Sunlight-Controllable Biopharmaceutical Production for Remote Emergency Supply of Directly Injectable Therapeutic Proteins
Abstract
Biopharmaceutical manufacturing requires specialized facilities and a long-range cold supply chain for the delivery of the therapeutics to patients. In order to produce biopharmaceuticals in locations lacking such infrastructure, a production process is designed that utilizes the trigger-inducible release of large quantities of a stored therapeutic protein from engineered endocrine cells within minutes to generate a directly injectable saline solution of the protein. To illustrate the versatility of this approach, it is shown that not only insulin, but also glucagon-like peptide 1 (GLP-1), nanoluciferase (NLuc), and the model biopharmaceutical erythropoietin (EPO) can be trigger-inducibly released, even when using biologically inactive insulin as a carrier. The facilitating beta cells are engineered with a controllable TRPV1-mediated Ca2+ influx that induces the fusion of cytoplasmic storage vesicles with the membrane, leading to the release of the stored protein. When required, the growth medium is exchanged for saline solution, and the system is stimulated with the small molecule capsaicin, with a hand-warming pack, or simply by using sunlight. Injection of insulin saline solution obtained in this way into a type-1 diabetes mouse model results in the regulation of blood glucose levels. It is believed that this system will be readily adaptable to deliver various biopharmaceutical proteins at remote locations.
1 Introduction
Engineered cellular systems have enormous potential for providing prosthetic replacements for missing or flawed physiological processes,[1, 2] as exemplified by the clinical success of chimeric antigen receptor (CAR)-T cells, in which an engineered receptor is introduced to enhance the ability to eliminate cancerous cells.[3, 4] Correction of chronic metabolic diseases is a next-level challenge, as biological systems function in tightly regulated closed-loop circuits and any therapeutic interference should be fully integrated and adaptable to the circuit.[5] In particular, the major hurdles facing endocrine regulation include not only its robust feedback loop, but also the need for rapid response to physiological changes.[6] Synthetic biology-based therapies have been reliant on small molecule inducers and transcription-based systems, both of which involve a significant lag time in the response.[7, 8] Recently the focus has shifted to traceless physical stimuli as inducers, such as light, heat, magnetic fields, and radio waves,[8-11] which can provide an immediate and localized stimulation, thereby enhancing the response kinetics of cellular therapies. The lag time can be further reduced by using a regulated secretory pathway instead of a transcriptional pathway. In contrast to the usual direct secretion that follows immediately upon co-translational import into the endoplasmic reticulum (ER), in regulated pathways, the newly synthesized proteins are sorted into intracellular storage vesicles, which fuse with the membrane to release their cargo only after calcium-elicited depolarization.[12-14] Selective sorting of proteins into storage vesicles is among the properties that distinguish endocrine cells from most other cells.[15, 16] Notably, a variety of bioactive proteins and peptides are stored and released in this manner to enable rapid homeostatic responses.[6, 17] In beta cells this mechanism is based on specific ER-localized receptors that recognize a conserved sequence in the proinsulin peptide and separate this protein from all other secreted peptides into storage granules, where maturation and crystallization occur.[12, 14] Furthermore, by fusing the C-peptide sequence of proinsulin with proteins of interest, it is possible to use the conserved sorting sequence to divert the fusion proteins into storage vesicles, as demonstrated with reporter proteins Gaussia luciferase and nanoluciferase (NLuc),[18] and fluorescent proteins,[19, 20] as well as bioactive cytokines (IL-10) and small peptides,[21] which might be of potential therapeutic interest. The release from intracellular storage vesicles depends on the calcium-dependent interaction of synaptotagmin with the SNARE complex, which initiates the fusion of the large dense core vesicles with the membrane and the subsequent release of their cargo.[13, 22] In pancreatic beta cells, the influx of calcium into the cytoplasm occurs in response to glucose-dependent membrane depolarisation and the subsequent opening of voltage-gated calcium channels. We demonstrated previously that electric current can be employed as a direct activator of voltage-gated channels to achieve membrane depolarization.[8] Furthermore, we also showed that glucose-insensitive beta cells can be generated, thus providing an important avenue for engineering pancreatic beta cells as a platform for rapid protein delivery from prosthetic implants.[23] To increase the modes of control and to enhance the operation of such rapid-release cells, in this work we utilized the transient receptor potential cation channel subfamily V member 1 (TRPV1), which can respond to traceless and non-invasive physiological stimuli, including heat,[24] radio waves,[25] and magnetic fields.[9] Previous work showed that, although active, the channel is not efficient or practical for transcriptional control of gene expression[10] as multiple negative feedback mechanisms exist.[26] However, here we show that the channel can efficiently generate quick induction pulses for triggering storage granule-based protein secretion from pancreatic beta cells through glucose-independent calcium influx into the cytoplasm. We provide evidence for the biopharmaceutical relevance of this rapid-release system, which can be precisely controlled through simple, easily accessible, non-invasive inducers, such as a hand-warming pad or concentrated sunlight, and we utilize it to develop a new biopharmaceutical production process in which stored protein therapeutics can be secreted within minutes into a saline solution that is directly injectable after membrane filtration to remove the cells and adjust the injection volume.
2 Results
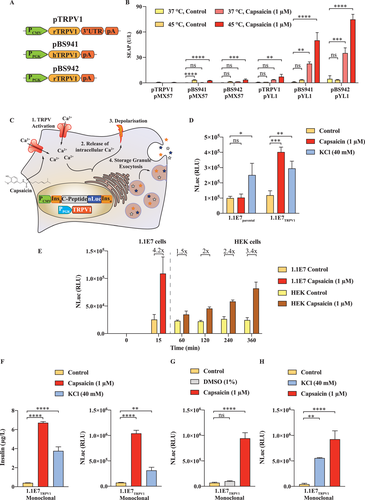
2.1 Engineering Capsaicin-Sensing Cells for Trigger-Inducible Protein Secretion within Minutes after Stimulation
In most cells, such as human embryonic kidney (HEK)-293, intracellular calcium influx activates transcriptional factors such as the nuclear factor of activated T-cells (NFAT) and serum response factor (SRE). These transcriptional factors provide an avenue to reroute the second messenger into the transcription of reporter genes. To establish the best-performing capsaicin receptor vector (Figure 1A), we transfected HEK-293 cells with different receptor constructs and either a pure NFAT reporter plasmid (pMX57) or a combinatory NFAT-SRE-CRE operated plasmid (pYL1) and exposed the cells to either heating for 1 h at 45 °C, or for 1 h to 1 µm capsaicin, or a combination of both treatments. We observed superior performance from the receptor constructs without a 3'-UTR, and the highest reporter gene expression was observed upon combined stimulation of the rTRPV1 (pBS942) (Figure 1B). In contrast to HEK-293 cells, in endocrine cells, the calcium influx also triggers storage vesicle fusion with the cell membrane and rapid release of the cargo protein (Figure 1C). By inserting NLuc between the C-peptide and the insulin alpha chain it is possible to target the recombinant protein in the storage compartment of pancreatic beta cells.[8] We engineered a 1.1E7-based stable cell line that expresses either only NLuc (1.1E7parental) or NLuc in combination with our engineered rTRPV1 expression vector (1.1E7TRPV1). Both cell lines were inducible by nonspecific KCl membrane depolarization, and responded with the release of NLuc into the supernatant; however, only the TRPV1-positive cell line was inducible with capsaicin (Figure 1D). The major advantage of a rapid release system is the lack of a transcriptional phase lag, as is evident from the observation that the engineered beta cells were strongly activated after only 15 min, whereas the capsaicin-activated HEK-293 cells (Figure 1E) responded hours after induction (Figure 1E). This is enabled through intracellular storage vesicles that are highly specific to endocrine cells, can be loaded with proteins such as insulin, and are present in the engineered 1.1E7TRPV1 cells (Figure S1, Supporting Information). To further improve the dynamic range of the cell line, we isolated a monoclonal line showing the highest induction fold as measured using luciferase assay (Figure 1F). As expected, the cell line showed a rapid and strong induction of insulin secretion, in addition to the NLuc reporter, upon capsaicin treatment (Figure 1F). We confirmed that DMSO, used to solubilize the inducer compound, had no effect (Figure 1G). Further, the induction was not abolished by pre-treatment of the cells with KCl as a depolarizing agent, indicating that the treatment order does not influence the capsaicin inducibility of the cells (Figure 1H).
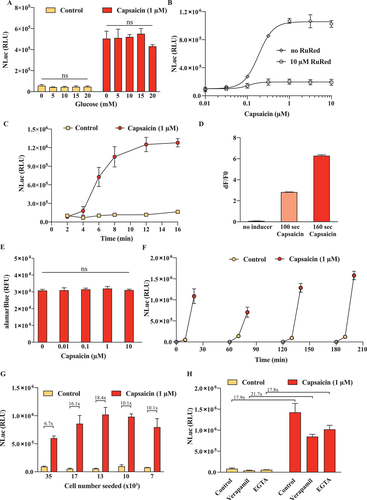
2.2 The 1.1E7TRPV1 Cell Line Shows Favorable Biomedical and Biopharmaceutical Parameters
Robustness and controllability are critical for biomedical and bioproduction processes. A major concern for this cell line would be its potential glucose sensitivity, which is an intrinsic property of pancreatic beta cells. As glucose concentrations may fluctuate in vivo, as well as in cell culture and in a bioreactor, it is essential that the engineered beta cell line should not be influenced by glucose. Therefore, we tested the inducibility by glucose and also examined whether glucose interferes with induction by capsaicin (Figure 2A). No glucose concentration-dependent effect was found. Next, we characterized the capsaicin dose-response kinetics (Figure 2B) and observed strong induction even at sub-micromolar capsaicin concentrations. In addition, induction was blocked by a specific inhibitor of the rTRPV1 channel (Figure 2B). Next, we characterized the time-dependent increase of reporter protein in the supernatant and found that the stimulation was nearly maximal after just 10 min, (Figure 2C); this well reflects the rapid calcium influx into the cells (Figure 2D). Possible calcium overload through the direct influx of calcium into the cells can have a detrimental effect on cell viability,[27] so we stimulated the cells with up to 10 µm capsaicin, then washed the cells and incubated them for 24 h. No decrease in metabolic activity was observed (Figure 2E). Since therapeutics delivery would be needed multiple times per day, it is essential that the system can recover and be restimulated soon after the initial pulse. We found that upon incubation for 1, 2, and 3 h after the initial stimulation, the cells were able to regain their inducibility and secretory activity upon repetition of the capsaicin-induced depolarization (Figure 2F). Further, a longer incubation period resulted in greater secretion during the subsequent induction phase, suggesting a greater degree of recovery from the initial stimulation (Figure 2F). Another important parameter that can influence the cellular response is the initial seeding density, as a high density may lead to decreased cell viability due to insufficient resources. Although there was some decrease after prolonged culture at excessive cell densities, inducibility was still retained, with only a minor decrease in absolute output (Figure 2G). In addition, the performance of the cells was largely unaffected by inhibitors of known intracellular calcium signaling inducers, such as verapamil and egtazic acid (EGTA), which is consistent with the direct influx of calcium ions through the activated TRPV1 channel (Figure 2H). Overall, these results demonstrate that the engineered fast protein release cell line is rapidly and reversibly inducible independently of glucose concentration, and robust to interference with calcium signaling, but can be specifically inhibited by TRPV1 channel inhibitors.
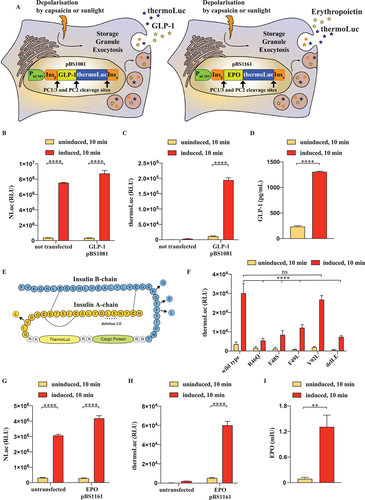
2.3 Replacing the C-Peptide Portion of Proinsulin Enables Generalizable Rapid Therapeutic Protein Release from Engineered Beta Cells
To demonstrate that our rapid-release system could potentially be extended to other proteins besides insulin and NLuc, we envisioned replacing the C-peptide region of our modified insulin construct (pBS1039) with a sequence encoding human glucagon-like peptide 1 (GLP-1) (pBS1081) or human erythropoietin (EPO) (pBS1161) as model biopharmaceuticals (Figure 3A). The synthesized peptide chain contains prohormone convertase (PC) 1/3 and PC2 cleavage sites for separation of the individual protein products after sorting in the storage granules (Figure 3A). After transfection of the GLP-1 encoding constructs in our 1.1E7TRPV1 line, we first verified the correct function of the cells by measuring the concentration of the NLuc reporter before and after stimulation (Figure 3B). The special thermostable nanoluciferase (thermoLuc) reporter gene[28] encoded by the pBS1081 construct was confirmed to be present only in the transfected cells and was inducibly secreted upon activation of the cells (Figure 3C). Additionally, we also verified that the GLP-1 peptide is expressed and that GLP-1 secretion is induced by depolarization of the cells (Figure 3D). The synergism of GLP-1 with insulin, used as a carrier sequence for sorting the storage granules, provides an example of the successfully engineered co-secretion of biologically active peptides, as real-life therapeutic interventions might benefit from multiple proteins used simultaneously. For applications where bioactive insulin would be detrimental, we envisioned introducing previously described loss-of-function mutations in the insulin carrier peptide, that would abolish its biological activity,[29, 30] but not the targeting into the storage granules[31] (Figure 3E). We used a reporter vector, encoding for thermoLuc, for evaluating the inducible secretion of insulin mutants. Based on this screen we identified the V92L Wakayama insulin, a variant of highly reduced receptor affinity and biological activity,[29] as the best suitable candidate showing secretion levels comparable to wild-type insulin (Figure 3F). Transfection of V92L modified insulin containing EPO encoding sequence in the C-peptide portion in the 1.1E7TRPV1 cells did not disturb inducible secretion (Figure 3G) and as expected thermoLuc was only present in the transfected cells (Figure 3H). Additionally, erythropoietin was inducibly released rapidly after activation of the cells (Figure 3I). These results expand the range of potential therapeutics that could rapidly be released from engineered beta cells and establish a generalizable rapid protein secretion system.
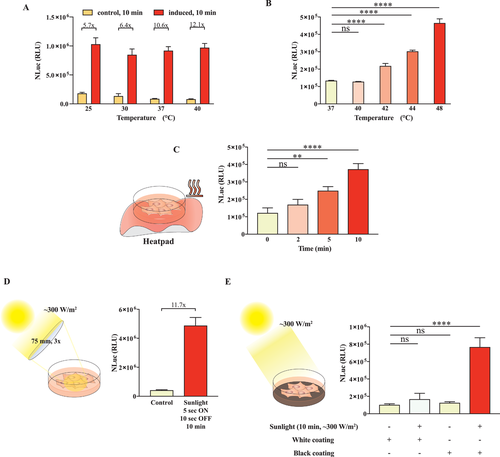
2.4 Activation of Rapid Protein Secretion can be Achieved Using Readily Available Energy Sources Such as Heat Packs and Sunlight
Energy efficiency and sustainability are important considerations for future biopharmaceutical processes. An important aspect of our engineered cell line is its dual control mode and sensitivity not only to the small molecule capsaicin, but also to the traceless physical stimulus heat. Small molecule inducers are difficult to deliver precise and are influenced by many factors, including bioavailability and pharmacokinetic properties. Therefore, a traceless and more controllable mode of activation of the cells, such as heat, is desirable for practical applications. Heat can be generated from many sources including electricity and chemical reactions, as well as by concentrating sunlight or utilizing environmental warmth. First, we established that capsaicin-mediated depolarization of the cells is not influenced by moderate temperatures in the range of 25–40 °C, suggesting that usual variations in environmental conditions would not randomly affect the system, and that specific, intentional warming would be necessary (Figure 4A). Next, to characterize the temperature inducibility of the system we briefly exposed the cells to various temperatures. At 42 °C and above, we observed an immediate increase in the secretion, as measured in terms of the NLuc reporter concentration in the supernatant (Figure 4B). We confirmed that short thermal pulses at 48 °C are not cytotoxic by using alamarBlue to evaluate the cell viability, compared to that of cells kept at 37 °C (Figure S2, Supporting Information). We also used a commercially available chemical hand-warming package to directly increase the temperature of the cell culture container. This resulted in an exposure time-dependent increase of secretion by the cells, suggesting that the amount of secreted protein could be tuned in a straightforward way by this means (Figure 4C). Next, to examine the feasibility of using sunlight as an inducer of the system, we focused on two modes of sunlight concentration. First, when sunlight was directly focused onto the cells with a lens, their temperature increased, leading to an increase in secretion (Figure 4D). Second, when a black absorbing surface was placed beneath the cell culture container, the temperature was increased upon sunlight exposure, again leading to an increase in secretion. We demonstrated that the cells were fully inducible by a black absorbing surface, but not by a white reflective surface or when protected from sunlight (Figure 4E). Both sunlight and thermal stimulation by a short heat pulse treatment to 48 °C were able to repeatedly activate the system just hours after a prior induction (Figure S3, Supporting Information). Overall, these findings open potential new avenues for constructing energy-efficient biopharmaceutical processes or for controlling designer cell therapies in patients. The rapid response and the need to apply relatively high levels of thermal energy make the system orthogonal to nonspecific stimulation, while at the same time such short heat pulses have been reported not to be detrimental to the surrounding tissue.[32]
2.5 1.1E7TRPV1 Cells Tolerate Non-Adherent Culture Conditions and Diverse Cell Culture Media while Maintaining Sunlight-Inducibility
Scalability is important in biopharmaceutical manufacturing and efficient production often requires the use of suspension cultures. Previous reports have established that multiple cell lines can be cultured in stirred bioreactors.[33] To demonstrate that our engineered cells show potential for adaptation to suspension cultivation, we first grew the cells on specialized AggreWell plates, inducing the formation of 3D spheroid organoid-like structures at the bottom of each inverted micro pyramid (Figure 5A). These structures showed continued expression of our engineered cassette (YPet marker; Figure 5A). The yield of these spheroids could be increased by transferring them from the AggreWell plates into a solar-powered bioreactor system, which can support a higher cell density per mL of medium (Figure 5B). Although the viability of the unattached organoid-like structures indicated the feasibility of suspension growth, we next examined whether the use of specialized plates could be avoided. We compared the viability of the cells cultured in different media, both as adherent cultures and in suspension. While the choice of the medium had a significant effect in adherent culture, with medium containing fetal bovine serum or a serum replacement being preferred, the differences were negligible in the case of suspension-cultivated cells, and the viability of the cells was only slightly reduced in comparison to the best-performing adherent cell culture, at least during short cultivation (Figure 5C). Another important parameter is the inducibility of secretory activity. We compared four different culture media and used cells that were either adherently grown and detached for this experiment, or cells were grown in suspension culture. Although adherently grown cells showed stronger induction, the suspension-cultured cells also showed increased secretion upon activation (Figure 5D). The default medium condition in RPMI1640 with fetal bovine serum (FBS) resulted in the greatest absolute NLuc concentrations and was also associated with higher metabolic activity and viability of the cells (Figure 5C). Cells grown in OptiPro with serum replacement were less inducible when grown adherently, and were not inducible when grown in suspension culture (Figure 5D).
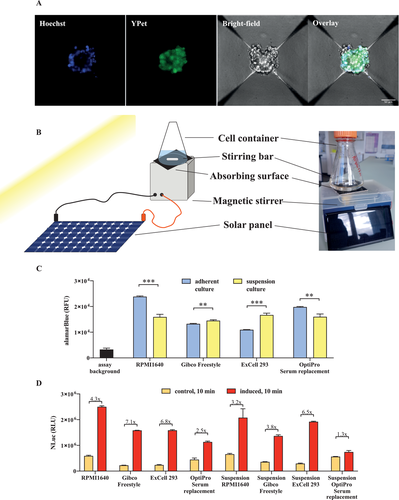
2.6 Smartphone Camera Assay Enables the Quantification of Nanoluciferase as a Measure of Insulin Concentration
A major advantage of this system is that targeting of NLuc reporter protein to the inducible-secretory pathway could enable a straightforward means for quantification of insulin. This simple determination would be beneficial for the implementation of a portable personalized supply of therapeutic protein. We envisioned an assay that includes a light-protected chamber in which the solution to be analyzed is positioned below a cell phone with fixed exposure parameters for consistent image acquisition (Figure 6A). The obtained pixel intensity information is then recorded without further processing, yielding a visible image of the enzymatic activity in each sample (Figure 6A,B). The pixel intensity for test samples 1 to 4, which are serial dilutions of the sunlight-induced secretion product from 1.1E7TRPV1 cells, provides a raw score (Figure 6A–C) from which the relative light units in the sample can be calculated (Figure 6D). The values of the detected increase of the relative light units were nearly identical to those measured with a commercial Tecan M1000 luminescence plate reader (Figure 6E). We also measured the insulin concentration in the samples (Figure 6F). The values increased in parallel with the NLuc values, confirming that the insulin concentration could be estimated from the NLuc measurements. This assay provides a simple and efficient tool for rapid screening of therapeutic protein concentrations secreted from the granular storage compartment of beta cells. Next, in order to confirm the functionality of the rapidly released insulin, we used an in vitro insulin receptor assay consisting of the insulin receptor (pIR), a TetR-Elk1 transcription factor activated by this receptor (pMkP37), and a TetR-controlled inducible promoter for the expression of SEAP reporter protein (PtetO7-SEAP) (Figure 6G). Upon addition of an insulin-containing sample to the assay system, we observed a dose-dependent SEAP response (Figure 6H). In addition, we analyzed control medium and medium from 1.1E7TRPV1 cells warmed by exposure to light irradiation over a black absorbing surface. The medium from the warmed cells showed higher SEAP levels, which could be further increased ≈20-fold by membrane concentration, during which the biological activity of insulin was preserved (Figure 6I). We also verified that the effect of volume adjustment on insulin concentration was similar in induced and uninduced samples (Figure S4, Supporting Information). Finally, we confirmed that the biological activity of the insulin-containing medium was not affected by the application of short heat pulses at temperatures above 37 °C (Figure S5, Supporting Information).
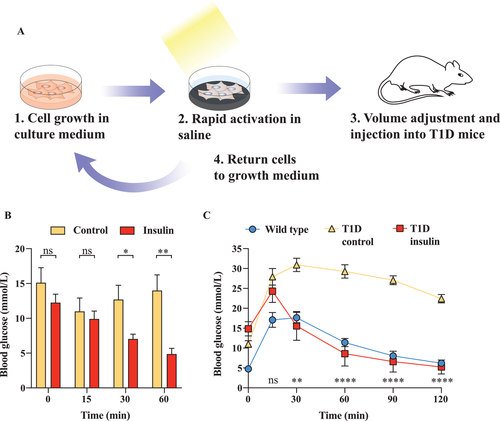
2.7 Rapid Release of Insulin upon Sunlight Activation in Physiological Saline Yields Therapeutic Injectable Solution for Treatment of Experimental Type 1 Diabetes in Mice
As a translational proof-of-concept, we tested our envisioned protein production process employing 1.1E7TRPV1 cells for the production of an injectable insulin saline solution and used it in an experimental type-1-diabetes model (Figure 7A). We transferred the engineered beta cells to calcium-supplemented phosphate-buffered saline and warmed them by exposure to light irradiation over an absorbing black surface. When the supernatant was collected in membrane filter tubes and injected into streptozotocin-induced experimental type-1 diabetic mice, we observed a significant decrease in blood glycemia of the treated mice (Figure 7B). Additionally, the injection of supernatant prevented hyperglycemic excursions and restored normoglycemia in a glucose tolerance test (Figure 7C). Overall, these data support the idea that our system could be used to provide emergency injectable solutions of therapeutic proteins at remote locations.
3 Discussion
Current biopharmaceutical processes are technically demanding, especially for the purification of the final proteins, and therefore there is a need for a way to produce and deliver therapeutic proteins conveniently in remote areas where production facilities or a cold supply chain would not be available. To meet this need, we envisioned an environmentally friendly system based on engineered cells maintained in a standard growth medium. In our scenario, the growth medium would be replaced by physiological saline and the cells would be exposed to an inducer, triggering the rapid release of a biopharmaceutical stored in intracellular vesicles when required. After collection of the physiological saline, the cells would be returned to the standard growth medium, where the vesicular stores would be transcriptionally replenished within hours. The saline solution could be either directly injected into the patients, or concentrated using membrane filter tubes if a higher dose or different injection volume were required. In order to estimate the concentration of the released therapeutic protein, the reaction could be conducted back-to-back using two containers – one for the protein to be injected, and the other for the luminescent reporter NLuc, which can be rapidly quantified, for example by means of a simple smartphone assay. Such a system would offer many advantages, especially for emergency use in remote locations: 1) it can rapidly release large amounts of therapeutic protein within minutes into injection-ready saline uncontaminated by potentially hazardous materials such as phenol red from the growth medium, growth factors, and cellular metabolic by-products, 2) it does not require sophisticated equipment, and 3) it can be triggered by an inexpensive, traceless physical stimulus such as a heat pad or sunlight. Scale-up of the system should also be feasible, since increasing the area of the containers would proportionally increase the amount of absorbed sunlight.
In order to develop such a system, we focused on recent applications of cellular engineering technologies and synthetic biology systems employing therapeutic cells capable of correcting chronic diseases.[1, 34] One of the most common and hard-to-correct chronic endocrine diseases is type 1 diabetes, which has enormous societal and economic costs.[35] Several studies have already provided translational proof of concept for the application of engineered designer cells to treat diabetic mice without the use of actual beta cells.[10, 11, 36, 37] Such systems employ closed-loop control based on the glucose concentration[37] or traceless physical stimuli[10, 11] or are intended to integrate with the lifestyle of the patient by using caffeine as an inducer.[36] However, to achieve clinical relevance, cellular therapies need to be rapidly adaptive and inducible. Such a level of control has been achieved through direct electrical stimulation[8] or exposure to blue light.[23] However, plastic implants may suffer from immune rejection and the blue light system may lead to unwanted nonspecific activation, so there is a need to improve the available control mechanisms. Here, we demonstrate that beta cells engineered with the TRPV1 channel can be controlled by the application of short pulses of high temperature or by capsaicin. Previous attempts to apply the TRPV1 channel to control protein expression have encountered difficulties, likely due to the existence of polymodal negative feedback loops. This is problematic for transcription-based systems, which require a significant activation time, but not necessarily for a system employing rapid protein release from vesicular stores, as presented here. The small molecule capsaicin is often used in transdermal patches, so it could potentially be a part of standard medical procedures or be integrated into patients’ lifestyles, as such patches are often used to treat chronic pain, which is a common comorbidity of diabetes. Furthermore, localized heating can be generated through focused ultrasound[38] or with magnetic fields,[9] so the possibility to control the cells by heat opens up an additional avenue for remote operation. Indeed, simple methods such as hand-warming pads, that require no specialized training, equipment, or handling could be used to activate the cellular implant in the case of an emergency. This would be a useful backup since the generation of heat from sunlight via black absorbing surfaces is weather-dependent and might be blocked by clouds. In addition, intense sunlight could potentially be harmful to the cells, if no UV protective filter is available. Cells could be engineered with a range of therapeutic proteins, including IL10 as reported previously.[21] Such systems would be particularly useful to treat various medical emergencies requiring rapid response, such as anaphylactic shock or other strong allergic reactions, epileptic seizures, and controlling blood-pressure spikes. The response dynamics of this rapid release system could be especially advantageous, as many effector proteins are released in bursts, and are then rapidly degraded to baseline levels. Therefore, we believe systems based on the triggered release of therapeutic proteins stored in secretory vesicles of engineered therapeutic cells have enormous potential as the next step in cellular therapies.
4 Experimental Section
Plasmid Construction and Purification
The gene expression vectors used in this study were produced through standard molecular cloning techniques using commercial restriction endonucleases (New England Biolabs, Ipswich, Massachusetts, USA; high-fidelity enzymes were used whenever possible), and the restriction products were ligated with T4 DNA ligase (New England Biolabs, cat. No. M0202L). To reduce background religation, dephosphorylation of the backbones was done with Antarctic phosphatase (New England Biolabs, cat. No. M0289L) before adding them to the reaction mixture. Phusion High-Fidelity DNA Polymerase (New England Biolabs, cat. No. M0530L) was used in PCR reactions. Detailed information on the restriction enzymes and primers used for generating the plasmids is presented in Supporting Information Table S1. XL10 Gold chemically competent cells (New England Biolabs, cat. No. C2992) were transformed and kept in liquid culture until confluency in order to amplify the plasmids for cell culture experiments. A plasmid miniprep kit (Zymo Research, Irvine, California, USA, cat. No. D4054) was used to extract the plasmids from the bacterial cultures.
Cell Culture
Pancreatic beta cells 1.1E7 (cat. No. 10070101-1VL, Sigma-Aldrich, Saint Louis, MO, USA) were cultivated in Roswell Park Memorial Institute 1640 medium (RPMI; cat. No. 72400-021, Thermo Fischer Scientific, Waltham, Massachusetts, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, Saint Louis, USA, cat. No. F7524, lot no. 022M3395) and penicillin (100 U mL−1) and streptomycin (100 µg mL−1) solution (Thermo Fischer Scientific, Gibco, cat. No. 15140122) at 37 °C under a humidified atmosphere containing 5% CO2. Human embryonic kidney cells (HEK-293T, ATCC: CRL-11268) were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and penicillin (100 U mL−1) and streptomycin (100 µg mL−1). Passaging of confluent 1.1E7 cultures was done through the detachment of cells with 0.05% trypsin-EDTA (Life Technologies, Carlsbad, California, USA; cat. No. 25300-054) for 5 min at 37 °C. Cells were transferred to a 10 mL cell culture medium and centrifuged for 1 min at 200 × g, then the supernatant was discarded and the cells were resuspended in fresh medium. Cell counts and viability were quantified using an electric field multichannel cell counting device (Casy Cell Counter and Analyzer Model TT, Roche Diagnostics GmbH, Rotkreuz, Switzerland) or a CellDrop brightfield cell counter (DeNovix, Wilmington, Delaware, USA). The concentration was adjusted to a standard cell density (1 × 106 cells mL−1), and the cells were reseeded in culture plates at suitable volumes according to the surface area at a seeding density of 3 × 104 per cm2. For experiments in Figure 5, the cells were cultured at 300 × 103 cells per well in 6-well AggreWell plates and visualized 24 h after seeding. The following media were used: Opti Pro SFM (Thermo Fischer Scientific, Gibco, cat. No. 12309050) supplemented with 10% KnockOut serum replacement (Thermo Fischer Scientific, Gibco, cat. No. 10828010); FreeStyle 293 Expression medium (Thermo Fischer Scientific, Gibco, cat. No. 12338018); EX-CELL 293 serum-free medium (Sigma-Aldrich, SAFC, cat. No. 14571C). For suspension experiments, the cells were grown either on suspension culture plates or under adherent conditions for 24 h in the indicated media and then detached. Transfections were conducted using Lipofectamine 3000 (Thermo Fischer Scientific, cat. No. L3000001), FuGene HD, and ViaFect (Promega, cat. No. E2311and cat. No. E4981) transfection reagents in accordance with the manufacturer's instructions at a 1:3 w/v plasmid to reagent ratio.
Heat Treatment of Engineered Cells
Cells were seeded at 3 × 104 per cm2 and cultured for 24 h in a humidified atmosphere at 37 °C and 5% CO2. Before the experiment, the cells were washed twice with either cold or prewarmed RPMI1640 medium, and then a fresh cell culture medium was added to each well. Temperature was controlled with a plate thermoblock (Eppendorf Thermomixer). Induction experiments at different temperatures were conducted by prewarming the medium. Stimulation with sunlight was done either by using a 3x, 10 cm diameter optical lens to focus the light or by attaching a black absorbing material to the bottom of the cell culture plate. For stimulation using a hand-warming pack (EAN; 8033355780244), the cell culture plate was positioned on top of the pack 5 min after activation, when the pack had reached a stable temperature. For reversibility experiments, the cells were stimulated with temperature or sunlight and a black surface for 10 min and then separated into 3 groups, which were restimulated after 1 h, 2 h, or 3 h.
Capsaicin Treatment of Engineered Cells
Cells were seeded at 3 × 104 per cm2 and cultured for 24 h in a humidified atmosphere at 37 °C and 5% CO2. Before the experiment, the cells were washed twice with either cold or prewarmed RPMI640 medium, and then fresh cell culture medium was added to each well. Stimulation with capsaicin was done either by adding capsaicin (Sigma-Aldrich, cat. No. M2028) dissolved in DMSO to the fresh cell culture medium at the desired concentration of 1 µm and then incubating the cells for 10 min in this medium or by dropwise addition of a 2x concentrated medium to the cell culture well for microscopy experiments. For experiments involving a comparison of the secretory activity in different media, the cells were incubated for 24 h after seeding, then the medium was exchanged twice before the addition of the first medium for 15 min, followed by the second medium (with capsaicin or KCl or DMSO) for 15 min and then a third medium for the last 15 min. HEK293T cells were stimulated for 1 h in a capsaicin medium and then incubated for 24 h in the inducer-free medium. For reversibility experiments, the cells were stimulated with capsaicin for 10 min and then separated into 3 groups, which were restimulated after 1 h, 2 h, or 3 h. For inhibitor experiments, the cells were pretreated with 50 µm verapamil or 750 µm ethylene glycol tetraacetic acid (EGTA) for 20 min before stimulation. For temperature-dependent capsaicin sensitivity, the cells were incubated with a medium prewarmed to the indicated temperatures for 10 min.
Cell Viability Determination
To determine the percentage of dead cells, Trypan Blue (Gibco, cat. No. 15250061) was mixed with cells resuspended in the medium at a 1:1 ratio in a CellDrop brightfield automated cell counter. Detection of metabolic activity of the cells as an indicator of cell viability was done by adding a 1:10 dilution of alamarBlue cell viability reagent (Invitrogen, cat. No. DAL1025) to the cell culture medium, followed by incubation for 2 h. Fluorescence was measured at 560/590 nm excitation/emission. For experiments regarding capsaicin-induced toxicity, the cells were incubated for 12 h in inducer free medium before the measurement.
Microscopy
For microscopy experiments, the cells were plated on cell culture treated chambered 8-well coverslips µ-Slide ibiTreat (Ibidi GmbH, Munich, Germany, cat. No. 80826) or cell culture treated 6-well AgriWell plates (Stem Cell Technologies, cat. No. 34421). Fluorescence signals were acquired with the 405 and 488 nm laser lines and 450 and 525 nm emission wavelengths (Hoechst33342, 405/450; Ypet, 488/525) on a Nikon WF6+A1 confocal system. For calcium measurements, the cells were stained with Rhod-2 AM fluorescent dye (cat. No. R1244, Invitrogen) and the increase in fluorescence was visualized on a Nikon X1 spinning disk confocal microscope equipped with Nikon Apo 60x oil immersion objective. The dye was stored as a frozen 5 mm solution in DMSO and diluted in a medium shortly before usage to 5 µm working concentration. After incubation for 15 min, a fresh medium was added to the cells. Baseline fluorescence was determined by acquiring an image every 5 sec for 60 sec and then 2x capsaicin medium was added dropwise to the wells and further imaging was immediately commenced at 5-sec intervals. The change of fluorescence intensity was calculated as dF/F0 = (F(t) – F0)/F0 from the obtained images using Fiji to quantify the pixel intensity. For confocal immunocytochemical analysis of insulin storage granules in 1.1E7TRPV1, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% TritonX-100. Insulin staining was done with rabbit anti-insulin (ab282459, Abcam), followed by donkey anti-rabbit Alexa594 (A-21207, Thermo Fisher Scientific).
Nanoluciferase (NLuc) and Thermostable Nanoluciferase (thermoLuc) Quantification
The Nano-Glo Luciferase Assay System (Promega, Madison, Wisconsin, USA, cat. No. N1110) was used to measure the concentration of NLuc and thermoLuc after centrifugation in cell culture supernatants according to the manufacturer's protocol. In brief, 7.5 µL from each well or sample was mixed with 7.5 µL of a buffer and substrate mixture in a 50:1 ratio in a 384-well plate (Greiner BioOne, Kremsmünster, Austria, cat. No. 781076 and Carl Roth, Karlsruhe, Germany, cat. No. CEL1.1) and incubated at room temperature (21 °C) for 10 min. Luminescence was quantified using a Tecan M1000 plate reader (Tecan Group Ltd., Switzerland). For smartphone-based quantification, the pixel intensity was determined as the average pixel intensity in the area of each well when localized directly below the camera, using Fiji image processing software from the obtained images. The measured background pixel intensity was subtracted from each well to obtain the Smartphone RLU values.
Insulin Quantification
Insulin in the cell culture supernatant was quantified using a mouse insulin ELISA Kit (Mercodia, cat. No. 10-1247-01) according to the manufacturer's instructions. Concentrations were calculated from a standard curve. To each well, 10 µl of standard or sample was added, followed by 100 µl of enzyme conjugate solution. The plate was incubated for 2 h at room temperature, then washed six times, and 200 µl of TMB substrate was added for 15 min before the addition of 50 µl stop solution. Optical density was read at 450 nm after the addition of the stop solution. Bioactivity of rapidly released insulin from 1.1E7TRPV1 beta cells in saline was quantified via activation of the insulin receptor and rerouting of its downstream signaling via the MAPK pathway using an Elk1-TetR fusion transcription factor to a TetO7 –controlled minimal promoter for the SEAP reporter gene.
GLP-1 Quantification
Human GLP-1 in the cell culture supernatant was quantified using an Abcam Human GLP-1(7-36) SimpleStep ELISA Kit (Abcam, cat. No. ab184857) according to the manufacturer's instructions. In brief, the measured concentrations were determined using a standard curve. To each well 50 µl of standard or sample was added, followed by 50 µl of HRP conjugated antibody cocktail was added. The plate was incubated for 1 h at room temperature, then washed, and the TMB substrate was added. Optical density was read at 450 nm directly after the addition of the stop solution.
Erythropoietin Quantification
Human erythropoietin in the cell culture supernatant was quantified using a RayBio Human Erythropoietin ELISA Kit (Ray Biotech, cat. No. ELH-EPO-1) according to the manufacturer's instructions. Concentrations were calculated from a standard curve. To each well, 100 µl of standard or sample was added. The plate was incubated for 2.5 h at room temperature, then washed, and 100 µl of biotinylated EPO antibody was added. After incubation for one hour, the plate was washed, and HRP-conjugated streptavidin was added to convert the TMB substrate. Optical density was read at 450 nm after the addition of the stop solution.
Statistical Analysis
Detailed information on the sample size (n), the data presentation, and the corresponding statistical analysis are included in the corresponding figure captions. For analysis of statistical significance in comparison of two data sets, an unpaired Student's two-tailed t-test was used, whereas one-way ANOVA was applied when analyzing multiple comparisons. The analysis was done using Prism GraphPad 8 (GraphPad Software Inc., San Diego, California, USA).
Animal Experiments
For animal experiments and quantification of insulin in a cell phone-based assay, the cells were seeded and cultured at the usual densities in FCS-supplemented RPMI1640. Before stimulation, the cells were washed twice with phosphate-buffered saline (PBS) and then induced for 10 min at a distance of 40 cm using a Beurer IL35 IR Lamp (EAN: 4211125616113) at 150 W power to simulate consistent solar radiation. Upon completion of the induction phase, the supernatants were collected, pooled, and processed using 3 kDa Ultracel-3 membrane filter tubes (Merck Millipore, cat. No. UFC800308). Then, 1 mL of the final insulin solution was injected i.p. for a total of 0.6–0.8 µg insulin per animal as calculated from the smartphone-based assay results. The control animals were injected with saline. For the glucose tolerance test the T1D mice were injected with 2.5 g kg−1 body weight of D-glucose at the start of the experiment. Measured blood glucose values were capped at 33.3 mm. All experiments involving animals were performed according to the directive of the European Community Council (2010/63/EU), approved by the French Republic (project no. DR2018-40v5, APAFIS #16753), and carried out by Ghislaine Charpin-El Hamri (license no. 69266309) at the Institut Universitaire de Technologie of the Université Claude Bernard Lyon 1, F-696226, Villeurbanne Cedex, France.
Generation of Monoclonal Stable Cell Lines
The integration vectors with Sleeping Beauty recognition sites and encoding the desired integration cassette and both a resistance marker and a fluorophore were co-transfected with a hyperactive Sleeping Beauty transposase (SB100x) expression vector in a 10:1 (w/w) ratio. Fresh medium was added 24 h after transfection and the cells were cultivated for a further 24 h. Then, a selection medium containing antibiotics corresponding to the resistance encoded by the integrated vectors was added. Zeocin was used at a final concentration of 100 µg mL−1 and puromycin at 3 µg mL−1. After maintenance in the selection medium for three passages the cells showing the highest expression levels were selected by means of fluorescence-activated cell sorting based on the signal intensity of the encoded fluorophore. Monoclonal cell populations were obtained by sorting a single cell per well in a 96-well format, followed by expansion for 20 days and functional analysis.
Acknowledgements
The authors thank Mohamed Mahameed for experimental support and helpful discussions. The authors thank Tom Lummen for support in microscopy experiments, Aleksandra Gumienny for support in cell-sorting experiments, and the BioEM Lab of the University of Basel for TEM experiments. The study was funded in part by a European Research Council advanced grant (ElectroGene, no. 785800) and in part by the National Center of Competence in Research (NCCR) for Molecular Systems Engineering.
Open access funding provided by Eidgenossische Technische Hochschule Zurich.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
B.-A.S., M.M., and M.F. designed the project. B.-A.S. conducted the in vitro experiments. G.C. conducted animal experiments. B.-A.S., M.M., and M.F. analyzed the data and wrote the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




