Crystallographic Pathways to Tailoring Metal-Insulator Transition through Oxygen Transport in VO2
Abstract
The metal–insulator (MI) transition of vanadium dioxide (VO2) is effectively modulated by oxygen vacancies, which decrease the transition temperature and insulating resistance. Oxygen vacancies in thin films can be driven by oxygen transport using electrochemical potential. This study delves into the role of crystallographic channels in VO2 in facilitating oxygen transport and the subsequent tuning of electrical properties. A model system is designed with two types of VO2 thin films: (100)- and (001)-oriented, where channels align parallel and perpendicular to the surface, respectively. Growing an oxygen-deficient TiO2 layer on these VO2 films prompted oxygen transport from VO2 to TiO2. Notably, in (001)-VO2 film, where oxygen ions move along the open channels, the oxygen migration deepens the depleted region beyond that in (100)-VO2, leading to more pronounced changes in metal-insulator transition behaviors. The findings emphasize the importance of understanding the intrinsic crystal structure, such as channel pathways, in controlling ionic defects and customizing electrical properties for applications.
1 Introduction
Oxygen defects are crucial in developing functionalities in functional oxide materials. Specifically, oxygen point defects, or oxygen vacancies, often enhance the useful electric or magnetic properties of these materials.[1-4] This enhancement is attributed to their role in inducing local lattice distortion and charge doping, which significantly impacts the electronic structure of correlated oxides. Therefore, controlling the formation of oxygen vacancies is essential for designing highly functional oxide materials. The manipulation of oxygen vacancies in oxide film is commonly achieved through oxygen ionic transports, driven by the electrochemical potential. For example, researchers have used external electric fields or annealing under low oxygen partial pressure (pO2) to move oxygen ions out of the oxide film.[5-9] More recently, unidirectional oxygen transport has been promoted through heterojunction interfaces by assembling another oxide with different oxygen chemical potentials. Oxygen ions diffuse from an oxide film move to an adjacent growing oxide layer to equilibrate the oxygen chemical potentials, forming oxygen vacancies at the initial oxide film.[10-12] For example, the carrier concentration in LaNiO3 film was regulated by the migration of oxygen to growing top oxide layers,[10] and in LaTiO3 film, oxygen migration from a SrTiO3 substrate formed an oxygen-deficient conducting layer with high carrier mobility at the substrate.[11, 12] Therefore, understanding of oxygen ion dynamics offers new avenues for tailoring the electrical properties of oxide films, potentially leading to breakthroughs in oxide-based electronics. For example, studies have reported that the types[10] and the growth conditions[13] of the adjacent oxide layer can control oxygen transport and the formation of oxygen vacancies in the initial oxide film.
One of the important and intrinsic factors to consider in ionic transport in oxide thin film is the crystallographic orientation of the oxide. Vanadium dioxide (VO2) is such a system where the movement of certain interstitial ions, such as hydrogen and lithium,[14, 15] is reported to be facilitated toward (001) orientation due to its unique anisotropic channel structure along the rutile c-axis. However, research on how these channels influence the movements of oxygen ions, which is the intrinsic defects in oxide, is limited. VO2 is a typical system where electric switching characteristics are effectively controlled by oxygen vacancies.[13, 16-23] VO2 undergoes a dramatic metal-insulator (MI) transition ≈ 340 K accompanied by a structural transition, which rapidly changes from a monoclinic-insulating state at low temperatures (T < 340 K) to a rutile-metallic state at high temperatures (T > 340 K).[24, 25] Oxygen vacancies in VO2 play a crucial role in reducing the transition temperature and the resistance of the insulating state (RON/ROFF ratio) by stabilizing its rutile-metallic state even in low temperatures, a process termed “metallization”.[16, 20, 21] This phenomenon has spurred extensive research into the control of oxygen vacancies in VO2 for applications in switching and sensing devices.[26-29] Consequently, an in-depth investigation is essential to comprehend how VO2’s unique channel structure affects oxygen transport and the formation of oxygen vacancies within VO2 films. Experimentally, Jeong et al. and Altendorf et al. have shown the facet dependency of ionic liquid gating effect, with gate-induced metallization being effective in (001)- or (101)-VO2 film but having minimal effect on (100)- or (110)-VO2 films.[17, 30] However, the precise role of the open channels in oxygen transport and the resulting formation of oxygen vacancies in VO2 remains unexplored.
In this study, we designed a model system of TiO2/VO2 films with distinct orientations, (100)-VO2 and (001)-VO2, where the [001] channel aligns perpendicularly and parallelly to the surface direction, respectively (please note that all orientations are based on the rutile structure). Oxygen ions migrate from VO2 to the top TiO2 layer due to the chemical potential mismatch, traversing across the channels in anti-channeling (100)-VO2 films, while moving along the channels in channeling (001)-VO2 films. Following the oxygen transport, we performed atomic-scale characterization of VO2 using scanning transmission electron microscopy (STEM) combined with electron energy loss spectroscopy (EELS). This approach enabled us to compare the formation of oxygen vacancies in both (100)- and (001)-VO2 films despite their ultrathin structure (≈13 nm). Consequently, our research emphasizes the crystallographic orientation of VO2 films (i.e., the direction of structural open channels) as a crucial factor that affects the formation of oxygen vacancies through ionic transport and, as a result, modulation of the MI transition properties of VO2 films.
2 Results and Discussion
2.1 Design of Model Systems for VO2 Films with Two Crystal Orientations
To investigate the influence of the crystal orientation of the VO2 film in the oxygen ionic transport out from the film, we explored an appropriate model system by selecting representative crystal orientations expected to exhibit the most distinct differences in ionic transport (Figure 1). Ion movement in anisotropic tetragonal VO2 based on the rutile structure is most preferred toward [001] direction, along which empty channels are aligned, as shown in Figure 1a.[14, 17, 19] Thus, as depicted in Figure 1b, we prepared model samples of the anti-channeling (100)- and channeling (001)-VO2 films with the [001] channels parallel (left) and perpendicular (right) to the surface, respectively. To induce oxygen ionic transport from an oxide, another oxide with an identical crystal structure but different oxygen chemical potential was epitaxially grown on the oxide.[10-13, 23] In VO2, Park et al. and Lu et al. reported that when TiO2 is epitaxially grown on VO2 film under an oxygen-deficient environment, stoichiometric TiO2 can be grown by absorbing the oxygen ions from VO2, consequently transferring the oxygen vacancies to VO2 layer.[13, 23] Based on previous reports, we suggest the TiO2 top layers on the (100)- and (001)-VO2 films as model systems.
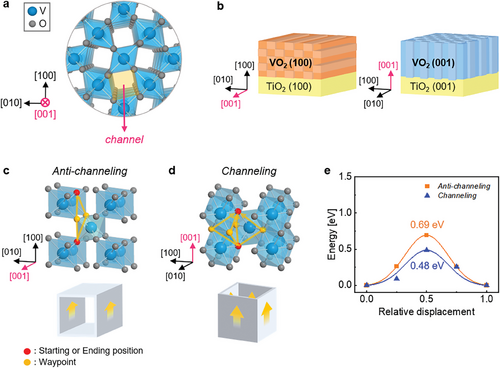
Utilizing atomic structure models and first-principles calculations (Figure 1c,d), we predicted the differences in oxygen migration behaviors in each VO2 film model sample. Oxygen migration occurs via oxygen vacancies in the lattice due to an oxygen-deficient environment during the top TiO2 layer's growth. We considered oxygen migration between planes oriented toward the film surface. In (100)-VO2, oxygen migrates to the same position on the subsequent (100) plane through the two nearest oxygen sites, as depicted in Figure 1c. In contrast, in (001)-VO2, oxygen moves through the four nearest oxygen sites to reach an identical position on the next (001) plane, doubling the path compared to (100)-VO2, as depicted in Figure 1d. This difference in migration paths is closely linked to the channel orientation. Oxygen vacancies travel along the edges of the VO6 octahedral chain, i.e., channel walls. The (100)-VO2 allows movement along only two channel walls perpendicular to the surface direction, as shown at the bottom of Figure 1c, while (001)-VO2 involves all four channel walls, as shown at the bottom of Figure 1d. Therefore, channeling (001)-VO2 facilitates oxygen migration with a higher probability than anti-channeling (100)-VO2. Additionally, we compared the oxygen migration barriers in each direction of VO2 thin films using first-principles calculations, as shown in Figure 1e. The VO2 structure was optimized considering its form as a thin film on TiO2 substrates (i.e., clamping on TiO2 substrates). The oxygen migration barrier in channeling (001)-VO2 is significantly lower at 0.48 compared to 0.69 eV in anti-channeling (100)-VO2. A lower migration barrier implies a substantially higher probability of migration. Therefore, given the crystallographic anisotropy of VO2, oxygen migration from VO2 to the top TiO2 layer is notably more efficient in channeling (001)-VO2 than anti-channeling (100)-VO2.
2.2 Epitaxial Growth of VO2 Films and Top TiO2 Layers for Oxygen Transport
To realize the model samples as mentioned in Figure 2, we prepared 13-nm-thick VO2 films on single-crystalline rutile (100)- and (001)-TiO2 substrates by pulsed laser deposition (PLD). The VO2 thickness of 13 nm is the optimal condition to prevent microstructural defects by strain relaxation[31-33] or interdiffusion from substrates[33, 34] that deteriorate VO2 characteristics, allowing us to focus on the effect of the crystallographic channel inside VO2 on oxygen transport. In the symmetrical X-ray diffraction (XRD) (Figure 2a,b), the TiO2 substrate peaks (denoted by closed arrow) are sharp and intense, while the VO2 film peaks (denoted by open arrow) are broadened due to their finite thickness.[35] The VO2 films exhibit a (200) peak with a TiO2 (200) peak in Figure 2a, and a (002) peak with a TiO2 (002) peak in Figure 2b, indicating that the intended (100)- and (001)-VO2 films were easily fabricated by changing the surface orientation of the TiO2 substrate. The layer fringes around the VO2 peak in both two films demonstrate that the VO2 films have very sharp interfaces and a uniform thickness of ≈13 nm, as estimated from the fringe period.
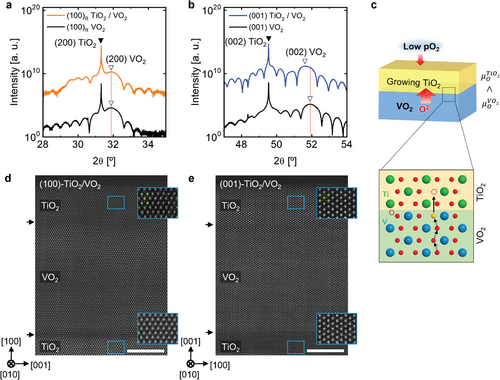
Subsequently, ≈7-nm-thick TiO2 layers were grown on the as-grown (100)- and (001)-VO2 films under a sufficiently low pO2 ≈8 mTorr to cause the oxygen transport from VO2 to TiO2 as depicted in Figure 2c.[13, 23] During this interfacial reaction step, the oxygen vacancies should be inevitably generated in VO2 near the interface region, and thus the oxygen ions inside the VO2 film diffuse to the interface region due to the gradient of the oxygen concentration. Interestingly, the c lattice parameter of VO2 increased by ≈0.6% in the case of TiO2 on the (001)-VO2 film, which indicates the favorable formation of oxygen vacancies in VO2, because the lattice expansion is one of the representative phenomena caused by the oxygen vacancies in VO2.[13, 17, 21]
The crystal orientation and crystallinity of the top TiO2 on the (100)- and (001)-VO2 films are shown in Figure 2c,d. The high-angle annular dark-field (HAADF)-STEM images demonstrate the perfect crystallinity in both TiO2 on the (100)- and (001)-VO2 films, as confirmed by the well-aligned Ti atom columns in their TiO2. It is quite intriguing that both TiO2 layers are epitaxially grown well with identical structures and orientations of their rutile TiO2 substrates because the TiO2 layers were grown at 150 °C, which is considerably lower than the conventional growth temperature of 400–500 °C.[19, 36] These epitaxial growths of 7-nm-thick TiO2 at a low temperature indicate that both (100)- and (001)-VO2 films supply oxygen ions to the top TiO2, as hinted by the previous reports.[13, 23] However, considering the different lattice expansion results in the two VO2 films in XRD after TiO2 growth, there is a difference in the degree of oxygen transport between the (100)- and (001)- VO2 films.
2.3 Local Lattice Strain Analysis on the VO2 Films using STEM
To investigate the lattice expansion inside the (100)- and (001)-VO2 films in more detail, we visualized the local lattice strain maps obtained by the geometric phase analysis (GPA; HREM Research Inc., Japan) of HAADF-STEM images (Figure 3).[37, 38] Here, only the lattice strains along the out-of-plane direction (z) are mentioned because the in-plane lattice parameters negligibly change after the oxygen transport owing to the tight constraint by interaction with their substrates (Figure S1, Supporting Information). The lattice spacing along the z-direction of the VO2 films was calculated with respect to the TiO2 substrates and lattice mismatch expressed by the lattice strain (i.e., ɛzz = (z − zsubstrate)/zsubstrate),[38] as shown in Figure 3c,d. Unlike the (001)-VO2 film, where the lattice strain is expressed by the green color in the entire VO2 film, the (100)-VO2 film exhibits a bimodal distribution, indicated by the red and green colors.
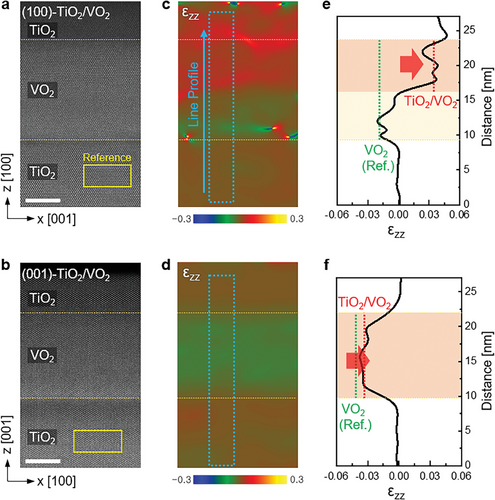
To quantitatively confirm the local lattice expansion, the values of ɛzz were extracted and profiled, as shown in Figure 3e,f. The actual strain can be elucidated by comparing the measured ɛzz (red dotted lines in e and f) and reference ɛzz (green dotted lines in e and f). The reference ɛzz was calculated by the lattice spacing of bulk VO2 using the Poisson's ratio (Supplementary 1). In (100)-VO2 film, the measured ɛzz at the lower part of VO2 is consistent with the reference ɛz, whereas the measured ɛzz at the upper part is higher (0.035) than that of the reference ɛzz (−0.017) indicated by the red arrow in Figure 3e, which corresponds to the lattice expansion of ≈5.3%. Alternatively, in the (001)-VO2 film, the measured ɛzz is homogeneous and higher (−0.035) than that of the reference ɛzz (−0.043), implying the lattice expansion of ≈0.8% in whole areas of the (001)-VO2 film. As the lattice expansion is mainly caused by the oxygen vacancies, the results of the strain analysis indicate that the oxygen vacancies in the (100)-VO2 film are preferentially formed at the upper region of VO2 near the interface, whereas the oxygen vacancies in the (001)-VO2 film are formed across the entire regions of VO2. The different distribution of the oxygen vacancies in the (100)- and (001)-VO2 films is consistent with the first-principles calculations shown in Figure 1, in which the oxygen ions are predicted to migrate more efficiently in the (001)-VO2 film than in the (100)-VO2 film.
2.4 Local STEM-EELS Analysis on the VO2 Films and TiO2 Layers
We verified the role of oxygen vacancies in the lattice expansion of the VO2 films. To confirm the distribution of oxygen vacancies in (100)- and (001)-VO2 films, we measured V L3,2- and O K-edge EELS by line scanning through the cross-sectional positions of the VO2 films, as shown in Figure 4a,b. In the 3d transition metal oxides, the L-edge of the transition metal and K-edge of oxygen obtained by EELS are known to be sensitive to oxygen vacancies, which change the chemical bonding states and reduce the valence states of the multivalent transition metals (i.e., from V4+ to V3+ in VO2).[39-41]
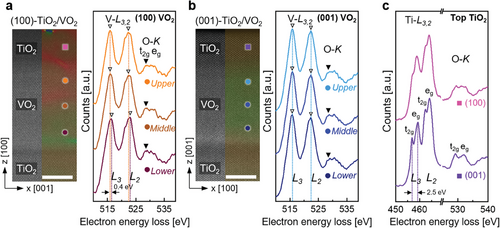
In the EELS results from (100)-VO2 (Figure 4a), different features are shown at the upper and middle parts compared to the lower part of the VO2 film. First, compared to the lower part, the V L3,2-edges of the upper and middle parts of VO2 shifted to the left by ≈0.4 eV (dotted lines in a, from 516.4 eV at the lower part to 516.0 eV at the upper and middle parts in the L3-edges), indicating the weaker binding energy with the introduction of oxygen vacancies.[39, 42] The increases of the L3/L2 intensity ratio in the upper and middle parts (open arrows “▽” in a, from 0.98 in the lower part to 1.02 in the upper and middle parts) depict the phenomena induced by the decreased oxidation state of V4+ to V3+ due to the oxygen vacancies.[42-44] Based on the V–O hybridization in VO2, the O K-edge spectra can also describe the changes in the oxidation state of the V ions.[43, 45] In the O K-edge, divided into the first t2g peak and second eg peak by crystal field splitting, the first t2g peak (closed arrows) at the upper and middle parts of VO2 is suppressed, compared to that of the lower part, which implies the decreased oxidation state of vanadium owing to the oxygen vacancies.[42, 43, 45] Therefore, in the (100)-VO2 film, oxygen vacancies are distributed in the upper half region, which proves that oxygen transport to the top TiO2 occurs only in the top local region of the (100)-VO2 film, where oxygen moves across the [001] channel which is parallel to the surface. In contrast, the (001)-VO2 film exhibits homogenous characteristics in its EELS spectra over the entire film region, as shown in Figure 4b. All V L3,2-edges in the entire area shifted to the left by ≈0.5 eV (dotted lines in b, 515.9 eV in L3-edge) with an increase in the L3/L2 intensity ratios (1.02–1.03) compared to the lower part of the (100)-VO2 film. Moreover, the first t2g peak of the O K-edges are suppressed at the entire VO2 area. For a detailed investigation of the non-stoichiometries in the VO2-x films after oxygen transport, the contents of oxygen vacancies were estimated to be x ≈ 0.002 and 0.13 in the lower and upper regions of (100)-VO2 film, respectively, and x ≈ 0.16 and 0.17 in the lower and upper regions of (001)-VO2 film, respectively. These estimates were obtained using a model-based quantification method on the EELS spectra (Figure S3, Supporting Information). These results indicate that oxygen vacancies were uniformly formed across the entire (001)-VO2 film and in greater quantities compared to the (100)-VO2 film, where vacancies were formed only in the upper region.
Therefore, in the (001)-VO2 film, where oxygens travel along the [001] channel which is perpendicular to the surface, oxygen vacancies are distributed throughout the entire VO2 film. The enhanced depth of the oxygen-deficient region in the (001)-VO2 film than that in the (100)-VO2 film confirms that the oxygen transport to the top TiO2 is facilitated along the [001] channel direction by the relatively favorable oxygen vacancy migration.
We also measured Ti L3,2-edge and O K-edge EELS on the top TiO2 layers of the (100)- and (001)-VO2 films to verify the different oxygen supplies from their bottom VO2, as shown in Figure 4c. In the top TiO2 on (001)-VO2 film (bottom of c), the Ti L3,2-edges and O K-edges are clearly split into two peaks (t2g and eg) due to the crystal field splitting of Ti 3d from the surrounding oxygens, and the energy splitting between the t2g and eg peaks in the Ti L3-edge (dotted lines in c) is 2.5 eV, which is consistent with the stoichiometric TiO2 reported by Grunes et al.[46] These results indicates that the TiO2 layer has a good stoichiometry with the Ti4+ ions despite the low pO2 of ≈8 mTorr owing to the sufficient oxygen transport from the entire region of the bottom VO2 film. In contrast, for the TiO2 on the (100)-VO2 film (top of c), the t2g–eg splits in Ti L3,2- and O K-edges almost disappeared, which directly confirms that the Ti3+ ions are ascribed to the oxygen vacancies.[47, 48] Additionally, XPS analysis, which is suitable for characterizing the chemical state of surface layers (up to a depth of ≈5 nm), also confirmed that the contribution of Ti3+ spectra is higher in (100)-TiO2/VO2 than in (001)-TiO2/VO2 (Figure S4, Supporting Information), similar to the EELS results. The TiO2 on the (100)-VO2 film exhibited good crystallinity in the HAADF-STEM images, as shown in Figure 2a, however, in reality, the (100) top TiO2 is nonstoichiometric owing to the oxygen vacancies because the (100)-VO2 film could not provide sufficient oxygen ions from the upper half of the VO2 film. Therefore, we directly determined the oxygen vacancies distribution in the local region of the VO2 film and top TiO2 through lattice strain and EELS analysis, which visualizes the differential oxygen transport between the (100)- and (001)-VO2 films. As predicted in Figure 1, oxygen transport was more effective when the [001] channel was perpendicular to the surface.
2.5 Differential tuning of Metal–Insulator Transitions in VO2 Films
To confirm the tuning of the MI transition properties of the (100)- and (001)-VO2 films after oxygen transport, we measured the temperature-dependent sheet resistance (Rs vs T) of the VO2 films before and after the growth of the top TiO2 layers, as shown in Figure 5a,b. In TiO2/VO2 films, the top TiO2 thin film has a negligible effect on the measurement of sheet resistance of VO2 film (Figure S5, Supporting Information) due to its much higher resistivity[19, 49] and high leakage current[50, 51] in its very thin thickness of 7 nm. Both the as-grown (100)- and (001)-VO2 films exhibit large resistance variations across three and four orders of magnitude, even at different transition temperatures owing to their strain from their substrates (Figures S1 and S2, Supporting Information).[13, 14, 16, 52] After growing the top TiO2 layers, different MI transition behaviors are noted on the (100)- and (001)-VO2 films (red arrows in a and b). In particular, the Rs at the insulating state decreased by an order in the (100)-VO2 film, whereas it decreased by three orders of magnitude in the (001)-VO2 film. Additionally, the transition temperature, based on the heating curve, decreases by 9 K (from 334 to 325 K) in the (100)-VO2 film, whereas it significantly decreases by 104 K (from 294 to 190 K) in the (001)-VO2 film. The decrease in the resistance of the insulating state and transition temperature of the VO2 films are ascribed to the oxygen vacancy that stabilized VO2 in the metallic–rutile phase.[16, 20, 21] Oxygen vacancies introduce extra electrons and destabilize the V-V dimerization of the insulating monoclinic phase, leading to the transition into the metallic rutile phase. We observed that the oxygen-deficient upper half region of the (100)-VO2 film was stabilized to the rutile structure, whereas the lower region has a monoclinic structure, as shown in Figure S6 (Supporting Information). This suggests the different changes of the MI transition in the (100)- and (001)-VO2 films owing to the different contents and distribution of the oxygen vacancies in VO2, as confirmed by the previous GPA and EELS analyses illustrated in Figure 5c,d. The MI transition of the (100)-VO2 is minimally suppressed because the local upper part of the film is metalized, whereas that of (001)-VO2 film is fully suppressed because the entire region of the film is metalized.
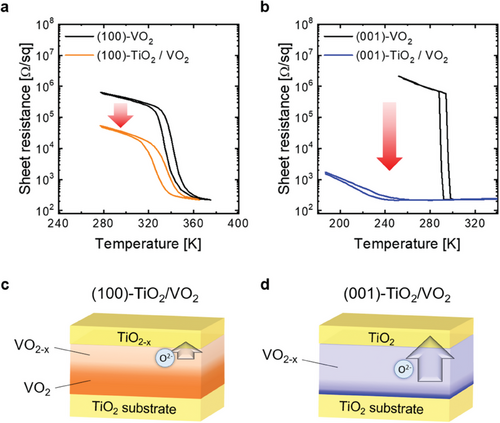
As other well-known possible factors for the changes in the MI transition properties, we also considered elastic strain changes and Ti doping after top TiO2 growth. However, RSM results confirmed that the in-plane strain state remained unchanged before and after TiO2 growth due to the rigid substrate (Figure S1, Supporting Information). Additionally, Ti doping would increase the transition temperature and resistance, which is the opposite of our observed changes.[53] Therefore, these factors are minor in the metallization of VO2 films after oxygen transport. Additionally, we considered the possibility of Magnéli phases VnO2n-1, which possess a lower oxygen stoichiometric ratio than VO2, as a cause for metallization. Given the estimated oxygen vacancy content in our VO2 films, there is a possibility of a phase transition to VnO2n-1 (5 ≤ n ≤ 8). However, in our study, the temperature and duration during the oxygen vacancy formation were very low at 150 °C and less than 10 min, respectively, which kinetically hinders the structural transformation to Magnéli phases.[20, 54] Z. Zhang et al.[20] annealed the insulating monoclinic VO2 at low temperatures of 450 °C with low pO2 and demonstrated that the annealed VO2 transformed into oxygen-deficient VO2-δ with δ ≈ 0.2, but did not transform into Magnéli phases despite having very similar stoichiometry to our cases. This is because the structural transition to Magnéli phases requires sufficient time and high temperature to order oxygen vacancies over long ranges. Therefore, we believe that the most reasonable factor causing changes in the electrical properties of both VO2 films is the formation of oxygen vacancies and their stabilization of the metallic rutile phase as we proposed. The differences in the distribution of these vacancies led to the different changes in the MI transition in the (100)- and (001)-VO2 films.
3 Conclusion
The metal-insulator transition of VO2 has been effectively modulated by introducing ionic defects within the lattice. Especially for the interstitial ions (e.g., hydrogen or lithium) or intrinsic ions (e.g., oxygen vacancies), techniques for ionic insertion or extraction using electrochemical potential from outside the film have been actively researched. Recently, the crystallographic orientation of VO2 films has been reported to significantly influence the ionic transport in VO2. However, previous studies are limited as they predominantly focus on comparing the role of the film's crystal orientation on the tuning of its electrical properties and lack a thorough investigation into the formation of internal ion defects. Consequently, the exploration into the inherent crystallographic channel structure of VO2 has been limited. Our research on the model system with two orientations of VO2 thin films, anti-channeling (100)-VO2 and channeling (001)-VO2, provides the fundamental understanding of the influence of the anisotropic channel pathway on defect formation within the film and tuning of the electrical switching performance by ionic transport.
In summary, we prepared two types of VO2 films based on their crystallographic channel structures and compared the oxygen transport and oxygen defect formations using STEM. First-principles calculation predicted the preferred oxygen migration in along the [001] channel in (001)-VO2 rather than across the channel in (100)-VO2. Local strain and EELS analysis using STEM not only confirmed oxygen migration from VO2 to top TiO2 but also demonstrated that the channel structure influences the distribution of the defect formation; oxygen vacancies were observed only in the upper half of (100)-VO2, while they distributed throughout the entire area in (001)-VO2 film. Consequently, the differential defect formation between the two orientations of VO2 films impacted the tuning of electrical switching properties. The (001)-VO2 films, where oxygen travels along the channels, showed a significant decrease of transition temperature and insulating resistance. We demonstrated that the crystallographic orientation of the VO2 film can serve as an important parameter when tuning the MI transition property of VO2 by greatly affecting oxygen transport. Our approach within VO2 films holds promise for broader applications in other oxide films with diverse anisotropic crystal structures, which could provide meaningful implications for the development of various electronic devices.
4 Experimental Section
First-Principles Calculation
First-principles density functional theory (DFT) calculations were performed using the Projector Augmented Wave (PAW) method and the generalized gradient approximation of Perdew, Burke, and Ernzerhof (PBE) for the exchange-correlation potential as implemented in the Vienna Ab-initio Simulation Package (VASP) code. The Brillouin zone integration used periodic boundary conditions and Monkhorst-Pack k-point sampling with a Г-centered k-point grid of up to 8 × 8 × 8. An energy cutoff of 450 eV was used for the plane-wave representation of the wavefunctions, and the 3s electrons of V and Ti ions were considered valence electrons. A Hubbard U correction term was applied to the V (U = 3.25 eV) and Ti (U = 3.00 eV) to properly reproduce the strong on-site Coulombic repulsion of 3d-electrons. The VO2 structures were optimized as thin films on TiO2 substrates. The (001)-VO2 matches lattice constants of the a- and b-axis to TiO2 and releases stress of the c-axis. The (100)-VO2 matches a lattice constant of the b-axis to TiO2 and releases stresses of the a- and c-axis. Migration barriers were calculated using the climbing image nudged elastic band (CI-NEB) method, implemented in the VASP transition state theory (VTST) code linked to the VASP code. Three images were utilized to represent intermediate configurations between the fixed endpoints, and a spring constant of 5 eV Å−2 was set between images.
Synthesis of VO2 Films and TiO2/VO2 Films
Both (001) and (100) TiO2/VO2 films were grown on (001) and (100) TiO2 substrates (Shinkosha CO., LTD) using pulsed laser deposition (PLD). Prior to film growth, polycrystalline TiO2 and V2O5 targets were prepared by sintering processes at 1100 °C and 620 °C, respectively, for 12 h. To grow high-quality heterostructures, TiO2 substrates were loaded into a high-vacuum chamber and evacuated to reach a base pressure of 1E-6 Torr. Subsequently, the V2O5 target was ablated using a KrF excimer laser (Coherent Compex Pro 102 F, λ = 248 nm) with a fluence of 1 J cm−2, a repetition rate of 1 Hz, and a target-to-substrate distance of 7 cm, at 300 °C and ≈ 12 mTorr. After growing the VO2 films, rapid quenching was performed to cool them down to 150 °C within 2–3 min. Once the substrate temperature reached 150 °C, the subsequent TiO2 films were grown on the VO2-buffered TiO2 substrates with a fluence of 1 J cm−2, repetition rate of 2 Hz, and distance of 7 cm under ≈ 8 mTorr at Tg ≈ 150 °C. Following the growth of heterostructures, the samples were cooled down to room temperature at a cooling rate of 20 °C min−1. To obtain high-quality VO2 films, the VO2 films were cooled down to room temperature without additional TiO2 growth process.
Structural Characterization
To characterize the information of out-of-plane lattice parameters in the VO2 and TiO2/VO2 heterostructures, symmetric 2θ-ω scans were performed using synchrotron radiation at 3D XRS (λ = 0.12 398 nm, energy = 10 KeV) beamline of the Pohang Light Source- ΙΙ (PLS-ΙΙ, Pohang, Republic of Korea). For the atomic-scale STEM and EELS analyses, cross-sectional TEM lamella samples of (100)- and (001)-TiO2/VO2 film on TiO2 substrate were prepared by mechanical polishing to a thickness of less than 10 µm. The polished samples were ion-milled using Ar+ ion beam (PIPS II, Gatan Inc.) at 3.5 keV for thinning and at low voltage (≈0.1 keV) for removing the surface damaged layers. The STEM imaging was performed using JEM-ARM200F (JEOL Ltd.) at Materials Imaging & Analysis Center of POSTECH, which equipped with a 5th order aberration corrector (ASCOR, CEOS GmbH) for forming 0.7 Å probe. The accelerating voltage and the convergent semi-angle of the beam are 200 kV and 28 mrad. The collection semi-angles were 54 to 216 mrad for high-angle annular dark-field (HAADF) imaging. The obtained raw images were radial difference filtered to remove the background noise (Filters Lite, HREM Research Inc.). The lattice strain maps are obtained by conducting geometrical phase analysis (GPA) from high-resolution HAADF-STEM images using GPA plug-in (HREM Research Inc.) implemented in the Digital Micrograph (Gatan Inc.). To determine a 2-D strain map, two of phase images should be calculated by selecting two non-parallel reciprocal lattice vectors from the Power Spectrum of the image. The angle of the x-axis and z-axis to the horizontal were defined as 0 and 90 ° in images. The reference regions were defined at the (100) and (001) TiO2 substrate regions whose lattice parameters are known. The STEM-EELS analysis was performed using JEM-2100F TEM (JEOL Ltd.) equipped with a spherical aberration corrector (CEOS GmbH). EEL spectra were obtained using an EEL spectrometer (Gatan GIF Quantum ER) at 200 kV with an energy resolution of 0.8 eV. The energy dispersion was 0.1 eV per channel with 2048 channels. The background spectra were removed in front of V L3,2-edge in VO2 and Ti L3,2- edge in TiO2 using the power-law method (DigitalMicrograph, Gatan Inc.)
Electrical Characterization
The temperature-dependent sheet resistance of both VO2 films and TiO2/VO2 heterostructures was measured using a Hall measurement system. For measuring sheet resistance, van der Pauw geometry was employed on square samples with dimensions of 5 × 5 mm2. Indium contacts were placed at the corners of the samples. The probing current ranged from 10 nA to 1 mA.
Acknowledgements
H.S. and K.Y.D. contributed equally to this work. This work was supported by the National Research Foundation (NRF) grant funded by the Korea government(MIST) (RS-2024-00355591), the National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (RS-2023-00258227), and the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (RS-2023-00257666). Y.P. was sponsored by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. K. Song acknowledges the work at the Korea Institute of Materials Science by the Fundamental Research Program of the Korea Institute of Materials Science (grant no. PNKA170).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




