The gonadotropins starting dose calculator, which can be adjusted the target number of oocytes and stimulation duration days to achieve individualized controlled ovarian stimulation in Japanese patients
Abstract
Purpose
To create a gonadotropin starting dose calculator for controlled ovarian stimulation, which can adjust the target number of oocytes and stimulation duration for each facility to achieve individualized controlled ovarian stimulation among the Japanese patients.
Methods
The patients received controlled ovarian stimulation using the gonadotropin-releasing hormone antagonist protocol, and oocytes were retrieved. Using single regression analysis, we selected age, anti-Müllerian hormone (AMH), and initial serum follicle-stimulating hormone as variables to predict the number of oocytes retrieved per gonadotropin dose (oocyte sensitivity index). Each variable was then analyzed using backward stepwise multiple regression.
Results
Age and AMH were selected as predictive variables from the backward stepwise multiple regression, and we developed a multiple regression equation. We decomposed the equation as the number of oocytes retrieved/(gonadotropin starting dose × stimulation duration days) and created a calculation formula to predict the gonadotropin starting dose from the target number of oocytes and stimulation duration days.
Conclusions
This is the first study to develop an individualized dosing algorithm for gonadotropins among Japanese patients. Our calculator will improve controlled ovarian stimulation performance and enable national standardization by allowing all physicians, regardless of their years of experience, to determine the appropriate starting dose of gonadotropins equally.
1 INTRODUCTION
Recently, the cumulative pregnancy and live birth rates (LBRs) per oocyte retrieval cycle have attracted attention worldwide, as key performance indicators of assisted reproductive technology (ART).1, 2 Important factors related to cumulative pregnancy and LBRs include the number of oocytes and embryos available for transfer.3-6 These reports suggest that a higher number of oocytes may lead to an increase in cumulative live births.
However, if the number of oocytes is too high, an increased risk of developing ovarian hyperstimulation syndrome (OHSS)7 and increased costs may occur.8, 9 In addition to, the number of oocytes, cultured embryos, and frozen embryos are counted by the insurance system for ART in Japan, and the prediction of the number of oocytes is important from the viewpoint of ART results of each facility, patient satisfaction, and revenue from the additional insurance points. To obtain the target number of oocytes, controlled ovarian stimulation (COS) should be initiated with an appropriate gonadotropin starting dose and sufficient duration of stimulation to prolong the follicle-stimulating hormone (FSH) window in which many follicles develop.10 In this study, we developed a calculator to set the starting dose of gonadotropins using patient age and AMH as predictors to obtain the target number of oocytes, referring to the study by La Marca et al.11 which has developed a nomogram to determine a gonadotropin starting dose. In the nomogram of La Marca et al., multiple regression analysis was performed with the objective variable as the number of oocytes per starting dose of gonadotropins (oocyte sensitivity index [OSI]), and the explanatory variables as AMH, age, and initial FSH level from patient data with the gonadotropin-releasing hormone (GnRH) agonist protocol of COS.11 Oocyte sensitivity index is defined as the number of oocytes retrieved per total dose of gonadotropin, and is used as a dynamic marker of ovarian response.12 Anti-Müllerian hormone (AMH) and antral follicle count (AFC) can predict ovarian reserve in controlled ovarian stimulation, but cannot always predict actual ovarian reactivity. The oocyte sensitivity index shows actual ovarian reactivity using the number of oocytes retrieved and the total gonadotropin dose.12 The oocyte sensitivity index is also known to correlate with age, AMH, AFC, and body mass index (BMI).13 With reference to these studies, we considered developing a calculator that can set the starting dose of gonadotropins in the antagonist protocol. Similar to La Marca's nomogram, various individualized dosing algorithms have been reported, and individualized starting doses of gonadotropins have been determined using explanatory variables, such as AMH, AFC, age, initial serum FSH level, and body weight.14-23 Among these variables, AFC was excluded because it may change, depending on the physician's subjectivity and skill. Anti-Müllerian hormone, initial serum FSH, age, and body weight were selected as explanatory variables to predict the OSI. In contrast, in a study of Japanese women, AMH, which has a positive correlation with oocyte count, and age, which has a negative correlation with oocyte count, were selected as factors associated with oocyte number.24 The total gonadotropin dose used in the OSI was the starting dose multiplied by the duration of COS in the fixed-dose protocol. Therefore, the prediction model of OSI by multiple regression formula was decomposed by setting OSI as the number of oocytes recovered/(starting dose of gonadotropin × duration days of COS), and a formula to predict the starting dose of gonadotropin from the target number of oocytes and duration days of COS was created. The duration of COS was defined as the period from the start of gonadotropin administration to the day of the trigger administration.
This calculator has the potential to enable all physicians, regardless of their clinical experience, to achieve the optimal scheme in COS. This study aimed to develop the first individualized dosing algorithm for gonadotropins for Japanese patients. Insurance coverage for infertility treatment began in Japan in April 2022, and standardization of medical care is required. To reduce the differences in the quality of COS due to empirical rules between facilities and physicians, a calculator to determine the starting dose of gonadotropins for COS based on objective indices is required in clinical practice.
2 MATERIALS AND METHOD
We performed two studies to create a gonadotropin starting dose calculator from the OSI and to verify its accuracy.
2.1 Study 1: Development of gonadotropin starting dose calculator model
The patient population consisted of 100 individuals with 100 cycles who were 36.16 ± 4.53 years of age and underwent in vitro fertilization (IVF) between April 2020 and March 2022. This study was performed on patients who provided informed consent. Patients with hypothalamic dysfunction, endometriosis, history of ovarian surgery, or a history of pelvic radiotherapy and/or chemotherapy were excluded. We analyzed patients who underwent controlled ovarian stimulation using the GnRH antagonist protocol. We performed a sequential protocol in which urinary FSH (uFSH) (FSH Asuka; ASKA Pharmaceutical, Tokyo, Japan) or recombinant FSH (rFSH) (Gonal F; Merck BioPharma, Tokyo, Japan) was administered for 6–8 days from the start of stimulation, and highly purified human menopausal gonadotropins (hMG) (HMG Ferring; Ferring Pharma, Tokyo, Japan) was administered later for 2–12 days. The gonadotropin starting dose was determined using the La Marca's nomogram11 based on each patient's age and AMH. This nomogram is objected to in-patients between the ages of 25 and 40 years, and the starting gonadotropin dose can be determined between 75 and 230 IU. From the nomogram, if the gonadotropin dose was determined between 112.5 and 187.5 IU, we administered 150 IU. If the gonadotropin dose was determined to be between 187.5 and 230 IU, we administered 225 IU. The starting dose was 300 IU for all patients who were > 40 years of age and had <3.0 ng/ml AMH level, and for all the patients who were < 40 years of age, but had very low AMH level as 0.5 ng/ml under a setting of FSH starting dose >225 IU on the nomogram.
Using this method, the initial gonadotropin dose was determined and administered. Gonadotropin-releasing hormone antagonists (Ganirest; MSD, Tokyo, Japan) were started at a dose of 0.25 m/day when the primary follicle reached approximately 14 mm and continued until the primary follicle reached 18–20 mm. When the primary follicle reached 18–20 mm, gonadotropins and GnRH antagonist dose of 0.25 m/day were terminated, and on the same day or the next day, 250 μg of choriogonadotropin alpha (Ovitrelle; Merck biopharma, Tokyo, Japan) or 600 μg GnRH agonist (Suprecur; CLINIGEN, Tokyo, Japan) was administered as a trigger. Oocyte retrieval was performed 34–36 h after the trigger. Blood samples were collected during the study for the assessment of AMH, FSH, luteinizing hormone (LH), estradiol, and progesterone. The serum concentrations of AMH were measured at screening before the start of the cycle and were used to determine the starting dose of gonadotropins. AMH was measured using an automated Elecsys AMH assay (Roche Diagnostics, Switzerland). Serum samples for the assessment of endocrine parameters (FSH, LH, estradiol, and progesterone) were collected at the start and end of stimulation.
The primary outcome was the number of oocytes retrieved per total dose of gonadotropin (OSI).The oocyte sensitivity index shows the number of oocytes retrieved/total dose of gonadotropin (IU). From patient characteristics (Table 1), variables predicting OSI, age, AMH, body weight, and initial serum hormone levels (E2, FSH, LH) were used as objective indicators that can be measured at the start of COS, regardless of the doctor's technique. Single regression analysis was performed for these variables. Variables associated with OSI were analyzed by multiple regression analysis and the backward stepwise method.
| Age, years | 36.16 ± 4.53 |
|---|---|
| Body weight, kg | 56.25 [43.00–94.00] |
| Smokers, % | 0 |
| AFC, follicles | 9.18 ± 5.38 |
| AMH, ng/ml | 3.63 ± 2.85 |
| Initial serum FSH, IU/l | 7.58 ± 2.57 |
| Initial serum LH, IU/l | 5.78 ± 2.28 |
| Initial serum E2, pg/ml | 32.56 ± 15.27 |
| Serum LH on trigger day, IU/l | 2.24 ± 2.11 |
| Serum E2 on trigger day, pg/ml | 4740.07 ± 2326.22 |
| Serum P4 on trigger day, pg/ml | 1.61 ± 1.27 |
| Stimulation duration days, days | 13.13 ± 2.29 |
| Total gonadotropins administered, IU | 2770.05 ± 879.81 |
| Starting gonadotropins dose, IU | 209 ± 48.02 |
| Number of retrieved oocytes per woman | 12.53 ± 7.61 |
| Rates of OHSS incidence, % | 0 |
| OSI (Ovarian Sensitivity Index) × 103 | 4.10 [0.42–23.64] |
- Note: Data are presented as mean ± standard deviation or median [max-min].
In the backward stepwise method, the selection of variables was applied using Wald p < 0.05 for inclusion and p > 0.1 as exclusion criteria. A final multivariate regression model was developed for the number of oocytes retrieved per gonadotropin dose. Validation of the regression model was estimated using the concordance index (C-index), assuming that it is concordant if the predictive OSI level is within ±1 of the real OSI level. For sensible models, C-index varies between 0.5 and 1.0 (the higher, the better). The total dose of gonadotropins used for OSI was defined as the starting dose of gonadotropins multiplied by stimulation duration days, and the optimal starting dose of gonadotropins to retrieve the target number of oocytes was expressed by the following formula: Target number of oocytes/the regression formula/stimulation duration days. From this formula, we set the target number of oocytes and stimulation duration and created an application that calculates the optimal starting dose of gonadotropins from age and AMH.
2.2 Study 2: Accuracy verification of gonadotropin starting dose calculator
The patient population consisted of 12 individuals with 12 cycles who were 35.42 ± 3.55 years of age and underwent IVF between April 2022 and September 2022 (calculated group n = 12). We analyzed patients who underwent controlled ovarian stimulation using the GnRH antagonist protocol. We performed a mono-protocol using rFSH (Gonal F; Merck BioPharma, Tokyo, Japan). At the start of COS, we set the target number of oocytes to 10, the stimulation duration to days, and determined the starting dose. The COS was performed on a set of stimulation durations (days). In a control group (n = 21) who were 35.14 ± 3.64 years of age and underwent IVF between June 2019 and October 2019, the starting dose of rFSH was fixed as 225 IU, and COS was performed on any stimulation duration days.
This study was performed on patients who provided informed consent. Patients with hypothalamic dysfunction, endometriosis, history of ovarian surgery, or a history of pelvic radiotherapy and/or chemotherapy were excluded. The concordance rate between the number of retrieved oocytes (actual number of oocytes) and the preset target number of oocytes as ten was evaluated. We calculated the mean absolute error (MAE) and root-mean-squared error for the difference in the number of retrieved oocytes. Furthermore, we also calculated the C-index and examined the accuracy of the gonadotropin starting dose calculation model, assuming that it is concordant if the actual number of oocytes fall within the range of the predicted number of oocytes (10 ± 2). The t-test and chi-square test were used, as appropriate. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
3 RESULTS
3.1 Study 1
A total of 100 cycles of 100 patients were available for statistical analysis. Patient characteristics are presented in Table 1. The mean age of the patients was 36.16 ± 4.53 years. Table 2 shows the results of the single regression analysis with OSI as the objective variable. Single regression analysis showed that statistical significance was reached for age, AMH level, and initial serum FSH level. Table 3 shows the results of the multiple regression analysis and backward stepwise method. In the backward stepwise multiple regression, only age and AMH were selected as predictive variables.
| Variables | Regresssion coefficient | Standard error | Adjusted R-square | p value |
|---|---|---|---|---|
| Age, years | −0.0004 | 0.0001 | 0.1923 | <0.05 |
| AMH, ng/ml | 0.0012 | 0.0001 | 0.5622 | <0.05 |
| body weight, kg | −0.0001 | 0.0000 | 0.0110 | 0.15 |
| Initial serum FSH, IU/l | −0.0007 | 0.0002 | 0.1277 | <0.05 |
| Initial serum LH, IU/l | 0.0002 | 0.0002 | 0.0037 | 0.24 |
| Initial serum E2, pg/ml | 0.0000 | 0.0000 | −0.0088 | 0.72 |
- Note: Statistical significance was reached for age, AMH, initial serum FSH and body weight.
| Multiple regression analyses | Backward elimination | |||||||
|---|---|---|---|---|---|---|---|---|
| variables | Regresssion coefficient | Standard error | adjusted R-square | p value | Regresssion coefficient | Standard error | p value | adjusted R-square |
| Age, years | −0.0002 | 0.0001 | 0.59 | <0.05 | −0.00019331 | 0.0001 | <0.05 | 0.59 |
| AMH, ng/ml | 0.0010 | 0.0001 | <0.05 | 0.00108384 | 0.0001 | <0.05 | ||
| Initial serum FSH, IU/l | −0.0002 | 0.0001 | 0.19 | - | - | - | - | |
- Note: Age and AMH were selected as predictive variables. Backward selection of variables was applied, using Wald p < 0.05 for inclusion and p > 0.1 for exclusion as criteria.
Figure 1 shows this application. We can set the target number of oocytes to A, stimulation duration days to B, and we can also set age to C, and AMH to D. Additionally, the unit of the starting dose of FSH is calculated as E, and F shows the risk of OHSS by using a level gauge. The retrieval of >15 oocytes is associated with a moderate risk of OHSS, and the retrieval of >25 oocytes is associated with a high risk of OHSS.7 The following link provides access to a gonadotropin starting dose calculator:
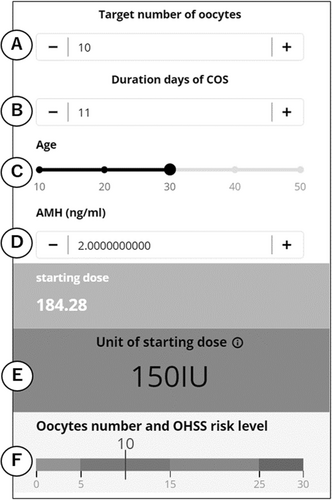
https://www.calconic.com/calculator-widgets/fsh/62c3f68141bd400029ac7eda?layouts=true
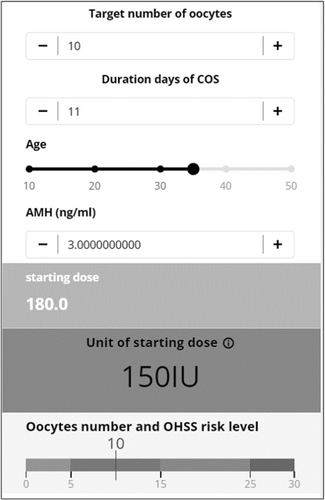
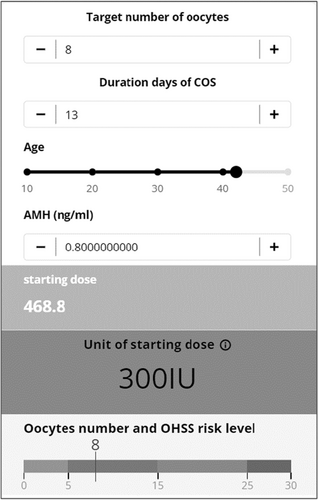
For example, in a 35-year-old patient with 3.0 ng/ml of AMH level, the starting dose was 150 IU if the target number of oocytes was ten and the number of stimulation days was 11 (Figure 2). In a 42-year-old patient with 0.8 ng/m of AMH level, the starting dose was 300 IU when the target number of oocytes was eight and the duration of COS was 13 (Figure 3).
3.2 Study 2
Table 4 shows a comparison between the calculated and control groups. The characteristics of the participants were similar and not statistically different between the two groups. The concordance rate of the retrieved oocytes was statistically high in the calculated group, assuming that it is concordant if the actual number of oocytes falls within the range of 10 ± 2. Table 5 shows a comparison of the actual number of oocytes retrieved by the gonadotropin starting dose calculation model, the predicted number of oocytes preset as 10, and the results of the accuracy analysis. The mean difference was 1.00, and the median difference was 1.00. The MAE was 3.25, RSME was 180, and C-index was 0.734 (95% CI, 0.253–1.000); therefore, the error seemed to be small, and accuracy of this model seemed to be high. These results suggest that the gonadotropin starting dose calculation model is highly accurate in predicting the number of oocytes.
| Calculated group (n = 12) | Control group (n = 21) | p-value | |
|---|---|---|---|
| Age, years | 35.42 ± 3.56 | 35.14 ± 3.64 | ns |
| Body weight, kg | 54.00 [46.00–72.00] | 51.00 [43.00–103.00] | ns |
| Smokers, % | 0 | 0 | ns |
| AFC, follicles | 6.25 ± 3.82 | 8.38 ± 4.10 | ns |
| AMH, ng/ml | 2.44 ± 1.44 | 4.15 ± 2.84 | ns |
| Initial serum FSH, IU/l | 7.88 ± 1.98 | 6.70 ± 1.45 | ns |
| Initial serum LH, IU/l | 5.35 ± 2.19 | 5.22 ± 1.91 | ns |
| Initial serum E2, pg/ml | 32.84 ± 13.77 | 30.77 ± 14.66 | ns |
| Serum E2 on trigger day, pg/ml | 3246.09 ± 2014.28 | 3625.81 ± 1728.30 | ns |
| Serum P4 on trigger day, pg/ml | 1.55 ± 0.59 | 1.61 ± 2.30 | ns |
| Stimulation duration days, days | 12.75 ± 1.82 | 13.76 ± 2.07 | ns |
| Total gonadotropins administered, IU | 2875.00 ± 929.26 | 3096.43 ± 466.05 | ns |
| Starting gonadotropins dose, IU | 225.00 ± 63.96 | 225.00 ± 0.00 | ns |
| Number of retrieved oocytes per woman | 9.42 ± 1.78 | 12.29 ± 5.45 | ns |
| The percentage of mature oocytes, % | 91.15 | 87.98 | ns |
| The fertilization rate, % | 78.64 | 67.40 | 0.04 |
| Rates of OHSS incidence, % | 0 | 0 | ns |
| OSI (Ovarian Sensitivity Index) × 103 | 2.74 [2.00–8.67] | 4.44 [0.99–8.89] | ns |
| Concordance rate, % | 75.00 | 28.57 | 0.01 |
- Note: Data are presented as mean ± standard deviation or median [max-min]. Patient background data of two groups in Study 2 are presented as mean ± standard deviation or median [max-min]. The concordance rate of oocytes retrieved was assumed that it is concordance if the actual number of oocytes falls within the range 10 ± 2. The t-test and chi-square test were used as appropriate.
| Actual number of oocytes | Predicted number of oocytes | |
|---|---|---|
| Mean number | 9.42 ± 1.78 | 10 ± 0 |
| Median number | 9.50 [7–13] | 10 [10–10] |
| Mean of difference | 1.42 ± 1.31 | |
| Median of difference | 1.00 [0–3] | |
| MAE (Mean Absolute Error) | 3.25 | |
| RMSE (Root-Mean-Squared Error) | 1.80 | |
| Concordance index (C-index) | 0.734 (95% confidence interval 0.253–1.000) | |
4 DISCUSSION
Several studies have shown that the optimal number of oocytes is 5–15.25-27 As a representative, Sunkara reported that LBR increases up to 15 oocytes when fresh embryo transfer is performed, but does not increase further after 15 oocytes.28 In contrast, as reported by Polyzos, with regard to the cumulative LBR (CLBR), which includes both fresh and frozen embryo transfers, it can be seen that CLBR increases with the number of oocytes.4 Furthermore, the higher the number of oocytes, the higher the number of euploid embryos obtained.29 However, the risk of OHSS increases when the number of oocytes increases. Lyan reported that the risk of OHSS increases when the number of oocytes exceeds 15.7 These reports suggest that the optimal number of oocytes is 5–15, but if CLBR is used as the outcome, a higher number of oocytes may be more effective. However, the risk of developing OHSS may also increase; therefore, it is important to obtain the optimal number of oocytes that are necessary and safely feasible for each patient to achieve a live birth.
A single-center cohort study of 17 948 cycles in China found that live births increased with increasing oocyte number.30 To reach a CLBR of 50%, a patient needs at least ten oocytes at age 38, 15 oocytes at age 40, and 20 oocytes at age 42.30 It has also been shown that the likelihood of obtaining one or more regular euploid blastocysts depends on the number of mature oocytes retrieved, and the estimated probability of a mature oocyte developing a euploid blastocyst decreases with age.
Other reports have calculated the number of oocytes needed to increase CLBR at each age.31, 32 What all these emphasize is that the number of oocytes required to produce a live birth increases with age. The primordial follicle steadily declines throughout life, with a rapid decline occurring when a woman reaches approximately 38 years of age.33
Therefore, it is important to obtain an adequate number of oocytes at each age during single-oocyte retrieval. To obtain an optimal number of oocytes, age, AMH level, and initial FSH level are useful patient parameters related to oocyte number. Various parameters, such as age, AMH, AFC, FSH levels, and smoking, have been used in previous studies. Among these, AMH, FSH, and age are appropriate parameters that are objective and can be quickly ascertained during outpatient consultation.34-37
A study on Japanese women has also shown that AMH levels correlate well with oocyte number in patients undergoing COS for IVF, and can predict the risk of ovarian hyperstimulation syndrome in these patients.24 Similar study also performed a regression analysis to evaluate the correlation between AMH and oocyte number. As a result, AMH, which is positively correlated with oocyte number, and age, which is negatively correlated with oocyte number, were selected as relevant factors in the stepwise model. This result was similar to that of this study. In addition to, the oocyte number increased in a dose-dependent manner with FSH.38, 39
It has also been reported that patients with a poor response may experience an average of one to two more oocytes retrieved with increased FSH doses, which may significantly reduce cycle cancellation rates in cases of inadequate follicle growth.40
Furthermore, a Cochrane review evaluating the effect of individualized gonadotropin dose selection using markers of ovarian reserve in women undergoing IVF/ICSI showed that in predicted high responders, lower doses of FSH reduced the overall incidence of moderate and severe OHSS.8
From the same study, the European Society of Human Reproduction and Embryology (ESHRE) guideline: ovarian stimulation for IVF/intracytoplasmic sperm injection (ICSI) states that it is unclear whether high-gonadotropin doses >150 IU are recommended, and gonadotropin doses >300 IU are not recommended for predictable poor responders. However, the authors reported a higher number of oocytes and more embryos available for transfer at higher doses in the poor, normal, and high groups.41 However, there are reports that FSH dose increases are limited.42, 43 There are also reports that increased FSH doses do not necessarily contribute to increased oocyte numbers.44 Regardless of the number of oocytes retrieved and the age of the patient, some studies have reported that the LBR decreases as the total dose of FSH increased.45 The subgroup analysis of a similar study, which was limited to patients with good prognosis, age < 35 years, BMI <30, and without a diagnosis of reduced ovarian reserve, endometriosis, or ovulation disorders, had similar results. This may be due to the high number of fresh embryo transfer cases included in the study, which may have been due to overstimulation with FSH, resulting in elevated estradiol and P4 that may have affected endometrial decidualization.46-48
Thus, excessive FSH dosing is not an effective strategy, as it does not have a positive effect on fresh embryo transfer and increases costs, owing to overstimulation. Therefore, the starting dose of FSH in relation to the total FSH dose is important in COS. Various algorithms have been reported to determine individualized doses of gonadotropins in COS using various parameters, such as AMH, AFC, age, weight, and smoking.11, 14-23
Among these, one of the most useful is the nomogram, that uses AMH, age, and FSH levels to determine the starting dose with an optimal oocyte number of nine.11
This nomogram is very useful for personalized dosing in COS; the only drawback is that it is limited to the GnRH agonist protocol, and the oocyte count setting is only nine.
Currently, COS is not only an agonist protocol but also an increasing number of antagonist protocols worldwide. The ESHRE guideline: Ovarian stimulation for IVF/ICSI also recommends an antagonist protocol, owing to the low risk of OHSS.41 It has also been reported that gonadotropin sensitivity may differ between Asians and Westerners,49 and that the distribution of FSH receptor polymorphisms may differ.50, 51 For these reasons, we cannot easily use algorithms for gonadotropin starting dose determination by Westerners among the Japanese population, and this calculator, which was created using Japanese data in this study, is considered more effective than previous studies when used in Japan.
The target number of oocytes varied from facility to facility. For example, in order to achieve a normal fertilization rate of 60% for conventional IVF, 65% for ICSI, a blastocyst development rate of 40%, and an implantation rate of 35%, which are the competencies in the Vienna consensus, 12 oocytes for c-IVF and 11 for ICSI are needed if the patient is ≤39 years.52 In contrast, the number of oocytes targeted may vary, depending on the key performance indicators of each facility.
Based on the above, it is thought that optimal COS can be achieved by setting the target number of oocytes and then calculating the amount of FSH required to achieve that target, especially the FSH starting dose.
Considering the duration of COS, it has been reported that the number of developing follicles, serum E2 levels, and number of oocytes retrieved reached a peak at 11 days of stimulation and gradually decreased at shorter or longer than 11 days.53 Additionally, in the GnRH antagonist protocol, it has been reported that a short duration of stimulation of COS causes a decrease in oocyte maturity and pregnancy rate.54 In contrast, there have been reports of reduced live births when the duration of COS is long (>13 days).55 The duration of COS has been found to be negatively correlated with gonadotropin dose, shortening with higher doses and prolonging with lower doses.53 The duration of COS may vary among facilities. The duration of COS before oocyte retrieval may increase or decrease due to differences in clinical hours and holidays, as well as patient preferences. In these situations, our calculator can set the number of days of COS and starting dose of gonadotropins required to achieve the target number of oocytes in that period.
Our calculator will improve COS performance and enable national standardization by allowing all physicians, regardless of their years of experience, to determine the appropriate starting dose of gonadotropins equally.
By setting a target number of oocytes at each facility and calculating the individualized doses needed to achieve this target, gonadotropin overdosage can be avoided, thereby preventing shorter stimulation days, increasing the risk of complications, and reducing extra costs. Similarly, it can prevent setting doses that are too low; thus, preventing the probability of cycle cancellation and prolongation of the duration of COS.
This study has two limitations. First, the inclusion of urinary gonadotropins (UGs). Because UGs contain protein impurities, FSH activity may vary from lot to lot. Therefore, it may be desirable to study a mono-protocol using only rFSH. However, in actual clinical practice, rFSH, uFSH, and hMG are often used, and we believe that the calculator created in this study is acceptable for clinical use. Another limitation is the use of recombinant human chorionic gonadotropin (hCG) and GnRH agonists as triggers of oocyte maturation. In this study, we did not consider the effect of different triggers on the results; however, because it has been reported that there is no difference in the number of oocytes retrieved between hCG and GnRH agonist triggers, it is thought that there is no effect on OSI.56-58
This is the first study to develop an individualized dosing algorithm for gonadotropins in Japanese patients. Further accumulation of cases is required to develop a standardized calculator that can be used throughout Japan. Prospective studies are needed to verify the accuracy of this calculator by actually using it in clinical practice. Most importantly, each facility should establish the number of oocytes required for each patient based on the ART results and develop an algorithm for determining the starting dose necessary to obtain the number of oocytes at each facility.
ACKNOWLEDGMENTS
We would like to express our deep gratitude to Merck BioPharma for their support of this study. A special gratitude I give to MB Lumius providing permission to present the gonadotropins starting dose calculator in this article. We would like to thank Editage (www.editage.com) for English language editing.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this article. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (facilityal and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study. This article does not contain any studies with animal subjects performed by any of the authors. The protocol for the research project, including human subjects, has been approved by a suitably constituted Ethics Committee, ‘Medical corporation Kobanawa Clinic ethic screening committee’.




