured melyrids with a distinct vestiture. They are regarded as the most diverse and least taxonomically understood subfamily of Melyridae (Majer 37). Dasytines can be recognized by their relatively well-sclerotized body, absence of extrusible glands, apical maxillary palpomere variable but mostly conical, elytra mostly completely covering the entire abdomen, legs and antennae rarely modified, basal tarsomere as long or longer than tarsomere 2 on all legs, claws variable but usually with ungual appendages, basal two ventrites free, and median lobe of aedeagus simple (Majer 41; Mayor 45). Larvae are found in leaf litter or rotting plant matter and are usually predaceous, although some are entirely herbivorous, predatory and herbivorous, or scavenge on small arthropods at some point during their development (Fiori 14; Kolibáč et al. 25). The adults visit flowers, often in large numbers, to feed on pollen and nectar. Due to their seemingly high host specificity, frequently high abundance, and strongly setose body, melyrids have been regarded as likely important pollinators of some angiosperms such as daisies (Asteraceae), legumes (Fabaceae), and mustards (Brassicaceae) (Mawdsley 44). Dasytines are distributed worldwide, although they are the most diverse in arid and semi-arid regions (Lawrence & Leschen 31). Approximately 1500 described species are placed into 50 genera (Mayor 45; Kolibáč et al. 25).
Here, we describe the first melyrid beetle from Cenomanian Charentese amber from the Charente-Maritime department in southwestern France. The fossil represents the earliest described member of Melyridae from the Mesozoic of Europe and documents the early diversity of the group in the Cretaceous.
Geological setting
Charentese amber is a collective name applied to late Albian and early Cenomanian fossil resin collected from several sites in southwestern France, namely in the Charente-Maritime and Charente departments. With more than 1500 arthropod inclusions discovered to date (Perrichot et al. 56, 57), including insects, arachnids, crustaceans and myriapods, it represents one of the most significant amber deposits from the Cretaceous of Europe. Around 40 well-preserved beetle fossils are known from the deposit, and a further 15 or so specimens are too fragmentary to be determined (Peris et al. 50).
The amber inclusion described herein originates from the Plage de la Vierge locality at the Fouras Peninsula, Charente-Maritime, which is a lignitic deposit at times rich in fossil wood and vertebrate remains within a glauconitic sand and clay. The locality is exposed only occasionally during low tides (Néraudeau et al. 47, fig. 1). The location and stratigraphy of the deposit have been discussed in detail in a number of previous publications, which have assigned the amber bed to the ‘lithological subunit B2ms’ (Néraudeau et al. 47; Perrichot 55; Perrichot et al. 57; Cockx et al. 7), or more recently to the unit ‘B2c/d’ based on palynomorph evidence (Peyrot et al. 58). Based on this latter publication, amber from Fouras is considered early Cenomanian in age (c. 96–100.5 Ma). Palaeontological and sedimentological studies indicate that the Fouras amber locality was an estuarine lagoon-like environment (Néraudeau et al. 47; Perrichot et al. 57). Coastal or riparian forests in the vicinity were dominated by gymnosperms (mostly Cheirolepidiaceae and Cupressaceae), abundant ferns, and rare aquatic angiosperms (Peyrot et al. 58).
To date, Fouras amber has yielded c. 110 organismic inclusions, mostly arthropods, including a beetle originally assigned to the family Trogossitidae (Peris et al. 50) and now considered to belong to Thymalidae (Peris 48), as well as a member of the family Thanerocleridae (Tihelka et al. 63). The fossil studied herein originates from a highly fossiliferous amber piece that holds 53 arthropods and three conifer fragments including the thaneroclerid beetle mentioned above, three other fragmentary beetle specimens, wasps belonging to the families Bethylidae and Sclerogibbidae (Cockx et al. 7; Perkovsky et al. 54), and conifer remains assigned to the genus Pagiophyllum Heer (Cheirolepidiaceae), which may have secreted the resin (Moreau et al. 46).
Material and method
Specimen preparation and photography
Given that the amber piece IGR.FRS-7 contained many fossils embedded within different amber flows, it was cut into several smaller fragments using a scalpel blade to enable easier observation of the inclusions. Each fragment containing at least one organic inclusion was embedded into mineralogical-grade epoxy Epotek 301-2 using a vacuum chamber. The resulting blocks were polished for an optimal view of the fossils. A total of 34 fossiliferous fragments were prepared and numbered IGR.FRS-7.1–7.34. The melyrid specimen described herein is preserved in fragment IGR.FRS-7.2 deposited in the collection of the Geological Department and Museum of the University of Rennes, France.
A Zeiss Discovery V20 stereomicroscope was used for observation and photography. Green epifluorescence microphotographs were taken using the Zeiss Axio Imager 2 light microscope in eGFP mode.
X-ray microtomography
Due to the generally quite opaque nature of Charentese amber, high-resolution X-ray microtomography (micro-CT) was used to reveal fine morphological detail of the fossil. The specimen was scanned using Zeiss Xradia 520 versa at the micro-CT laboratory of the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences in Nanjing, China. A CCD-based ×4 objective was used, which provides isotropic voxel sizes of 2.9478 μm with the help of geometric magnification. The acceleration voltage for the X-ray source was 50 kV. To improve signal-to-noise ratio, 3001 projections over 360° were collected, and the exposure time for each projection was 2 s. The tomographic data were analysed using AVIZO (v. 2019.01; gold-coloured reconstructions) and VG Studio (v. 3.0; Volume Graphics; silver-coloured reconstructions).
Systematic palaeontology
Order COLEOPTERA Linnaeus, 34Suborder POLYPHAGA Emery, 12Superfamily CLEROIDEA Latreille, 30Family MELYRIDAE Leach, 33Subfamily DASYTINAE Laporte, 29Tribe INCERTAE SEDISGenus PROTODASYTES nov.
LSID
urn:lsid:zoobank.org:act:2C7906D2-3968-440A-B3D5-70462AF4E303
Derivation of name
Generic name is a combination of Greek proto- (meaning ‘giving rise to’), and Dasytes Paykull, in reference to the putatively basal position of the new genus in relation to the tribe Dasytini.
Type species
Protodasytes cretaceus sp. nov.
Diagnosis
Body elongate, subcylindrical, setose. Pubescence moderately long, not decumbent, or scale-like. Clypeus and labrum transverse. Maxillary palpomere subovate. Eyes lacking interfacetal setae, moderately projecting. Antennae moniliform, somewhat serrate on inner side, with apical three segments slightly widened. Postocular portion of the head capsule half as long as the length of eyes. Frons with deeply incised and moderately arching longitudinal furrows laterally. Pronotum transverse, no wider than elytra in humeral portion, with submarginal grooves laterally, connected to a transverse furrow running along pronotal posterior margin. Distinct setose lateral fringe on pronotum and elytra present. Epipleura complete, widest at humeri. Tarsomeres on all legs subequally wide. Tarsomere 4 slightly shorter than 3 on all legs. Claws symmetrical, lacking free membranous appendages.
Protodasytes cretaceus sp. nov.Figures 1-3
LSID
urn:lsid:zoobank.org:act:C1A31868-5634-4FE8-9B8E-D97BFB1879D1
Derivation of name
The specific epithet is derived from the Latin creta (= chalk, chalky), in reference to the Cretaceous age of the fossil.
Holotype
IGR.FRS-7.2, sex undetermined.
Diagnosis
As for genus.
Description
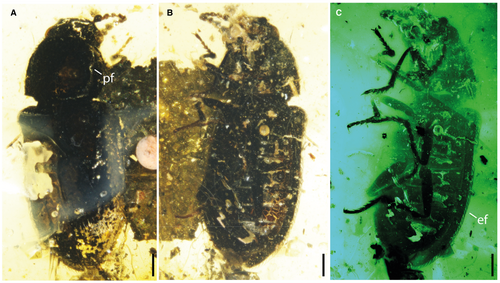
Body elongate and slender, moderately cylindrical, 2.29 mm long from clypeus to elytral apex, 0.88 mm wide across elytral humeri. All surfaces black, antennae and tarsi with dark brown tints. Pubescence moderately long, erect, densely scattered on the surface and margins of pronotum and elytra (Fig. 1).
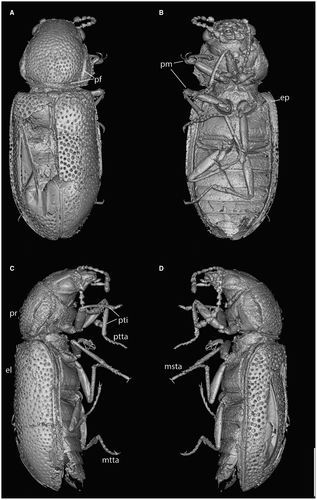
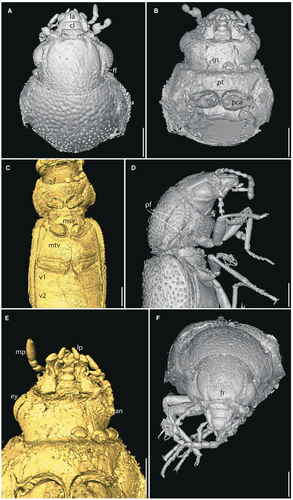
Head almost prognathous, not distinctively subrostrate, 0.37 mm long, 1.5-fold wider than long, broadest at eyes, which are as wide as the base of the pronotum (Fig. 2). Frontoclypeal suture visible. Clypeus trapezoid, wider than long, with a medial transverse furrow. Labrum transverse, subpentagonal, with a rounded apex. Mandibles moderately elongate (Fig. 3A, B). Maxillary palpi 4-segmented; basal palpomere short and narrow; palpomere 2, 2.5-fold longer than the preceding segment and about twice as broad apically; palpomere 3 barrel-shaped, approximately as long as wide; palpomere 4 as long as the preceding two segments together, elongate and subovate, broadest medially. Labial palpi 3-segmented, apical segment obclavate (Fig. 3E). Eyes large, moderately projecting, finely faceted, without interfacetal setae. Distance between eyes representing 2.6-fold the eye length. Antennal insertions visible from above, separated from eyes by 0.5-fold the eye length. Antennae 11-segmented, moniliform, somewhat serrate on inner side, with apical three segments slightly broadened and forming an indistinct club, reaching to pronotal–elytral junction, with short erect setae. Antennomere 1 bulbous, 1.2-fold longer than following segment; antennomeres 2, 3 elongate and subequal; antennomeres 4–8 somewhat triangular and slightly serrate on inner side, approximately subequal in length; antennomeres 9–11 slightly wider than preceding segments, 1.1-fold wide than the preceding segments and not distinctly serrate, gradually lengthening apically; antennomere 11 obclavate, 1.2-fold longer than the preceding segment. Postocular part of the head capsule half as long as the length of eyes. Frons and vertex flat, with deep circular and irregularly arranged punctures. Sides of frons next with deep moderately arching longitudinal furrows, running slightly longer than the length of eyes. Ventral side of head lacking subantennal grooves. Gular sutures well-separated.
Pronotum transverse and broadest medially, 0.22 mm long, 2.0-fold wider than long. Anterior margin of pronotum shallowly emarginate, with an indistinct elevation. Lateral margins not serrated, but with a densely setose fringe. Pronotal disc densely punctate with deep circular pits separated by no less than 3-fold their diameter. Each side of disc with a sharply delineated groove extending almost the entire length of the pronotum. Outer part of the furrow lying on the pronotal margin and the inner and deeper part found in the lateral height of the pronotal disc and connected to a transverse furrow running along the pronotal posterior margin (Figs 1A, 2A, 3D: pf), although this may be a CT-reconstruction artefact. Posterior margin straight, slightly elevated. Scutellar shield subtriangular, not abruptly elevated. Precoxal portion of prothorax 0.7-fold the maximum width of procoxae. Procoxae strongly transverse, projecting well below prosternum, narrowly separated, externally broadly open, separated by narrow and apically acute prosternal process reaching to the middle of the procoxae. Mesoventrite with mesocoxal cavities strongly transverse and sub-contiguous, separated by less than a quarter of their width. Metathorax with discrimen extending to over half the length of the segment. Postcoxal lines absent. Metacoxae narrowly separated, extending laterally to meet sides of body.
Legs slender and elongate. Protrochanters exposed. Femora broadest medially, with a shallow apical groove for the reception of tibiae. Tibiae of prothoracic legs moderately curved (Fig. 2C: pti), straight on meso- and metathoracic legs, presence and shape of tibial spurs cannot be assessed. Tarsi 5-segmented, slightly shorter than tibia, all tarsomeres subequally wide. Tarsomeres 1 and 2 subequally long, tarsomeres 3 and 4 gradually shortening, tarsomere 5 as long as the preceding two segments together (Figs 2, 3). Claws symmetrical, lacking free membranous appendages.
Elytra 1.70 mm long, 1.7-fold as long as their combined width, as wide as pronotum at its broadest point, parallel-sided, gradually narrowing in the posterior half, completely covering abdomen. Disc with deep and irregularly arranged circular pits, separated by more than twice their diameter. Suture bordered with an elevated ridge. Margin with densely setose fringe (Fig. 1C: ef). Epipleura complete, widest at humeri. Elytral apices meeting at suture.
Abdomen with 5 ventrites gradually shortening apically.
Remarks
Two phoretic mites (Oribatida: Brachypilina) are present on the pro- and mesothoracic legs of the holotype (Fig. 2B: pm).
Discussion
Systematic position of Protodasytes gen. nov.
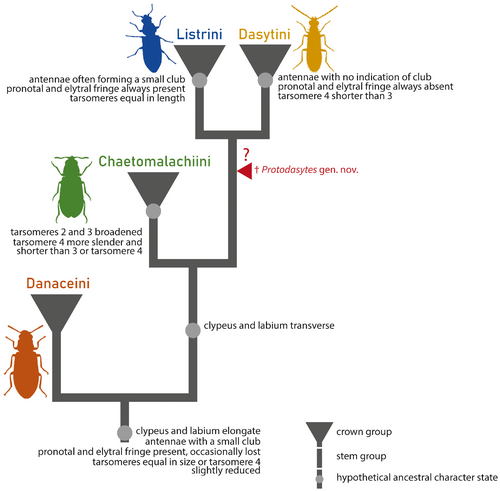
The fossil can be unambiguously assigned to the melyrid subfamily Dasytinae on the basis of the presence of a distinct clypeus; procoxae prominent, nearly contiguous, open posteriorly; body lacking obvious eversible vesicles; tarsomere 1 not shorter than tarsomere 2 on all legs; confused elytral punctation that does not form striae; and first two ventrites not connate (Mayor 45; Lawrence et al. 32; Gimmel et al. 16). In its current sense, the subfamily Dasytinae includes the following four tribes: Danaceini, Chaetomalachiini, Listrini and Dasytini (Gimmel et al. 16). Protodasytes gen. nov. possesses an unusual combination of characters that precludes an unambiguous placement into either of the modern tribes. It is excluded from Danaceini by its transverse clypeus and labrum and symmetrical claws. Placement in the tribe Chaetomalachiini is ruled out by tarsomeres 2–3 not distinctly broadened and segment 4 not distinctly slenderer than segment 3. It does not belong to Listrini, because its tarsomeres are not subequal in length and claws lack free membranous appendages. Although dasytines often have appendiculate claws, Protodasytes gen. nov. has symmetrical claws that lack extensive modifications but possess a basal tooth.
Protodasytes cretaceus gen. et sp. nov. is mostly similar to the tribe Dasytini in its serrate to moniliform antennae; eyes not pubescent; tarsomere 4 slightly shorter than tarsomere 3 on all legs but not distinctly narrower; claws symmetrical, with a basal tooth (Majer 37, 41; Mayor 45). However, unlike all modern Dasytini, Protodasytes gen. nov. has a distinct lateral fringe on the pronotum and elytra. Moreover, the indistinct antennal club of the new genus is unique within crown Dasytini. Both characters, the presence of a pronotal and elytral fringe and a small antennal club, are present in the tribes Danaceini, Chaetomalachiini and Listrini (Majer 38, 39). Given that in Majer's (41) cladogram, Listrini + Dasytini formed a clade, with Chaetomalachiini sister to both and Danaceini as the earliest diverging clade within the subfamily, both characters can be regarded as plesiomorphic. Given that it shares characters present in both Dasytini and Listrini, we regard Protodasytes gen. nov. as a ‘fossil link’ between the two tribes (Fig. 4). Majer (41) provided a list of morphological characters supporting the sister relationship between Dasytini and Listrini, and this relationship has also been recovered by molecular analyses based on four genes (Gimmel et al. 16). However, the same analysis also recovered a non-monophyletic Danaceini and Chaetomalachiini, and further recovered the tribe Amauronioidini as closely related. Deeper relationships between melyrid tribes vary between molecular studies that are based on only a few genes (Bocáková et al. 5), given that they may be susceptible to phylogenetic artefacts due to insufficient phylogenetic signal and the limits of the phylogenetic inference software (e.g. Young & Gillung 70; Tihelka et al. 64). Given the unresolved higher classification of Dasytinae, we choose to provisionally treat Protodasytes gen. nov. as tribe incertae sedis.
Fossil record of Melyridae
The earliest fossil melyrid is the Middle Jurassic Sinomelyris praedecessor from Daohugou Beds in north-eastern China. The fossil has been assigned to the subfamily Melyrinae mostly based on its habitus characters, but a more precise systematic placement was not possible (Kolibáč & Huang 23). The only other Mesozoic melyrid known to date is the Albian Acanthocnemoides sukatshevae from Siberian Taimyr amber. The fossil, known from two fragmentary specimens, has been originally assigned to the family Acanthocnemidae (Zherikhin 1002), but a recent re-examination of the type material supported its inclusion in the dasytine tribe Danaceini (Kolibáč & Perkovsky 24). Two undescribed specimens from Lower Cretaceous Lebanese amber, apparently belonging to Malachiinae and Dasytinae, were mentioned by Kirejtshuk & Azar (21). Further undescribed specimens from Albian Spanish amber were mentioned by Peris et al. (51). A single Albian–Cenomanian melyrid has been mentioned from Burmese amber in the collections of the Natural History Museum in London (Rasnitsyn & Ross 60). This makes P. cretaceus gen. et sp. nov. the earliest described member of Melyridae from the Mesozoic of Europe.
Soft-winged flower beetles are more abundant in the Cenozoic fossil record. They are known from Eocene Baltic and Rovno ambers (Spahr 62; Hieke & Pietrzeniuk 20). Majer (42) reviewed the melyrid fauna of Baltic amber, and since then several new species have been described (Kubisz 26; Tshernyshev 66, 67). Two malachiine species are known from Eocene Oise amber (Kirejtshuk & Nel 22). The recent species Attalus lusitanicus Erichson, 1000 reported from Eocene–Oligocene Bembridge Marls is herein removed from the family and treated as Polyphaga incertae sedis, given that it is represented by a single poorly preserved specimen lacking the important diagnostic characters that would support its assignment to this particular species. Fossils described from the late Eocene Florissant Formation in Colorado are all in need of revision (Wickham 68, 69; Mawdsley 43), given that many are very fragmentary. Troglops punctatissima Théobald in Piton & Théobald, 1935 was described from the late Oligocene of Puy-de-Dôme, France, based on only a single elytron without any informative diagnostic characters (Piton & Théobald 59), and as such it is excluded from the family and treated as Polyphaga incertae sedis. Malachius vertummni Heer, 19 from the Miocene of Oeningen, Germany (Heer 19) is excluded from Melyridae due to the lack of diagnostic characters and is treated as Polyphaga incertae sedis. Subfossil soft-winged flower beetles are also known from Quaternary sediments (e.g. Elias 10; Elias et al. 11), these specimens are not included in the overview of the described fossil Melyridae species given in Table 1.
| Species | Subfamily | Tribe | Deposit | Age |
|---|---|---|---|---|
| JURASSIC | ||||
| Sinomelyris praedecessor Kolibáč & Huang, 23 | Melyrinae | incertae sedis | Daohugou Beds, Haifanggou Formation, northeast China | Callovian |
| CRETACEOUS | ||||
| Acanthocnemoides sukatshevae Zherikhin, 1002 | Dasytinae | Danaceini | Taimyr amber, Taimyr Peninsula, Begichev Formation, Far North Russia | Albian |
| Protodasytes cretaceus gen. et sp. nov. | Dasytinae | incertae sedis | Charentese amber, Fouras Peninsula, southwestern France | Cenomanian |
| EOCENE | ||||
| Colotes constantini Kirejtshuk & Nel, 22 | Malachiinae | Colotini | Oise amber, Le Quesnoy, northern France | Ypresian |
| Colotes impexus Kirejtshuk & Nel, 22 | Malachiinae | Colotini | Oise amber, Le Quesnoy, northern France | Ypresian |
| Colotes sambicus Kubisz, 26 | Malachiinae | Colotini | Baltic amber, Baltic Sea coast | Priabonian |
| Dasytes ochraceus Tshernyshev, 67 | Dasytinae | Dasytini | Baltic amber, Baltic Sea coast | Priabonian |
| Palpattalus baltiensis Tshernyshev, 66 | Malachiinae | Attalini | Baltic amber, Baltic Sea coast | Priabonian |
| Palpattalus eocenicus Tshernyshev, 66 | Malachiinae | Attalini | Baltic amber, Baltic Sea coast | Priabonian |
| Protocephaloncus perkovskyi Tshernyshev, 66 | Malachiinae | Troglopini | Rovno amber, Ukryantar factory material, Ukraine | Priabonian |
| Collops desuetus Wickham, 69 | Malachiinae | Malachiini | Florissant Formation, Colorado, USA | Priabonian |
| Collops extrusus Wickham, 69 | Malachiinae | Malachiini | Florissant Formation, Colorado, USA | Priabonian |
| Collops priscus Wickham, 69 | Malachiinae | Malachiini | Florissant Formation, Colorado, USA | Priabonian |
| Malachius immurus Wickham, 1001 | Malachiinae | Malachiini | ||
| Eudasytites listriformis Wickham, 68 | Dasytinae | incertae sedis | Florissant Formation, Colorado, USA | Priabonian |
| Eutrichopleurus miocenus Wickham, 68 | Dasytinae | Listrini | Florissant Formation, Colorado, USA | Priabonian |
- Quaternary subfossils and undescribed material are omitted from the checklist.
Soft-winged flower beetles: potential pollinators of early angiosperms?
The earliest unequivocal fossil angiosperms date back to the Early Cretaceous (Friis et al. 15). Their diversification during the mid-Cretaceous, when they replaced the previously dominant gymnosperms (Benton 4), is believed to have drastically altered food chains and led to the diversification of many terrestrial animal lineages in an event known as the Cretaceous Terrestrial Revolution (Lloyd et al. 36). Together with thrips, lacewings, flies, and scorpionflies (Labandeira et al. 27, 28), beetles were among the first pollinators of gymnosperms during the Mesozoic (Cai et al. 6; Liu et al. 35). Beetles associated with pollen are known from Cretaceous ambers (Bao et al. 3; Peris et al. 53; Peris & Jelínek 49), and it has been suggested that the diversification of angiosperms may have contributed to the radiation of phytophagous beetles (Farrell 13; Ahrens et al. 1).
Adult soft-winged flower beetles are believed to be exclusively pollinivorous and nectarivorous, although predation and scavenging has been reported as well (Mayor 45). In summer, they can be collected in large numbers on the inflorescences of diverse angiosperm groups such as daisies, legumes, and mustards. as well as on some gymnosperms such as pines. Mawdsley (44) listed 68 genera of plants belonging to 26 families from North America visited by dasytines. Large quantities of pollen that adhere to the beetle's setose elytra and abdomen are transported between flowers during the course of normal feeding (Hawkeswood 18). At least two Nearctic members of the phlox family Polemoniaceae depend primarily on pollination by soft-winged flower beetles (Grant & Grant 17), and dasytines have also been demonstrated to contribute to the pollination of sunflowers in South Africa (du Toit 65). The body of Protodasytes gen. nov. is densely setose and possesses deep grooves, which are frequently found in pollinating beetles (Cai et al. 6). The occurrence of diverse monocots and eudicots at Fouras (Peyrot et al. 58) suggests that like its modern relatives, P. cretaceus gen. et sp. nov. may have visited and pollinated early angiosperms in the Cenomanian of western Europe. Further specimens associated with pollen would be required to directly confirm this association. At the very least, P. cretaceus gen. et sp. nov. provides an important calibration point for the Mesozoic diversification of dasytines.
Conclusion
The diversification of angiosperms in the Aptian–Albian is believed to have propelled many lineages of phytophagous beetles to their present-day diversity (Farrell 13; Ahrens et al. 1; Peris et al. 52). Dasytine soft-winged flower beetles are a diverse group of flower-visiting cleroid beetles that feed on nectar as well as angiosperm and gymnosperm pollen (Mawdsley 44). Here we describe the third Mesozoic dasytine beetle, from Cenomanian (c. 96–100.5 Ma) Charentese amber from the Plage de la Vierge locality in the Fouras Peninsula, southwestern France, representing the first record of the family in the Cretaceous of Europe. Protodasytes cretaceus gen. et sp. nov. possesses an unusual combination of characters that exclude it from the crown group of all four dasytine tribes and most likely belonged to the stem of the lineage Listrini + Dasytini. As such, P. cretaceus gen. et sp. nov. provides evidence that pollinivorous dasytines began to diversify by the Late Cretaceous. The fossil provides an important calibration point for testing the co-diversification between beetles and angiosperms in the Cretaceous.
Acknowledgements
We are indebted to Prof. Didier Néraudeau (University of Rennes), who collected and gave access to the amber piece containing the specimen described herein. We also thank David Peris (University of Bonn) for the preliminary identification of beetles in the amber piece studied herein. Our thoughts go to Katya Sidorchuk (1981–2019), who identified the phoretic mites. Financial support for field studies and collection of Charentese amber was provided by the French National Research Agency grant BLAN07-1-184190 (project AMBRACE to D. Néraudeau), and CNRS-INSU grant Interrvie (project NOVAMBRE 2 to D. Néraudeau and V. Perrichot). Support for the present study was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (41672011 and 41688103), and the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706). We are grateful to Dr Yan Fang and Ms Su-Ping Wu for technical help with microtomography. We thank Matthew Gimmel and two anonymous reviewers for their valuable comments.
Open Research
Data archiving statement
This published work and the nomenclatural acts it contains, have been registered in ZooBank: http://zoobank.org/References/3E78BC27-0411-48E7-BE08-D15608EB1073. Micro-CT scans (full-resolution image stack in .tiff format and model in STL format) are available from Mendeley Data: https://doi.org/10.17632/zwrcvtjn6n.1.




