Trophic niche isotope analysis of the fish assemblages in a subtropical river, Southern China
Abstract
Freshwater fishery resources in Chinese rivers have been markedly impaired as the results of overfishing, damming, and watershed development. However, little is known about trophic ecology of fish assemblages in the rivers of China. In this study, fishes were collected from an upstream, midstream, and downstream site of the Moyangjiang River, Southern China, to investigate trophic niche structure using stable isotope analysis. We calculated several trophic niche metrics using δ13C and δ15N ratios of the fish assemblages at each study site. Results showed no significant differences in the δ13C ratios between the pelagic and benthic fish assemblages at each study site. This suggests a homogenous dietary base shared by all fish at each site, likely as the result of continuous water column mixing in this shallow and fast-moving river. The upstream fish assemblage had the greatest species abundance and trophic diversity. The fish assemblage at the midstream site displayed the smallest trophic niche space (total area of the δ13C and δ15N bi-plot space) and trophic diversity. The midstream site also showed the most depleted 13C, suggesting a more degraded habitat compared with the other two study sites. The fish assemblages at the downstream site displayed the greatest basal resource diversity, largest CR, and trophic niche space and trophic diversity. However, the fish assemblage at the downstream site also displayed the lowest species abundance, redundancy, and evenness of trophic spacing. These findings suggest that the trophic niche of the fish assemblage at the downstream site, which received resources from upstream flow, experienced greater watershed development and greater in situ production, demonstrating positive and negative impacts by anthropogenic activities. Future studies should also gather information on fish production, watershed development, and water quality, to aid in the interpretation of the stable isotope analysis of fish trophic niches.
1 INTRODUCTION
Fish assemblages are important components of river food webs (Power, 1990). Fishes are consumers of various natural resources. They are preyed upon by top predators and used as a protein source or for recreational fisheries by humans (Chen et al., 2009; Forsberg et al., 1993). All fishes in a specific habitat are positioned within a food web characterized by species composition, trophic base, trophic position (TP), and interactions (Paine, 1980). Therefore, the trophic niche of a fish assemblage is indicative of ecosystem function and stability, which in turn are influenced by natural perturbations and human activities. The trophic niche of fish assemblages along the river continuum may be dramatically different as the results of changes in hydrology, plant canopy, and land use (Doctor et al., 2008; Finlay, 2004; Rasmussen & Trudeau, 2007).
Carbon and nitrogen stable isotopes are convenient tools for studying food webs (Bearhop et al., 2004; Fry, 1991; Kling et al., 1992). The carbon stable isotope ratio (δ13C) of organic matter reflects the origin of primary production (Peterson & Fry, 1987). During the transfer of organic matter from one trophic level to another, carbon isotope fractionation appears to be negligible, so δ13C is used as an indicator of the trophic base of the food web (Post, 2002). The nitrogen stable isotope ratio (δ15N) of consumers increases consistently during each trophic transfer (Minagawa & Wada, 1984; Post, 2002) and is an indicator of a consumer's TP (Bearhop et al., 2004; Post, 2002). As the stable isotope signature of consumers reflects the chemical compounds assimilated from their diets over space and time, analyses of a consumer's δ13C and δ15N can provide spatially and temporally integrated information on trophic niches (Jackson et al., 2011; Layman, Arrington, et al., 2007). Stable isotope analysis has been successfully applied to provide a better understanding of the food web structure of fishes in rivers and streams, with the majority of studies focused on North and South America (Fry, 2002; Pilger et al., 2010). Layman, Arrington, et al. (2007) proposed a set of trophic niche metrics to describe community trophic structure. A number of studies have used this approach to quantify food web structure (Cooper & Wissel, 2012; Masese et al., 2018; Suriyamongkol et al., 2022), ecosystem fragmentation (Layman, Quattrochi, et al., 2007), hydrological changes (Delong et al., 2011), and species invasion (Quintana et al., 2023). More recently, Bayesian approach has been used to quantify Layman's trophic metrics (Brush et al., 2016; Cutting et al., 2016; Jackson et al., 2011; Wang et al., 2018).
Fisheries resources in Chinese rivers have been greatly depleted due to overfishing, damming, and environmental pollution (Chen et al., 2009, 2012). The rivers and streams in the southwestern Guangdong Province of China are ideal for studying the trophic ecology of fishes. The fish assemblages in the region are characterized by high species richness (The Pearl River Institute of Fishery Science, 1991). Although some of the rivers, especially the headwaters, have been protected from human influence, the mid and lower reaches have experienced various magnitudes of ecological change (Chen et al., 2009). There is an urgent need to gather basic information on the distribution and trophic structure of freshwater fish for biological conservation and restoration (Chen et al., 2012). There are increasing studies on few trophic structure of riverine fish assemblages in China using the stable isotope approach (Chen et al., 2010; Qin et al., 2021; Zhang et al., 2007, 2019).
The objectives of this study were to (1) characterize the trophic niches of the fish assemblages at upstream, midstream, and downstream sites in the Moyangjiang River using δ13C and δ15N, and (2) assess if there were any significant differences in trophic niche along the river. The combination of downriver environmental changes and rich fish resources makes the Moyangjiang River an ideal system by which to examine the responses of fish trophic niche to anthropogenic disturbances. The distribution of δ13C and δ15N of the three fish assemblages and six niche metrics were used to determine the trophic niches at upstream, midstream, and downstream sites.
2 METHODS
2.1 Study sites
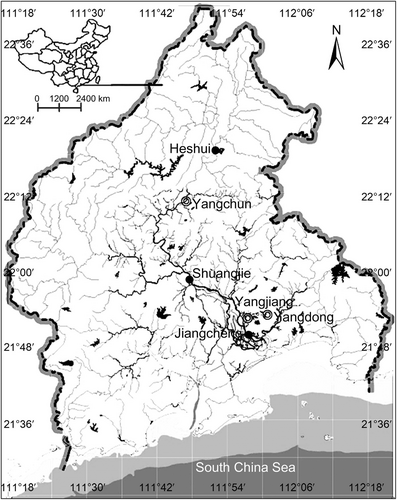
Moyangjiang River is located in Guangdong Province, China, and enters the South China Sea (Figure 1). The climate is humid subtropical and nearing a tropical climate in the far south. Winters are short, mild, and relatively dry, whereas summers are long, hot, and wet. The average annual air temperature of the drainage basin is 23°C. The average annual river discharge is ~8.8 × 109 m3. The main channel of the river is nearly 200 km long and encompasses a drainage basin of 60,910 km2. The headwaters of the Moyangjiang River originate in the mountains of western Guangdong Province and flow through an undeveloped region. The midstream is typically shallow and land use in the watershed is dominated by agriculture. The downstream portion of the watershed is dominated by dense residential and agricultural development. As a consequence, water quality degrades downriver as chemical contamination increases (Yuan & Zhu, 2010).
2.2 Sample collection and processing
Fish samples were collected from three study sites along the Moyangjiang River from 17 to 19 July of 2011. The study sites were located upstream, midstream, and downstream in the main river channel (Figure 1). Fishes were caught using gillnets, dipnets, and seine nets. The sampling effort yielded a total of 422 fish specimens from six orders, 14 families, 27 genera, and 27 species. The number of species collected was highest at upstream (18), followed by midstream (14), and downstream (nine) (Appendix Tables A1–A4).
All samples were stored on ice in the field and shipped to the laboratory for identification and processing. In the laboratory, the dorsal muscle tissue from each fish specimen was collected. After lipids were removed from the muscle tissue following the technique of Bligh and Dyer (1959), samples were dried at 60°C and ground to a fine powder. Between 0.3 and 0.5 mg of dry material was loaded into tin capsules and analyzed for carbon and nitrogen stable isotopes.
2.3 Stable isotope analysis
Stable isotope analysis was conducted at Washington State University. Samples were combusted in a Costech elemental analyzer (Valencia). N2 and CO2 gases from combusted samples were analyzed for 15N/14N and 13C/12C with a continuous flow isotope ratio mass spectrometer (GV Instruments Isoprime). Ovalbumin was analyzed in sample runs as a routine standard. The isotope ratio is expressed in the conventional delta (δ) notation, defined as the per mil (‰) deviation from the isotope standard, Peedee Belemnite formation for carbon, and the ambient atmosphere for nitrogen. Routine precision for replicate samples was ±0.3‰ for δ15N and ±0.1‰ for δ13C.
2.4 Data analysis
At each study site, we calculated fish TP as per Post (2002) and six community-wide trophic metrics, as proposed by Layman, Arrington, et al. (2007), were calculated using the Bayesian approach in R. The six niche metrics are measures of the total extent of spacing and trophic redundancy within a δ13C and δ15N bi-plot (Layman, Arrington, et al., 2007). The δ13C range (CR) is the difference between the species with the most enriched and most depleted δ13C ratio and is a measure of basal resource diversity. The δ15N range (NR) is the difference between the species with the most enriched and most depleted δ15N ratio, and is a measure of trophic length within a food chain. The total area (TA) of the δ13C and δ15N bi-plot space (convex hull) describes the total space occupied by a community. TA is now often represented using Bayesian standard ellipse area (SEA). The mean distance to the δ13C-δ15N centroid (CD) provides a measure of trophic diversity. The mean nearest-neighbor distance (MNND) is the mean of the Euclidean distances to each species' nearest neighbor in bi-plot space and is thus a measure of the overall density of species packing. Small MNND suggests increased trophic redundancy. Finally, the SD of nearest-neighbor distance (SDNND) is a measure of the evenness of specie packing in bi-plot space. Low SDNND values means more even distribution of trophic niches. More recently, a Bayesian approach has been used to quantify Layman's trophic metrics (Jackson et al., 2011).
2.5 Statistical analysis
Statistical analyses were performed using SigmaPlot software (Version 12.5, Systat Software, Inc.). Data sets were first examined for normality using the Shapiro–Wilk procedure. Data sets that passed the normality test were analyzed using one-way analysis of variance (ANOVA) followed by a pairwise comparison (Holm–Sidak method) or T test for the two sample comparison. Data sets that failed the normality test were analyzed using the nonparametric procedure (Kruskal–Wallis ANOVA on ranks) followed by pairwise comparisons (Dunn's method) or the Mann–Whitney U Statistic for two sample comparison. A two-way ANOVA was used to examine the interactive effects of study sites (upstream, midstream and downstream) and habitats (pelagic and benthic community) on the carbon isotope compositions of fish (indicators of resource use). Statistical differences were considered to be significant at p < 0.05.
3 RESULTS
3.1 Isotope signatures of the three fish assemblages
The average δ13C ratio of the fish assemblages was −21.8‰ at the upstream site, −23.7‰ at the midstream site, and −22.8‰ at the downstream site (Figure 2). A significant difference in the δ13C ratios was found among the three study sites (ANOVA, df = 2, F = 9.08, p < 0.001). Multiple comparison showed that the difference occurred between the upstream and the midstream sites (Dunn's method, t = 4.26, p < 0.001). The δ15N ratios of the fish assemblages ranged from 8.1‰ to 15.6‰ at the upstream site, from 10.2‰ to 14.0‰ at the midstream site, and from 9.3‰ to 14.0‰ at the downstream site (Figure 2). The site average δ15N ratios varied from 12.2‰ to 12.8‰ (Kruskal–Wallis analysis, H = 5.19, p = 0.07). As the number and identity of fish species was different among the three sites, we also compared the average isotope ratios of the five species found at all three sites (Figure 2). These species include sharpbelly (Hemiculter leucisculus), mud carp (Cirrhinus molitorella), crucian carp (Carassius auratus), beauty loach (Traccatichthys pulcher), and sharphead sleeper (Eleotris oxycephala), which are common indigenous fish in south China (Appendix Table A2–A4) Unlike the fish assemblages, there were no significant differences in the average δ13C of the five fish species among sites (ANOVA, F = 1.56, p = 0.24). Similar to results for the fish assemblages, for the five fish species examined, δ15N did not differ significantly among the three sites (ANOVA, F = 0.15, p = 0.86, Figure 2).
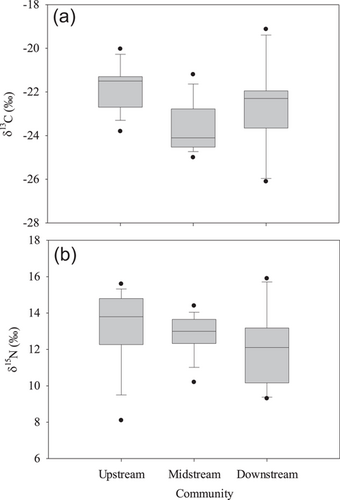
When isotope data from individuals of the same species among sites were compared, there was a significant difference in δ13C (Kruskal–Wallis, H = 15.10, p ≤ 0.001) and δ15N (ANOVA, F = 140.73, p < 0.001) for crucian carp. The difference in δ13C occurred between upstream and downstream (Dunn's method, Q = 3.873, p < 0.05) and the differences in δ15N occurred in all site comparisons (Holm–Sidak method, all p < 0.005). Similarly, sharpbelly fish also showed significant differences in both δ13C (Kruskal–Wallis, H = 10.02, p = 0.007) and δ15N (Kruskal–Wallis, H = 33.33, p ≤ 0.001) among sites. For δ13C, the difference occurred between upstream and downstream (Dunn's method, Q = 3.16, p < 0.05). For δ15N, there was a significant difference between upstream and midstream (Dunn's method, Q = 17.55, p < 0.05), and between upstream and downstream (Q = 5.64, p < 0.05). Mud carp showed similar δ13C among sites (ANOVA, F = 1.39, p = 0.263), but differed in δ15N (Kruskal–Wallis, H = 12.47, p = 0.002). The difference occurred only between upstream and downstream (Dunn's method, Q = 3.53, p < 0.05). Only two sites contained sufficient individuals of sharphead sleeper and Amur catfish for statistical comparisons. There was no difference in δ13C (two-tailed T test, t = −0.551, p = 0.304) and δ15N (Mann–Whitney, T = 199.00, p ≤ 0.001) of sharphead sleeper between midstream and downstream. However, there was a significant difference in δ15N between the two sites (Mann–Whitney, T = 199.00, p ≤ 0001). For Amur catfish collected at upstream and midstream, there was no difference in δ13C (Mann–Whitney, T = 101.00, p = 0.089), but a significant difference in δ15N (Mann–Whitney, T = 120.00, p = 0.003).
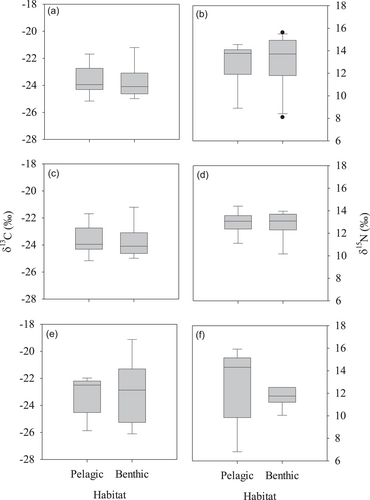
The stable isotope ratios of pelagic and benthic fish assemblages were compared to assess the types of resources available at each habitat (Figure 3). Average δ13C ratios ranged from −23.6‰ at midstream to −22.4‰ at upstream for pelagic fish and from −23.8‰ at midstream to −21.7‰ at upstream for benthic fish. For all of the study sites, the average δ13C ratio differed by <1.0‰ between pelagic and benthic fish assemblages. Results from two-way ANOVA on δ13C data with study sites and habitats as independent factors revealed a significant site effect (df = 2, F = 7.12, p = 0.002), but no significant effects due to habitat types (df = 1, F = 0.13, p = 0.719) and site and habitat interactions (df = 2, F = 0.24, p = 0.773; Table 1).
| Community | δ13C mean ± SD | δ15N mean ± SD | TP ± SD | N |
|---|---|---|---|---|
| Upstream | −21.8 ± 1.0 | 13.1 ± 2.0 | 3.7 ± 0.6 | 19 |
| Midstream | −23.7 ± 1.0 | 12.9 ± 1.1 | 3.2 ± 0.3 | 18 |
| Downstream | −23.0 ± 1.5 | 12.2 ± 1.9 | 3.8 ± 0.5 | 10 |
- Abbreviation: TP, trophic position.
3.2 Trophic niche
The TP of fish assemblages in Moyangjiang River was similar between the upstream and downstream sites and was lower at the midstream site (Table 2). There were general trends of decreasing trophic metrics values from upstream to downstream (Figure 4). The trophic space (SEAs) differed considerably among study sites, with the upstream SEAs more than twice as much as that in midstream and downstream. The high SEAs at the upstream was the result of the greater CR (dX range) and NR (dY range) than other sites. The CR at the upstream site was nearly twice as high as the midstream site and slightly higher than the downstream site. However, the CR is over 3‰ wider than the midstream site. Similarly, the mean distance to centroid (CD) was highest at the upstream, lowest at midstream and intermediate at downstream. The upstream–downstream trend of trophic metrics values also appeared to the MNND and the SDNND.
| Community | NR | CR | TA | CD | MNND | SDNND |
|---|---|---|---|---|---|---|
| Upstream | 7.5 | 3.5 | 13.36 | 1.75 | 0.69 | 0.50 |
| Midstream | 4.2 | 3.8 | 9.11 | 1.25 | 0.60 | 0.54 |
| Downstream | 6.6 | 4.3 | 14.57 | 2.04 | 1.28 | 0.77 |
- Abbreviations: CD, mean distance to centroid; CR, δ13C range; MNND, mean nearest-neighbor distance; NR, δ15N range; SDNND, SD of nearest-neighbor distance; TA, total area of niche space.
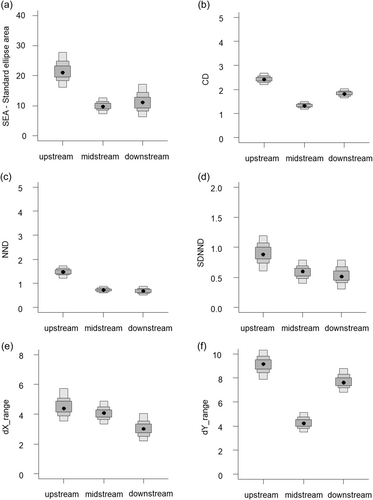
The CR was the same between upstream and downstream but greater at the downstream. Species with the most depleted and most enriched δ13C varied among sites (Figure 4). The NR was highest at the upstream, similar at the midstream and the downstream. The species with the highest δ15N ratio was mountain loach (upstream) and Japanese perch (midstream and downstream). The species with the lowest δ15N ratios were mud carp (upstream and midstream) and crucian carp (downstream).
4 DISCUSSION
Consumer trophic diversity increases with the increase in resource availability. The upstream site of the Moyangjiang River was dominated by a natural forest. As a result of the lack of agricultural land use and urbanization which may otherwise provide additional resources, the fish assemblage displayed a moderate degree of trophic diversity. This is evidenced by the narrow CR, a measure of basal resource spectrum, which led to reduced niche space (SEA). The midstream site displayed the same CR as the upstream, suggesting no increases in basal resources. The lowest average δ13C ratios at this site (Figure 2) imply that a source of 13C-depleted organic matter was present. However, the fish assemblage at the midstream site had the smallest NR, which led to the smallest trophic niche space occupied. The smallest NR also indicated a lower trophic level for the Japanese perch at this site. The poor environmental conditions in this river section (Yuan & Zhu, 2010) might have decreased edible basal resources and lowered the TP of the top predators inhabiting the midstream site. Further studies should collect hydrological and water quality data to verify these observations.
The highest CR of the fish assemblages at the downstream section of the Moyangjiang River suggest that resources supporting the river fish assemblages tended to be more diversified downstream. This is because the downstream region of a river is the recipient of resources from the upper river section and the watershed. In southern China, the downstream ^g of a river system often has a highly developed watershed, dominated by urbanization or/and agriculture and is therefore more polluted or eutrophic, with high in situ primary production. This is also the case for the Moyangiang River (Yuan & Zhu, 2010). The fish assemblage at the downstream site of the Moyangjiang River occupied a greater trophic space as indicated by greater δ13C-δ15N bi-plot spacing (TA) and greater mean distance to CD. However, fish species diversity at the downstream site was considerably less than the two upper river sites. This might suggest that anthropogenic pollution had eliminated environmentally sensitive species (de Carvalho et al., 2020; Dudgeon et al., 2006).
Despite increases in basal resources at the downstream site which provided greater trophic spacing for the fish assemblage, compared to the upstream and midstream sites, anthropogenic disturbances also decreased species diversity at the downstream site as indicated by lower species abundance, lower species redundancy (MNND), and a lower degree of evenness of distribution (SDNND). These measures revealed the negative impacts of environmental pollution on fish community structure. It appears that increasing system productivity accompanied by decreasing environmental quality induced by watershed changes may pose positive (fish yield) and negative impacts (fish diversity) to the riverine fish assemblages.
Trophic diversity may also be enhanced by hydrology, particularly water mixing. In general, there is a contrasting δ13C signature between planktonic and benthic algae in lakes and streams (France, 1995; Hecky & Hesslein, 1995), which may influence the isotope compositions of the pelagic and benthic fish. However, δ13C ratios of the pelagic and benthic fish assemblages in the Moyangjiang River were not significantly different. Our results suggest that the pelagic and benthic fish consume multiple sources of organic matter within their habitats, resulting in similar isotope signatures. It is possible that some pelagic and benthic fish also obtain a portion of their diets from other habitats, thereby resulting in consuming similar dietary items resulting in a similar stable isotope composition. Zanden and Vadeboncoeur (2002) reported that the pelagic fish in north-temperate lakes relied 65% on benthic secondary production. In this study, common carp (C. carpio), crucian carp, tilapia and mud carp may feed between the surface and bottom waters. Furthermore, suspension of benthic production due to rapid river flow and the settlement of pelagic primary production to a river bottom may provide additional dietary resources to the respective habitat.
5 CONCLUSIONS
Understanding the trophic niche structure of the fish assemblages in rivers is essential for wildlife conservation in inland waters. This study describes the trophic niche characteristics of the fish assemblages from a subtropical river in China. Our results and analysis reveal the existence of differences in the trophic niches of three fish assemblages and the resource diversity in this subtropical river. Anthropogenic influences are shown here to impart change to the fish trophic niche structure. These changes contain positive and negative aspects with regard to the fish trophic niche diversity and stability. There is a need to better define the relationship between human activities and the responses of trophic niche diversity in receiving water systems. This may be accomplished by the collection of biological and environmental data from the river channel and the human activities within the watershed.
ACKNOWLEDGMENTS
We appreciate the efforts of Dr. Thomas Dreschel for language editing. This study was supported by several grants (#2005A20105001, 2006B20701001, and B200601G02) from the Bureau of Science and Technology of Guangdong Province, China, a grant (#41376158) from the Natural National Science Foundation of China and a grant from China Agriculture System (CARS-50).
ETHICS STATEMENT
None declared.
APPENDIX A
| Order | Family | Species | Common name | Habitat | Main prey |
|---|---|---|---|---|---|
| Tetraodontiformes | Tetraodontidae | Takifugu rubripes | Japanese puffer | Pelagic | Invertebrates |
| Perciformes | Serranidae | Lateolabrax japonicus | Japanese seabass | Pelagic | Fish |
| Perciformes | Cichlaidae | Tilapia nilotica | Nile tilapia | Pelagic | Plankton, detritus |
| Perciformes | Basilewsky | Mastacembelus armatus | Zig-zag eel | Benthic | Small invertebrates |
| Perciformes | Eleotridae | Eleotris oxycephala | Sharphead sleeper | Benthic | Invertebrates |
| Perciformes | Gobiidae | Glossogobius giuris | Tank goby | Benthic | Algae, detritus, invertebrates |
| Perciformes | Channidae | Channa maculata | Blotched Snakehead | Benthic | Fish, invertebrates |
| Cypriniformes | Cyprinidae | Opsariichthys uncirostris bidens | Amur three-lips | Pelagic | Fish |
| Cypriniformes | Cyprinidae | Zacco platypus | Pale chub | Benthic | Crustaceans |
| Cypriniformes | Cyprinidae | Hemiculter leucisculus | Sharpbelly | Pelagic | Invertebrates |
| Cypriniformes | Cyprinidae | Rhodeus sinensis | Bony fish | Pelagic | Algae, detritus |
| Cypriniformes | Cyprinidae | Cirrhinus molitorella | Mud carp | Benthic | Algae, detritus |
| Cypriniformes | Cyprinidae | Osteochilus salsburyi | NA | Benthic | Algae, detritus |
| Cypriniformes | Cyprinidae | Pseudogobio vaillanti | Psudo gudgeon | Pelagic | Fish, invertebrates |
| Cypriniformes | Cyprinidae | Cyprinus carpio | Common carp | Benthic | Detritus, invertebrates |
| Cypriniformes | Cyprinidae | Carassius auratus | Crucian carp | Pelagic | Plankton, detritus |
| Cypriniformes | Cyprinidae | Traccatichthys pulcher | Beautiful hillstream loach | Benthic | Aquatic insects |
| Cypriniformes | Cyprinidae | Cobitis sinensis | Siberian spiny loach | Benthic | Algae, plant detritus |
| Cypriniformes | Cyprinidae | Hemibarbus nedius | NA | Pelagic | Invertebrates |
| Cypriniformes | Cyprinidae | Erythroculter ilishaeformis | Topmouth culter, | Pelagic | Fish |
| Cypriniformes | Cyprinidae | Sinibrama wui | Ray-finned fish | Pelagic | Plants |
| Cypriniformes | Cobitidae | Misgurnus anguillicaudatus | Pond roach | Benthic | Plankton, detritus, invertebrates |
| Clupeiformes | Clupeidae | Clupanodon thrissa | Chinese gizzard shad | Pelagic | Plankton |
| Clupeiformes | Engraulidae | Coilia mystus | grenadier anchovies | Pelagic | Invertebrates |
| Siluriformes | Siluridae | Silurus asotus | Amur catfish | Pelagic | Scavengers |
| Siluriformes | Amblycipitidae | Pelteobagrus fulvidraco | Yellowhead catfish | Benthic | Small fish, invertebrates |
| Anguilliformes | Anguillidae | Anguilla japonica | Japanese eel | Benthic | Invertebrates, small fish |
| Species | n | SL (cm) | WW (g) | δ13C | δ15N |
|---|---|---|---|---|---|
| Hemiculter leucisculus | 19 | 12.0 ± 3.8 | 25.4 ± 21.5 | −22.7 ± 1.8 | 9.5 ± 1.6 |
| Cirrhinus molitorella | 8 | 14.0 ± 1.6 | 64.3 ± 23.5 | −23.3 ± 3.7 | 8.1 ± 1.6 |
| Carassius auratus | 16 | 7.6 ± 2.2 | 16.9 ± 14.7 | −21.6 ± 0.9 | 14.0 ± 0.9 |
| Traccatichthys pulcher | 10 | 5.9 ± 0.7 | 3.7 ± 1.2 | −21.5 ± 0.6 | 15.6 ± 0.4 |
| Opsariichthys uncirostris bidens | 17 | 9.3 ± 2.4 | 15.2 ± 10.8 | −21.2 ± 0.5 | 15.2 ± 0.9 |
| Hemibarbus nedius | 14 | 10.1 ± 12.3 | 19.1 ± 12.2 | −21.3 ± 0.9 | 14.6 ± 1.3 |
| Sinibrama wui | 12 | 7.8 ± 1.5 | 8.7 ± 6.3 | −21.8 ± 2.8 | 12.9 ± 1.2 |
| Pelteobagrus fulvidraco | 6 | 7.8 ± 0.9 | 10.2 ± 3.8 | −21.3 ± 0.7 | 13.9 ± 0.8 |
| Tilapia nilotica | 6 | 13.4 ± 2.1 | 102.1 ± 42.8 | −22.8 ± 0.56 | 13.8 ± 0.4 |
| Pseudogobio vaillanti | 14 | 6.1 ± 0.6 | 3.2 ± 1.1 | −21.5 ± 0.9 | 15.1 ± 0.7 |
| Silurus asotus | 9 | 19.6 ± 4.6 | 79.4 ± 66.2 | −21.0 ± 0.6 | 14.1 ± 0.6 |
| Mastacembelus armatus | 15 | 21.1 ± 4.3 | 42.1 ± 18.9 | −21.5 ± 1.3 | 13.2 ± 1.3 |
| Cobitis sinensis | 11 | 5.8 ± 0.85 | 1.6 ± 0.4 | −21.3 ± 0.6 | 14.8 ± 0.4 |
| Osteochilus salsburyi | 4 | 7.5 ± 0.4 | 8.7 ± 1.9 | −23.8 ± 1.7 | 11.9 ± 1.6 |
| Zacco platypus | 14 | 6.5 ± 0.5 | 4.2 ± 1.2 | −22.5 ± 1.4 | 11.93 ± 1.84 |
| Rhodeus sinensis | 6 | 4.4 ± 0.3 | 1.9 ± 0.4 | −22.1 ± 2.6 | 13.8 ± 0.5 |
| Eleotris oxycephala | 1 | 9.7 | 16.7 | −20.3 | 12.3 |
| Misgurnus anguillicaudatus | 1 | −20 | 15.6 | ||
| Corbicula fluminea formosa | 4 | −23.4 ± 0.4 | 6.4 ± 1.3 | ||
| Sediment | 3 | −23.8 ± 0.1 | 3.9 ± 0.4 |
- Abbreviations: n, number of sample; SL, standard length; WW, wet weight.
- a River clam.
| Species | n | SL (cm) | WW (g) | δ13C | δ15N |
|---|---|---|---|---|---|
| Hemiculter leucisculus | 15 | 12.4 ± 2.3 | 23.2 ± 14.2 | −23.9 ± 1.4 | 12.1 ± 1.2 |
| Cirrhinus molitorella | 18 | 11.6 ± 2.7 | 41.3 ± 30.1 | −21.2 ± 3.2 | 10.2 ± 1.9 |
| Carassius auratus | 11 | 8.3 ± 1.8 | 22.4 ± 12.8 | −24.7 ± 2.5 | 12.9 ± 1.2 |
| Traccatichthys pulcher | 3 | 5.1 ± 0.2 | 1.9 ± 0.2 | −24.1 ± 0.7 | 13.9 ± 0.9 |
| Opsariichthys uncirostris bidens | 16 | 8.5 ± 1.5 | 10.0 ± 5.6 | −24.1 ± 0.6 | 13.1 ± 0.4 |
| Hemibarbus nedius | 13 | 9.7 ± 3.8 | 21.6 ± 27.3 | −23.2 ± 2.1 | 13.2 ± 0.6 |
| Sinibrama wui | 11 | 15.6 ± 4.1 | 90.7 ± 66.5 | −24.5 ± 1.4 | 11.1 ± 1.7 |
| Pelteobagrus fulvidraco | 12 | 9.8 ± 1.7 | 22.7 ± 11.6 | −25.0 ± 0.4 | 12.8 ± 0.3 |
| Eleotris oxycephala | 11 | 9.4 ± 2.0 | 18.7 ± 11.2 | −23.7 ± 1.5 | 12.4 ± 1.5 |
| Glossogobius giuris | 16 | 8.1 ± 3.4 | 13.8 ± 16.7 | −24.6 ± 1.5 | 13.4 ± 0.8 |
| Lateolabrax japonicus | 9 | 13.9 ± 2.1 | 53.7 ± 22.1 | −22.8 ± 1.9 | 14.4 ± 0.7 |
| Pseudogobio vaillanti | 12 | 6.3 ± 0.7 | 3.4 ± 1.3 | −24.1 ± 0.9 | 13.6 ± 0.7 |
| Silurus asotus | 7 | 21.3 ± 3.0 | 106.1 ± 44.9 | −22.2 ± 1.8 | 12.1 ± 0.5 |
| Takifugu rubripes | 7 | 7.4 ± 0.5 | 15.6 ± 3.5 | −24.1 ± 1.0 | 14.0 ± 0.2 |
| Coilia mystus | 3 | 15.1 ± 2.2 | 12.0 ± 4.3 | −24.1 ± 0.5 | 13.8 ± 0.5 |
| Clupanodon thrissa | 6 | 14.9 ± 1.3 | 56.1 ± 15 | −22.7 ± 1.1 | 12.7 ± 0.8 |
| Corbicula fluminea formosa | 6 | −26.7 ± 0.3 | 8.9 ± 0.4 | ||
| Sediment | 3 | −23.7 ± 0.7 | 3.9 ± 1.9 |
- Abbreviations: n, number of sample; SL, standard length; WW, wet weight.
- a River clam.
| Species | n | SL (cm) | WW (g) | δ13C | δ15N |
|---|---|---|---|---|---|
| Hemiculter leucisculus | 12 | 12.2 ± 1.7 | 23.5 ± 7.7 | −21.8 ± 3.7 | 14.0 ± 3.8 |
| Cirrhinus molitorella | 13 | 11.8 ± 2.3 | 43.4 ± 28.7 | −22.3 ± 2.3 | 12.4 ± 1.9 |
| Carassius auratus | 13 | 9.9 ± 2.1 | 39.6 ± 29.9 | −22.3 ± 0.5 | 9.3 ± 0.4 |
| Pseudogobio vaillanti | 5 | 14.4 ± 0.9 | 94.4 ± 24.8 | −22.0 ± 1.1 | 12.5 ± 0.7 |
| Traccatichthys pulcher | 6 | 10.0 ± 1.6 | 9.1 ± 4.7 | −26.1 ± 1.7 | 10.2 ± 3.1 |
| Lateolabrax japonicus | 9 | 15.7 ± 1.4 | 76.3 ± 23.5 | −22.5 ± 0.6 | 15.9 ± 1.0 |
| Tilapia nilotica | 3 | 11.5 ± 1.9 | 72.7 ± 36.1 | −22.0 ± 1.1 | 12.9 ± 1.1 |
| Glossogobius giuris | 6 | 8.0 ± 2.2 | 9.9 ± 8.2 | −24.7 ± 1.3 | 11.8 ± 1.07 |
| Eleotris oxycephala | 8 | 7.1 ± 1.2 | 9.6 ± 5.9 | −23.3 ± 1.4 | 11.2 ± 0.9 |
| Corbicula fluminea formosa | 17 | −25.9 ± 0.1 | 6.8 ± 0.2 | ||
| Sediment | 3 | −24.5 ± 0.3 | 5.7 ± 0.9 |
- Abbreviations: n, number of sample; SL, standard length; WW, wet weight.
- a River clam.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available upon request.




