Rapid, Tough, and Trigger-Detachable Hydrogel Adhesion Enabled by Formation of Nanoparticles In Situ
Abstract
Integrating hydrogel with other materials is always challenging due to the low mass content of hydrogels and the abundance of water at the interfaces. Adhesion through nanoparticles offers characteristics such as ease of use, reversibility, and universality, but still grapples with challenges like weak bonding. Here, a simple yet powerful strategy using the formation of nanoparticles in situ is reported, establishing strong interfacial adhesion between various hydrogels and substrates including elastomers, plastics, and biological tissue, even under wet conditions. The strong interfacial bonding can be formed in a short time (60 s), and gradually strengthened to 902 J m−2 adhesion energy within an hour. The interfacial layer's construction involves chain entanglement and other non-covalent interactions like coordination and hydrogen bonding. Unlike the permanent bonding seen in most synthetic adhesives, these nanoparticle adhesives can be efficiently triggered for removal by acidic solutions. The simplicity of the precursor diffusion and precipitation process in creating the interfacial layer ensures broad applicability to different substrates and nanoparticle adhesives without compromising robustness. The tough adhesion provided by nanoparticles allows the hydrogel-elastomer hybrid to function as a triboelectric nanogenerator (TENG), facilitating reliable electrical signal generation and output performance due to the robust interface.
1 Introduction
Hydrogels comprising of either natural or synthetic polymer networks with high volume fraction of water, have garnered interest toward extensive applications across various domains, ranging from commodity products to medical devices,[1] energy, and other engineering fields owing to their extraordinary characteristics including flexibility, biocompatibility, and permeability. One critical challenge in applying hydrogels is integrating them with other materials (e.g., other hydrogels, elastomers, tissues, plastics, metals, or glass), which is a key step to unlock the full range of established and emerging applications.[2-8]
The presence of water, which constitutes the majority of the hydrogel mass content, poses a substantial obstacle in integrating other materials on the surface. Water at the interface does not contribute to hydrogel adhesion and can impede intimate contact between the polymer network and the adherend. Therefore, bonding diversesubstances to water-rich hydrogels relies on the use of the chemical or physical properties of the hydrogel network. Existing bonding approaches have thus shown effective adhesion between hydrogels and various adherends through tailoring the chemistry of hydrogel networks. Examples of these include introducing a bridging molecule carrying reactive groups into the hydrogel network and the adhered surface,[9] synthesizing hydrogels with specific functional groups (e.g., carboxylic acid groups) that can form interface-bridging covalent bonds to living tissues,[10] or modifying the bulk hydrogel backbone with functional groups like catechol or nucleobases to impart excellent self-adhesiveness.[11-13] Despite their efficacy, they are restricted to specific functional groups from both the hydrogel and the adherend, and inevitably involve energy-intensive operations, as well as harsh processing conditions such as anoxic reaction environment.[14]
Another strategy that eliminates the requirement for specific functional groups in both the hydrogel and the adherend makes use of physically topological polymer entanglement.[15-17] This approach typically entails the introduction of stitching polymers into both the hydrogel and adherend matrices, followed by triggering the crosslinking of these stitching polymers to form a third network. The new polymer network acts as a suture, seamlessly stitching the two existing networks together at the molecular level. Some examples of stitching polymers utilized in this method include chitosan, cellulose, poly(4-aminostyrene), and alginate. Nevertheless, this process is typically slow to reach equilibrium, which is undesirable for time-sensitive applications. Additionally, this process is limited in the selection of third networks if two adherends are quite dissimilar in their compatibility and solubility, such as hydrogel-elastomer.
Although numerous strategies have realized strong bonds between hydrogels and various adherends, a fast yet versatile method to achieve robust hydrogel adhesion to various substrates has yet to be conceived of. Moreover, most reported adhesion methods are generally incompatible with both wet and dry surfaces[10, 18] and would create a permanent bond between hydrogels and other surfaces, which often causes a secondary damage during detachment, and severely hampers their integration into “smart” materials or into more flexible devices.[19-21]
Among various strategies toward hydrogel adhesion, the use of nanoparticles is facile, generalizable, repeatable, and applicable to wet surfaces such as biological tissues. The current state-of-the-art methods in using nanoparticles to this end, however, have scarcely reported strong adhesion.[22-25] This limitation may arise from not fully exploiting the scope of non-covalent interactions and the absence of topological entanglement between nanoparticles and polymer networks in hydrogels. Nanoparticles with both strong chemical bonds and in topological entanglement with the hydrogel networks will significantly improve their adhesion performance.
To this end, we report an interfacial precipitation-driven adhesion strategy to build a robust interface between hydrogels and adherends using nanoparticles formed in situ, such as ZIF-L (zeolite imidazole framework-L) nanosheets (Figure 1). We hypothesized that upon contact, the metal ions and ligands would approach the interface from opposite directions, forming coordination bonds, followed by an instantaneous homogeneous nucleation and subsequent growth into a dense layer of immobile nanoparticles. This dense layer would be localized at the interface between the two adherends, where the nanosheets can topologically entangle with the pre-existing polymer chains and form non-covalent bonds (coordination or hydrogen bonds) with the functional groups in the adherends. Moreover, taking advantage of the acid instability of the ZIF-L crystals would allow a pH-triggered detachment of the hydrogel-adherent hybrids. With this strategy, rapid, tough, and reversible adhesion between hydrogels and diverse substrates could be achieved, both in dry and wet conditions.
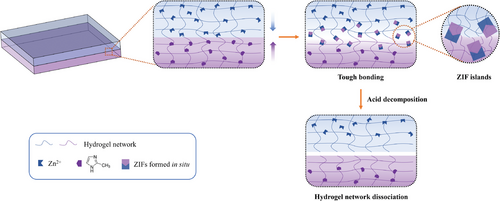
Furthermore, since the proposed strategy does not rely on specific types of functional groups from either the hydrogel or adherends, it is generally applicable to diverse hydrogels and substrates such as elastomers, plastics for surgically implantable devices, and even biological tissue. Additionally, we found that the interfacial nanoparticle was not specific to ZIF-L. The interfacially precipitated layer could also be formed by a variety of pH-sensitive nanoparticles assembled via coordination bonds, including various MOFs (metal–organic frameworks, that is, ZIF-L-Co, Tb2(BDC)3 (BDC: 1,4-benzenedicarboxylate)) and layered double hydroxide (LDH). The adhesion energy we can achieve is up to 902 J m−2. As a proof-of-concept, the hydrogel–elastomer hybrids with robust interfaces were employed in triboelectric nanogenerators (TENG). The enhanced and stable energy harvesting performance in this system corroborates the promise of our technology.
2 Results and Discussion
2.1 Fabrication and Structural Characterization of Hydrogel-Adherend Hybrids
The hydrogel-adherend hybrids were prepared by a simple soaking method. As an illustrative example, the adhesion of two Agar/polyacrylamide (Agar/PAAm) hydrogels using ZIF-L nanosheets formed in situ was demonstrated. The hydrogels were separately impregnated in aqueous solutions of Zn(NO3)2·6H2O and 2-methylimidazole (2-MeIm) for a certain time period (normally 10 min). This allowed for the diffusion of Zn2+ and 2-MeIm into the hydrogels to a certain depth, enabling them to interact with the hydrogel networks through attractive interactions such as hydrogen bonding or coordination. It is noted that the soaking process caused slight swelling of hydrogels (≈25% volume increase for Zn2+ solution and 8% for 2-MeIm solution), but no obvious degradation on their mechanical property was observed (Figure S1, Supporting Information). Subsequently, the two hydrogels were attached and compressed (Figure 1). The metal ions and ligands diffused into the counterpart hydrogel driven by their concentration gradients and formed the coordination bonds, which would be preceded by an instantaneous homogeneous nucleation and subsequently precipitation to form a layer of immobile ZIF crystals that topologically entangle with the polymer networks of both hydrogels. Chemical bonding on the interface (e.g., hydrogen bonding between hydroxyl groups in Agar and imidazole in ZIF particles) and ZIFs’ topological entanglement with the hydrogel networks led to the strong adhesion between two hydrogels. It is worth noting that the metal ion and ligand cannot be used directly as an adhesive without impregnation since ZIF crystals can be formed in a very short time period, which inhibits the penetration of precursors into the hydrogels and consequently prevents their interactions and topological entanglement with hydrogel networks.
Figure 2a shows the front view of two stacked hydrogels (with a dimension of 20 × 10 × 3 mm3) loaded with Zn2+ and 2-MeIm, respectively. After compression for a certain duration, the interface between two hydrogels turned from transparent to opaque in seconds, eventually becoming fully opaque white within 10 min, indicating the rapid formation of particles. These white particles were later verified to be ZIF-L crystals (vide infra). The side view and cross-sectional image (Figure 2b) revealed that ZIF-L nanosheets were mineralized in situ on the interface of the two hydrogels. The bright field optical microscope image (Figure 2c) clearly showed that the two hydrogels tightly adhered with an interfacial layer (dark area) of a 420 µm thickness. The cross-sections of the adhered hydrogels were further visualized by scanning electron microscopy (SEM) to investigate the structure and morphology of the interface (Figure 2d). Along the contacting surfaces of two hydrogels, a dense ZIF crystal layer (55 µm) was formed, which comprises 2D leaf-like ZIF-L nanoflakes (Figure 2f) with lateral dimensions of 2.5 ± 0.5 µm length and 1.0 ± 0.3 µm width (measured on intact particles) corroborating the tough and conformal interfacial adhesion. Away from this layer, there are hydrogel layers loaded with metal ion and ligand wherein ZIF crystals are dispersed much less densely among their matrices together with ≈10 µm pores in their freeze-dried state (Figure 2e,g). It is worth noting that the apparent ZIF-L thickness (dark area) measured by optical microscopy (Figure 2c) is much larger than that from SEM (420 µm vs 55 µm) due to ZIF nanoparticles being dispersed in a much greater depth into the hydrogel matrices with a gradient distribution, and less nanoparticles while farther away from the interface. Additionally, due to the inherent self-completing nature of interfacial growth, the free space between two hydrogels derived from rough hydrogel surfaces was gradually filled over time. This is attributed to the faster diffusion of the ZIF precursors through the free spaces compared to other parts in hydrogels. As a result, new crystallites were primarily formed at the free spaces, thereby facilitating in situ repair of film defects and resulting in a uniform film.
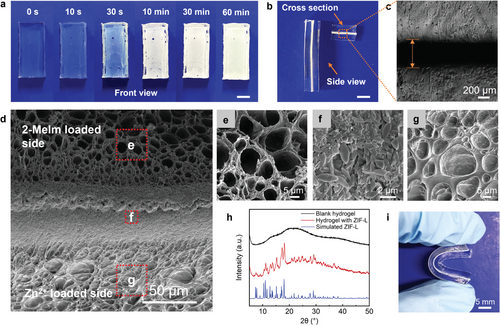
The crystals were confirmed to be ZIF-L by X-ray diffraction (XRD) (Figure 2h). The discrete, broad diffraction peak centered at 2θ = 20.9° (top, Figure 2h) is associated with the amorphous nature of the polymeric matrix of Agar/PAAm hydrogel. In contrast, the diffraction peaks with 2θ values of ≈7.3°, 10.9°, 12.7°, 15.1°, 17.2°, and 18.0° corresponded to the (110), (200), (211), (220), (310), and (222) planes of ZIF-L crystals (middle, Figure 2h), respectively, by comparing with the simulated spectrum of ZIF-L crystal (bottom, Figure 2h). This demonstrated effective in situ formation of ZIF-L crystals at the hydrogel interface.[26-28] It is noted that the flexibility is not compromised by the rigid ZIF-L crystals owing to the low mass fraction and small size of ZIF-L. The adhered hydrogels maintained their original softness and pliability to withstand mechanical deformations, such as repeated bending (Figure 2i).
Interestingly, we observed that, regardless of the synthetic conditions or hydrogel substrates, the Zn2+ loaded hydrogel consistently developed a thicker ZIF-L layer than 2-MeIm loaded hydrogel at the interfaces (35 µm vs 20 µm in Figure 2d), even though the concentration of 2-MeIm in the soaking solution is much higher than that of Zn2+ (3.8 m vs 0.38 m). We hypothesized that the diffusion of Zn2+ from one hydrogel matrix to another is slower than that of 2-MeIm due to their stronger interaction with polymer chains in hydrogel network, such as coordination of Zn2+ with hydroxyl functionalities in Agar or amides in PAAm. This can be demonstrated by the following mechanistic analysis.
First, the formation of ZIFs–hydrogel adhered interface was evidenced by structural analysis using SEM energy dispersive X-ray spectroscopy (SEM-EDS). SEM-EDS measurement was performed on the cross-section of two adhered polyvinyl alcohol (PVA) hydrogels instead of Agar/PAAm hydrogel to avoid the interference of nitrogen element from PAAm (Figure S2a, Supporting Information). Figure S2b (Supporting Information) depicts distinct element distributions of nitrogen (N), oxygen (O), and zinc (Zn) in the selected area. The strong signal of N is evenly distributed across two hydrogels, indicating 2-MeIm as the N source is free to diffuse from the loaded gel through the interface and further into Zn2+ loaded gel (Figure S2c,d, Supporting Information). However, in the case of Zn2+ (Figure S2e, Supporting Information), a strong zinc signal was observed only in the Zn2+ loaded hydrogel side while being almost excluded from ligand loaded hydrogel side, indicating zinc cations are reluctant to diffuse, likely due to coordination with the hydroxyl functionalities in PVA chains. To further track the precursors’ diffusion and interfacial nucleation associated precipitated layers, the elemental composition in each hydrogel layer was obtained by semi-quantitative EDS analysis. While defining atomic percentage of oxygen from PVA as a standard that is constant with the addition of precursors, the distribution of the ligands and metal ions could be inferred from the signal ratios of N/O and Zn/O in the respective hydrogels. In the 2-MeIm loaded hydrogel, the N/O and Zn/O ratios were ≈0.066 and 0.017, respectively (Figure S2f, Supporting Information). In contrast, the N/O and Zn/O ratios in Zn2+ loaded hydrogel were much higher, with the values of ≈0.300 and ≈0.280, respectively (Figure S2g, Supporting Information). This disparity is consistent with our hypothesis that 2-MeIm can diffuse much faster than Zn2+ ions within the hydrogel network. The difference between Zn and N distribution provided mechanistic insight into ZIF-L adlayer formation. The Zn2+ ions are unable to diffuse across the hydrogel interface as fast as 2-MeIm, which may prevent rapid dendrite growth caused by unregulated ion transport and thereby promoting homogeneous Zn nucleation and in situ crystal growth within hydrogel polymer network.
2.2 Adhesion Performance of Hydrogel Hybrids
We next optimized the hydrogel adhesion performance against a few variables. We found that two hydrogels being adhered after contacting for 60 s were strong enough to tolerate repeated swaying back and forth (Movie S1, Supporting Information). Standard 180° peeling adhesion tests were performed to quantify the adhesion energy (Figure 3a). Adhesion energy is defined as doubling plateau peeling force divided by sample width (2Fplateau/W).[18] Figure 3b presents the curves of peeling test of adhered hydrogels that indicated the change of adhesion energy as a function of curing time. The ZIF-L crystals were formed immediately after we compressed two precursor-loaded hydrogels together. The adhesion energy increased as the ZIF-L generation progressed, established to ≈ 73 J m−2 within 60 s, which might be strong enough for many practical applications. As the curing time increased, more metal ions and ligands diffused across hydrogel surfaces, forming thicker spatially confined ZIF-L layer and resulting in gradually enhanced interface bonding. After 60 min curing, the precursors in the hydrogels reacted sufficiently to approach a saturation point, giving an adhesion energy of ≈504 J m−2.
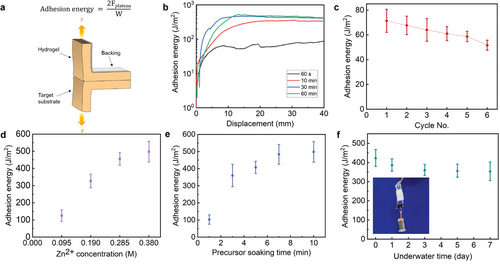
This result indicates that the ZIF-L adhesion exhibited a rapid increase in adhesion energy over the curing time. In this way, a fast adhesion can form at the initial stage and gradually build toward an ultrastrong adhesion over hours. This unique process provides an opportunity to integrate simultaneously strong and rapid adhesion with a fail-safe timing mechanism to prevent mistargeted adhesion, which would prove particularly advantageous in surgical operations since most surgeries are operated on dynamic tissue surfaces, making the risk of mispositioning adhesives unavoidable.[29] To test the fail-safe adhesion, we conducted cyclic attachment/detachment tests over a short timescale (60 s) for each cycle in one soaking. It is notable that the hydrogels can be repositioned multiple times without showing an obvious decrease in binding strength due to sufficient remaining precursors. The adhesion energy was ≈73 J m−2 for the first cycle, decreased slightly to 86% after 3 cycles, and 71% after 6 cycles (Figure 3c). Adhesion control at different timescales has the potential to aid clinical translation, as the initial adhesion is strong enough to hold tissues or hydrogels yet still has a time window for repositioning.[30] Based on these results, considering performance comparison and reproducibility, the bonded hydrogels with 60 min contacting time were selected for subsequent studies.
To demonstrate that the strong adhesion originated from in situ formation of ZIF-L, control experiments were conducted to rule out the contributions of Zn2+ and 2-MeIm individually to the overall adhesion strength. A pristine hydrogel was tested to adhere with the hydrogel loaded with Zn2+ or 2-MeIm, respectively. Meanwhile, another control experiment by applying a pre-synthesized ZIF-L suspension as an adhesive to two pieces of pristine hydrogel was also performed. In all control experiments, the adhered hydrogel samples peeled easily along the interface, with a low adhesion energy of < 10 J m−2, too weak for most application scenarios. The above results emphasized the critical role of the in situ growth of ZIFs in anchoring the hydrogel polymer networks as nanoparticle islands as well as enhancing interfacial adhesion. Additionally, in situations where soaking the hydrogel substrates in precursor solutions is not viable, an alternative method is direct surface casting to attain the desired result. Specifically, solutions of Zn2+ (0.38 m) and 2-MeIm precursor (3.8 m) were directly casted to the surfaces of two Agar/PAAm hydrogels for 10 min. After excess precursor solutions were removed, the two hydrogels were brought into contact and compressed for 60 min. Subsequent measurement of the adhesion energy indicated a comparability (472 J m−2, Figure S3, Supporting Information) to that achieved through the soaking strategy (504 J m−2).
Having established the rapid and robust adhesion of the ZIF-L layer, we reasoned that whether the adhesion strength varies with the amount of ZIF-L, that is, the thickness of ZIF-L layer formed in situ. Therefore, we decided to investigate two variables to change the adhesion performance: precursor soaking time and precursor concentration. The corresponding adhesion energies were measured by peeling tests, which were shown in Figure 3d (precursor Zn2+ concentration) and Figure 3e (precursors soaking time) for a 60 min curing time on Agar/PAAm hydrogels. While varying one parameter, the other remained constant. For different Zn2+ concentrations from 0.095 to 0.38 m, we maintained a constant molar ratio of Zn2+ to 2-MeIm (MZn2+:M2-MeIm = 1:10). It is noted that Zn2+ to 2-MeIm stoichiometries were not investigated because it may cause completely different nanoparticle morphologies that would complicate the analysis. As shown in Figure 3d, the adhesion energies were positively correlated with the precursor concentrations, presenting much lower adhesion energy for 0.095 m Zn2+ (≈125 J m−2) compared to that of 0.38 m Zn2+ (≈504 J m−2).
While inspecting the thickness of ZIF-L layers by SEM (Figure S4, Supporting Information), it is interesting to find that there is no observed ZIF-L layer at the Zn2+ concentration of 0.095 m (Figure S4a, Supporting Information). In contrast, higher concentrations presented dense ZIF-L layers with 32 µm thickness for 0.19 m concentration and 53 µm for 0.285 m (Figure S4c,e, Supporting Information), respectively. Further increasing Zn2+ concentration to 0.38 m did not largely raise the thickness of the ZIF-L layer (55 µm, Figure 2d). We hypothesized that the diffusion of ZIF precursors goes through the ZIF-L layer not in depth but in particle size due to crystal growth, which was demonstrated by the evolution of particle size from SEM. There are few particles observed in the SEM image for 0.095 m Zn2+ concentration (Figure S4b, Supporting Information). The dimensions of ZIF-L particles prepared at 0.019 and 0.285 m Zn2+ concentrations were found to be 0.7 ± 0.1 µm in length and 0.25± 0.05 µm in width, 0.9 ± 0.2 µm in length and 0.3 ± 0.1 µm in width, respectively (Figure S4d,f, Supporting Information), which were considerably smaller than that of the ZIF-L crystals prepared with 0.38 m Zn2+ concentration (Figure 2f, 2.5 ± 0.5 µm in length and 1.0 ± 0.3 µm in width). These trends in ZIF-L layer and particle size are correlated to the adhesion energies, indicating the interfacial layer and particle size are critical for interfacial bonding. This can also be explained by the interaction not only between nanosheet and hydrogel networks but nanosheets themselves. In such cases, breaking the gels apart requires breaking not only nanosheets–gel bonds but also nanosheets–nanosheets bonds.[31, 32] Larger nanosheets typically have larger volume to entangle polymer network in hydrogel, as well as larger contact area between adjacent nanosheets and thus stronger van der Waals forces or other intermolecular interactions, which explains higher adhesion energies in lager ZIF-L crystals.
In addition to concentration, as the precursor soaking time increased from 1 to 7 min, the metal ion and ligand diffused deeper into the hydrogels, resulting in a significant increase in adhesion energy from 102 to 484 J m−2. After 7 min, the adhesion energy reached a plateau, indicating saturated precursors in hydrogels (Figure 3e).
2.3 Underwater Adhesion and On-Demand Debonding of the Adhesion
Underwater adhesion is generally more challenging compared to dry adhesion since interfacial water separates the molecules of the two surfaces.[33] Effective expulsion of the hydrated layer on the substrate surface is the key to achieving wet adhesion.[33-37] In this study, the wet adhesion performance of the ZIF-L layer in binding hydrogels was thus investigated. We show the underwater adhesion of the metal ions loaded hydrogel (red color – from an added RhB dye) to ligand-loaded hydrogel (colorless – no dye added) (Movie S2, Supporting Information). One piece of Zn2+ loaded gel sat underwater and another piece of 2-MeIm loaded gel was placed onto it quickly. After pressing gently, the adhesion was observed with the evident change from transparent to opaque at the overlapped area within 120 s, very similar to the dry adhesion. The adhered hydrogels were strong enough to endure repeated swaying movements and torsions without delamination. In the case of the extended contact time of two hydrogels to 60 min, compared to that in dry conditions, the adhesion energy was slightly lower at Day 0 (Figure 3f, 422 vs 504 J m−2). The reduced adhesion may be attributed to the loss of metal ions and ligands diffusing from hydrogel surfaces into the surrounding water upon submersion.
The adhesion durability underwater is generally a serious concern since long-term water immersion causes inevitable swelling, adds stress along the bonding interface and consequently diminishes adhesion to other surfaces.[30] However, the ZIF-hydrogel hybrid system retained its underwater adhesion even after long-term immersion. We placed the adhered hydrogels in water at room temperature and then measured their underwater adhesion energy at predetermined time intervals. As shown in Figure 3f, the adhesion remained ≈84% of their original value (355 J m−2) after immersing in an excess of water and swelling for 7 days (≈300% of original hydrogel weight). The slight reduction in adhesion energy of the swollen hydrogels might have resulted from the diminished ability of hydrogels to dissipate energy. As a simple demonstration of the tough bonding in wet environment, we soaked the adhered hydrogel joint with overlay area of 3 cm × 1 cm in DI water for 7 days until it was fully swelled, and then we used it to bear a weight of 50 g (Figure 3f, inset). As a result, no interfacial failure was observed, which is in line with the results obtained from the peeling test. We anticipated that the stability of underwater adhesion was due to the rigidity of ZIFs in wet environment as well as they locked polymer networks once formed, as islands to bind two hydrogels tightly. Even though the polymer chains locked in the ZIF-L islands are not stretchable, those between neighboring islands are stretchable so that the adlayer would adapt to the swelling of gels. This stability makes it well-suited for practical applications and commercialization. Taken together, the above results indicate that the ZIF-L adhesive is capable of robust and long-term underwater adhesion.
On top of this, the ZIF-L interfacial layer is quickly removable, on-demand, when exposed to an acidic solution (pH 2.0) at the separation front of two adhered hydrogels. This decomposed the ZIF-L particles quickly and allowed the hydrogel hybrids to be easily detached without noticeable damage (Movie S3, Supporting Information). The visible white layer observed from the side in the movie is attributed to a minimal residue from the ZIF-L nanoparticles. Upon closer inspection, there was no evidence of cohesive failure after debonding, as indicated by the intact hydrogel layers. If a milder acidic solution (pH 5) is used, the ZIF-L layer still decomposes and consequently separates two adhered gels, albeit at a slower rate. This pH-triggered detachment is critical to repositioning misplaced bioadhesives as well as the retrieval of implanted devices.
2.4 A Generic Approach to Construct Robust Hydrogel-Adherend Interfaces
The hydrogel adhesion strategy enabled by the formation of ZIF-L nanosheet in situ is applicable to various substrates. Besides Agar/PAAm hydrogel–hydrogel adhesion, this strategy can be employed for hydrogel adhesion to various substrates with distinct surface properties, which include i) hydrogels with various chemical compositions, ii) elastomers, iii) plastics that normally used as blood-contacting biomedical devices, and iv) biological tissue. Furthermore, the versatility of the strategy can be further extended to other nanoparticles formed in situ such as different MOF crystals (ZIF-L-Co, and Tb2(BDC)3) and LDH nanoparticles.
Replacing the Agar/PAAm double network hydrogel with other single or double network hydrogels, including PAAm, alginate/PAAm (Alg/PAAm), chitosan/PAAm, poly(dimethyl acrylamide) (PDMA), PVA, and poly(hydroxyethyl methacrylate) (PHEMA), the adhesion energy ranged from 30 to 902 J m−2 (Figure 4a), demonstrating the versatility of the proposed method. The adhesion energy between two hydrogels generally correlated to their bulk fracture toughness. Indeed, we observed that the residue from one hydrogel was left on another one, indicating a cohesive failure of hydrogel occurred near the interface after peeling (Figure S5, Supporting Information). In addition, the PDMA hydrogel exhibited a bulk toughness of ≈20 J m−2, and the adhesion energy is ≈30 J m−2. In contrast, the Alg/PAAm hydrogel demonstrated significantly higher bulk toughness of 1456 J m−2, and the adhesion energy of ≈902 J m−2. Therefore, the interfacial failure most likely happened during the peeling test. Unlike the PDMA hydrogel with only single network, the Alg/PAAm hydrogel consisted of two interlocked networks: one comprising PAAm long-chain polymer networks that were cross-linked by covalent bonds, and another consisting of alginate chains were crosslinked by ionic bonds as mechanically dissipative components. During stretching, the covalent cross-links remained unaffected, while the ionic cross-links freely dissociated, resulting in the Alg/PAAm hydrogel displaying high dissipation characteristics.[38] Therefore, when two pieces of the Alg/PAAm hydrogel were adhered together, the ZIF-L nanosheets were in topological entanglement with the Alg/PAAm networks. This interlocking mechanism was strong enough to generate energy dissipation across a large volume of the hydrogels, resulting in a high adhesion energy. As for other hydrogels that were less dissipative, the measured adhesion energies are lower. These results confirm the universality of this strategy as well as the importance of hydrogel dissipative property and high bulk toughness in achieving robust interfaces.
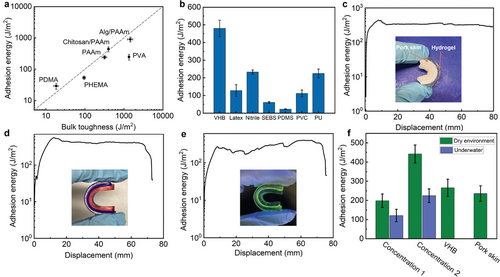
Our method also enabled strong adhesion between hydrogels and various elastomers/plastics. Existing methods of fabricating hydrogel–elastomer hybrids with robust interfaces normally involve modifying the surface chemistry or topology of elastomer to increase its surface energy and thus improve adhesion, which can be complex and require specialized equipment or expertise.[39-41] To fabricate hydrogel–elastomer hybrids, we treated the elastomer surface with 2-MeIm solution via a simple swelling-driven surface absorption process. Similar to adhesions between hydrogels, this technique used the solvent for 2-MeIm to swell the elastomer and thus introduce the ligands into the elastomers in a certain depth. The key to achieving high adhesion performance is to find suitable solvents that effectively swell the elastomers. The hydrogel layer, on the other hand, was treated with metal ion solution that would form a diffusion layer and enable the exchange of precursors at the hydrogel–elastomer interface once they are attached. Through this straightforward procedure, strong adhesion was achieved between the hydrogel and diverse elastomers/plastics (Figure 4b and Table 1), including VHB tape (adhesion energy, 480 J m−2), latex rubber (adhesion energy, 128 J m−2), nitrile rubber (232 J m−2), styrene–ethylene–butylene–styrene (SEBS, 60 J m−2), polydimethylsiloxane (PDMS, 22 J m−2) and polyvinylchloride (PVC, 112 J m−2) and polyurethane (PU, 225 J m−2).
| Entry | Hydrogel–adherend hybrid | Hydrogel solvent | Adherend solvent | Adhesion energy (J m−2) |
|---|---|---|---|---|
| 1 | Agar/PAAm– Agar/PAAm | H2O | H2O | 504 |
| 2 | Agar/PAAm–VHB | EtOH/H2O (1/1, v/v) | EtOH | 480 |
| 3 | Agar/PAAm–Latex | EtOH/Acetone (1/1, v/v) | EtOH/Acetone (1/1, v/v) | 128 |
| 4 | Agar/PAAm–Nitrile | EtOH/Acetone (1/1, v/v) | EtOH/Acetone (1/1, v/v) | 232 |
| 5 | Agar/PAAm–PDMS | EtOH/Acetone (1/1, v/v) | EtOH/Acetone (1/1, v/v) | 22 |
| 6 | Agar/PAAm–SEBS | THF/EtOH (3/2, v/v) | THF | 60 |
| 7 | Agar/PAAm–PVC | EtOH/Acetone (1/19, v/v) | Acetone | 112 |
| 8 | Agar/PAAm–PU | EtOH/Acetone (1/19, v/v) | Acetone | 225 |
| 9 | Agar/PAAm–pork skin | H2O | H2O | 320 |
- a) The hydrogel was soaked in Zn2+ solution for 10 min. The other adherend was soaked in 2-MeIm solution for 10 min. After pat-drying, the two adherends were attached and pressed for 60 min.
The conditions for the precursor soaking are summarized in Table 1. The discrepancy in adhesion energy among these materials can be attributed to the intrinsic properties of the distinct materials including the solubility in the selected solvent, crosslinking density, and other non-covalent interactions (hydrogen bonding and chain entanglement) between the materials and ZIF-L nanoparticles. For instance, the VHB tape is a hydrophilic acrylic polymer that can be readily swelled in ethanol and allows for adequate precursors absorption and ZIF-L formation within its polymer chain to enable chain entanglement between the materials and nanoparticles. Meanwhile, the functional ester or acid groups in acrylic chain forms strong interactions with Zn2+ (i.e., coordination) and imidazole (i.e., hydrogen bonding) in ZIF-L nanoparticles. This hypothesis can be verified by a seamless interface in the adhesion of VHB and Agar/PAAm as visualized by SEM (Figure S6, Supporting Information). Even after being subjected to extreme cooling under liquid nitrogen, the adhered materials remained intact, indicating a strong and robust bond between these two materials. In contrast, PDMS and SEBS are relatively hydrophobic rubbers that have a low swelling ratio in acetone, leading to low precursor loading as well as low conversion of ZIF-L. Most significantly, PDMS and SEBS have no functional groups to interact with any components in ZIF-L nanoparticles. Therefore, their adhesion energies were relatively low.
We further applied the strategy for bio-adhesion between hydrogel and biological tissue. The structural similarity of the permeable matrices of biological tissues with that of hydrogels allows for the potential of strong adhesion.[42] As a proof-of-concept, we used ZIFs to bond the Agar/PAAm hydrogel to pork skin. Significantly, the corresponding adhesion energy was as high as 320 J m−2 (Figure 4c).
We then investigated the generality of this adhesion strategy to a broad range of nanoparticles, such as ZIF-L-Co, Tb2(BDC)3, and LDH. Indeed, nanoparticles formed readily upon stacking two hydrogel segments, as evidenced by the visible color change observed in the cases of ZIF-L-Co and Tb2(BDC)3 and SEM images (Figure 4d,e; Figure S7a–d, Supporting Information). The interfaces exhibited adhesion energies as high as 413 J m−2 (ZIF-L-Co) and 348 J m−2 (Tb2(BDC)3). It is worth noting that the use of interfacially formed MOFs including ZIF-L, ZIF-L-Co, and Tb2(BDC)3 would inevitably compromise the optical transparency of hydrogel-adherend hybrids, as their sizes are larger than the wavelength of visible light as well as their expectedly high refractive index. For some specific applications, such as smart artificial skin, wearable electronics, and flexible displays, optical transparency is required for the transmission of optical information.[43-45] With this objective in mind, the selection of nanoparticles will be critical. It's remarkable that the adhered samples prepared using Mg-Al-LDH appeared significantly more transparent than those from MOFs (Figure S8, Supporting Information) due to their thin-sheet-like structure (Figure S7e,f, Supporting Information). SEM also evidenced the adhered interface enabled by LDH. Adhesion was evident between hydrogels at different concentrations, in both dry and wet conditions. Additionally, it was observed in the hydrogel–elastomer and hydrogel–pork skin systems (Figure 4f).
2.5 Triboelectric Nanogenerators Using Hydrogel Hybrid
Owing to its simplicity, versatility, and robustness, the design strategy for creating tough hydrogel–adherend hybrids enabled us to explore a set of unprecedented functions. For instance, the hydrogel–elastomer hybrids have been extensively employed in triboelectric nanogenerators (TENGs), which can transform ambient mechanical energy into electrical output. However, the energy harvesting performance of traditional hydrogel-based TENGs deteriorates during long-term use due to the naturally weak interfacial bonding strength between hydrogels and elastomers.[46] Therefore, improving the mechanical reliability between hydrophilic hydrogels and relatively hydrophobic triboelectric materials is important for optimizing high-performance TENGs.[47]
In this study, a TENG device was assembled from an elastomer (VHB) and a hydrogel using our bonding methodology. The TENG device in this study consisted of two VHB layers sandwiching a conductive hydrogel electrode (with 8 m LiCl). Based on the triboelectric series,[48] it has been found that nylon has the highest tendency to lose electrons when it comes into contact with VHB. Thus, VHB and nylon were employed as the triboelectrically negative and positive layers, respectively due to their different electronegativity.
Figure 5a schematically illustrates the working mechanism of the TENG in a two-electrode, contact-separation mode. Once the nylon film contacts the VHB film, electrification happens at the interface, electrons are injected from the surface of nylon layer onto the VHB layer, making the VHB layer negatively charged, and the nylon layer positively charged (Figure 5a-i). When there is a relative separation between those two materials, the static charges on the insulating VHB surface will induce the movement of the free ions in the ionic hydrogel to balance the static charges, forming a layer of excessive positive ions at the VHB/hydrogel interface (Figure 5a-ii). This will polarize the metal wire-ionic hydrogel interface, thus generating the same number of ions of the opposite charge in the interface. Electrons will then flow from the metal wire–hydrogel interface to the nylon film through the external circuits, generating a current signal. The electron flux will be stopped until all the static charges in the VHB film are screened, and the nylon film is neutralized (Figure 5a-iii). When the nylon layer gets in contact with the VHB cover again, the whole process will be reversed, and an electron flux will transfer to the hydrogel/metal wire interface through the external load (Figure 5a-iv). When repeating the cycles, a periodic alternating electrical output will be generated.
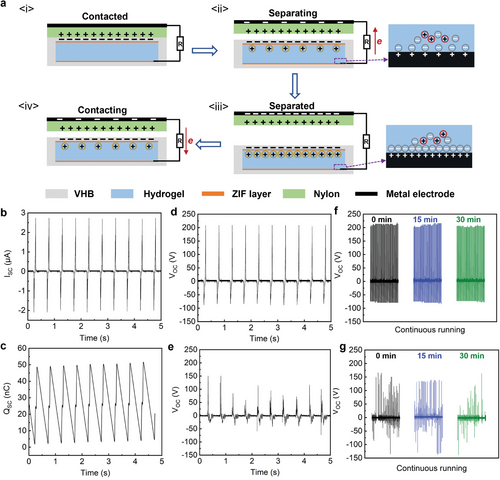
The energy-harvesting performance of the two-electrode TENG working at contact-separation mode was evaluated. The frequency (2 Hz) and speed (0.04 m s−1) of the periodically compressing cycle and the pressure (≈100 kPa) between the two contacting films were maintained constant by a linear motor during all subsequent tests. The TENG with an interfacial ZIF layer and an area of 4.5 cm2 generated the peak open-circuit voltage (VOC) of 210 V (Figure 5d), peak short-circuit charge quantity (QSC) of 51 nC (Figure 5b), and peak short circuit current (ISC) of 2.74 µA under continuous taping (Figure 5c). In the continuous operation test (Figure 5f), the TENG was continuously tapped under the same conditions as mentioned above for 30 min. The output voltage appeared to be highly stable during the whole period, indicating that the TENG is well-suited for long-term use.
In contrast, if the TENG were assembled without interfacial adhesion, the sticky surface of VHB can slow down the separation motion of the active object.[49] Thus, poor mechanical durability between triboelectric and hydrogel layer would lead to local delamination during repetitive compression, and thus hinder the charge transportation, resulting in an overall lower and less stable energy output. As shown in Figure 5e, the peak output voltage of the unmodified TENG was ≈150 V, which was 30% lower than the TENG with a ZIF layer. In addition, after operating for 30 min, a gradual decrease in output signals were observed (Figure 5g). Meanwhile, the VOC of the TENG with a ZIF layer was significantly higher than that without the layer (210 V vs ≈150 V). This enhanced electrical output performance originated from the positive surface potential of ZIFs, which facilitated the capturing of electrons from the positive friction layer during the triboelectrification process and suppressed the recombination between electrons and positive charges (induced charges on the electrode and charged ions from the air) through its high electron-capture properties.[50-53] Therefore, the use of a ZIF adlayer to generate interfacial bonding between triboelectric materials and ionic hydrogels was found to be highly effective in improving the output performance and stability of the TENG, thus providing a promising application in flexible, portable, and self-powered devices.
3 Conclusion
To conclude, we have developed a facile and universally applicable adhesion strategy for rapid and tough bonding between diverse hydrogels and other adherends under both wet and dry conditions using an in situ nanoparticle formation methodology. Topological entanglement and chemical bonding of nanoparticles with both hydrogel and adherend, coupled with significant mechanical dissipation of the bulk hydrogels are key factors that lead to the tough bonding with adhesion energy up to 902 J m−2. Furthermore, on-demand detachment can be realized by applying an acidic solution at the adhered interfaces. The working principle has been demonstrated to be applicable to a wide variety of MOF (ZIF-L-Zn, ZIF-L-Co, Tb2(BDC)3) and LDH nanoparticles, with a variety of sizes, forms, and surface chemistry. As a proof-of-concept, we then used this interface to assemble elastomers through hydrogel as a stretchable hybrid device for energy harvesting TENG application, with high electrical signal quality and mechanical resistance. With our demonstrated strong and tough adhesion by nanoparticles, we anticipate that integrating hydrogels with chemically and mechanically mismatched materials will be simple and fast, which should offer a plethora of options for the development of advanced wearables to future-generation medical tools and health monitoring devices.
Acknowledgements
M.Z. acknowledges UNSW Sydney for the PhD scholarship of the University International Postgraduate Award. J.X. acknowledges the financial support from Australian Research Council (ARC) (DP210101904). K.L. acknowledges the financial support from the ARC (DP210100422).
Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.




