Recent advances of in vitro culture systems for spermatogonial stem cells in mammals
Funding information
This work was supported in part by the Grant in -Aid for Scientific Research (B) from Japan Society for the promotion of Science to H.I
Abstract
Background
Spermatogonial stem cells (SSCs) in the mammalian testis are unipotent stem cells for spermatozoa. They show unique cell characteristics as stem cells and germ cells after being isolated from the testis and cultured in vitro. This review introduces recent progress in the development of culture systems for the establishment of SSC lines in mammalian species, including humans.
Methods
Based on the published reports, the isolation and purification of SSCs, identification and characteristics of SSCs, and culture system for mice, humans, and domestic animals have been summarized.
Results
In mice, cell lines from SSCs are established and can be reprogrammed to show pluripotent stem cell potency that is similar to embryonic stem cells. However, it is difficult to establish cell lines for animals other than mice because of the dearth of understanding about species-specific requirements for growth factors and mechanisms supporting the self-renewal of cultured SSCs. Among the factors that are associated with the development of culture systems, the enrichment of SSCs that are isolated from the testis and the combination of growth factors are essential.
Conclusion
Providing an example of SSC culture in cattle, a rational consideration was made about how it can be possible to establish cell lines from neonatal and immature testes.
1 INTRODUCTION
In mammals, spermatogenesis is a sequential, organized process of self-renewal and differentiation of spermatogonial stem cells (SSCs) that are found in the testis and that result in the continuous production of spermatozoa throughout the life of a man.1-4 Spermatogenesis protects genomic integrity and plays an essential role in the preservation of the species and genetic diversity.5 The processes in spermatogenesis are conserved among mammalian species. However, the transformation of spermatogenesis from self-renewing stem cells to mature spermatozoa is completely different and unique among species. The process lasts 35 days in mice,6 74 days in humans,3 and 63 days in cattle.7 For the duration of this transformation, the SSCs undergo mitotic multiplication, meiotic recombination of genetic material and morphological changes into spermatozoa.8 This is a highly productive process that begins at puberty in male animals and ultimately produces 100 million spermatozoa in adult men9 and 6000 million spermatozoa in mature bulls.10 Male fertility completely relies on the steady state of spermatogenesis in pubertal animals.
The development of a culture system and successful establishment of SSC lines in rodents has attracted much attention from researchers. Although SSCs from many mammalian species have been shown to proliferate for more than 6 months in the seminiferous tubules of immunodeficient mice, no germ cell (GC) line has been established in most mammalian species, other than mice. It is still unknown whether this lack of cell line is related to the lack of knowledge regarding culture conditions and the factors regulating and maintaining SSCs in culture.
This review summarizes the recent progress in the development of the culture system and possible challenges in establishing a SSC line in human and livestock species.
2 SPERMATOGONIAL STEM CELLS
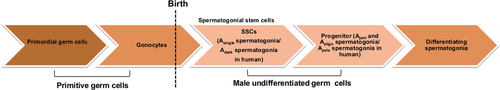
Spermatogonial stem cells originate from gonocytes, they are a derivative of primordial germ cells (PGCs), which are cells from a germ line lineage that arises from the extraembryonic mesoderm at the posterior end of the primitive streak. They migrate to the urogenital ridge, which forms gonads.11 The PGCs that cease their proliferation in the male genital ridge are called gonocytes. After birth, the gonocytes resume their proliferation, migrate to the basement membrane of the seminiferous tubules, and transform into SSCs. The transition of gonocytes to SSCs after birth occurs within 3 days in mice12 and 20 weeks in bulls.13
The SSCs have a unique ability for both self-renewal and cell differentiation toward spermatogenesis (Figure 1). The existing self-renewal model of SSCs was originally proposed by Huckins1 in rats and Oakberg6 in mice. This model proposes that only Asingle (As) spermatogonia act as stem cells and give rise to committed cells that divide into Apair (Apr) and Aalign (Aal) cells during spermatogenesis. The extended studies of the self-renewal model of As spermatogonia (As model) using genetic labeling, lineage tracing analysis, and live imaging have provided a striking observation that As spermatogonial cells represent heterogeneity14 and showed that populations of Apr and Aal SSCs change their behavior during regeneration and acquire stem cell potential. The actual cell number of SSCs having stem cell potential is very low, with ~2000 cells per testis, as calculated by using a pulse-labeling strategy14 and ~3000 cells per testis by using a serial transplantation assay.15 This number is very low, compared to the As model based on morphological characteristics,16 which was estimated at ~35 000 cells per testis. These findings support the heterogeneity of As SSCs in states of morphological similarity. In humans, the spermatogonial renewal model was first proposed by Clemont in 1966.17 The model postulates that the Adark and Apale spermatogonia, similar to Apr and Aal in rodents, occur in the human testis and that the Adark spermatogonia are mostly undifferentiated and reserved as stem cells, whereas the Apale spermatogonia were renewing and were spermatogonia in the early stages of differentiation.
3 SPERMATOGONIAL STEM CELL NICHE IN THE TESTIS
Adult stem cells can self-renew only in a specialized microenvironment called a niche, which provides architectural support, growth factors, and extrinsic stimuli for SSCs.18, 19 The SSCs reside in the basement of the seminiferous tubules and constitute a niche that is surrounded by Sertoli cells, Leydig cells, and peritubular myoid cells.20 The Sertoli cells seem to play a particularly important role in the SSC niche because numerous factors, such as glial cell-derived neurotrophic factor (GDNF), fibroblast growth factor 2, kit ligand, activin A, and bone morphogenic protein 4 (BMP4), are produced by Sertoli cells and affect the self-renewal, proliferation, and differentiation of the SSCs.21 Recent evidence suggests that As, Apr, and Aal spermatogonia can be found along the peritubular blood vessels and are preferentially located in a specific compartment that serves as the niche.22, 23
4 IDENTIFICATION OF SPERMATOGONIAL STEM CELLS
4.1 Transplant assay of the isolated spermatogonial stem cells
The first transplant assay for the identification of SSCs in mice was performed by Brinster and Zimmermann.24 The recipient mice are depleted for endogenous SSCs through treatment with the anticancer drug, Busulfan, and the transplanted donor-derived SSCs result in complete spermatogenesis. This assay provides functional and quantitative analyses of SSCs, in which donor-derived colonies are generated from single transplanted SSCs.15 In addition, cross-species transplants between mice and rats,25 as well as mice and hamsters,26 results in complete spermatogenesis and the production of healthy offspring. Surprisingly, the transplant of GCs from non-rodent species (Table 1)27-31 (ie, rabbits and dogs32), as well as pigs, cattle, and horses,33 shows the colonization of cells in the mouse testis, but there is a lack of complete spermatogenesis. This finding raises questions regarding whether transplants can be used as a bioassay for the determination of stem cell potential in non-rodent species.34
| Species | Donor-derived spermatogenesis | Reference |
|---|---|---|
| Pig (homologous) | Complete | Honaramooz, Megee, Dobrinski27 |
| Goat (homologous) | Complete | Honaramooz, Behboodi, Megee, et al28 |
| Cattle (autologous) | Complete | Izadyar29 |
| Cattle (homologous) | Not demonstrated | |
| Cattle (homologous) | Not demonstrated | Hill, Dobrinski30 |
| Goat | Complete with the integration of a transgene (adenovirus) | Honaramooz, Megee, Zeng, et al31 |
Apart from the identification of SSCs, a transplant technique has been used for multiple applications, including the restoration of infertility, generation of transgenic and knockout animals, and the evaluation of the culture system and cell markers.35, 36 The transplant of human SSCs into immunodeficient mice was first shown by Nagano, Patrizio, and Brinster.37 The isolated SSCs could colonize and survive for 6 months in mouse testes. The number of SSCs was significantly reduced 2 months after the transplant and no cell differentiation into meiosis was observed. The xenotransplant of human SSCs to the mouse testis by using cultured cells shows a potential regenerative technique for fertility preservation in patients with cancer. Similarly, the autotransplant of SSCs in prepubertal patients with cancer has been considered to be a feasible way to restore infertility after cancer treatment.38
4.2 Biochemical characterization of the spermatogonial stem cells
Defining the SSC populations by using biochemical markers that distinguish them from spermatogonia in other stages of differentiation was a great tool for the isolation of potential SSCs and the development of culture systems in rodents. In recent years, several molecular markers have been identified for SSCs in rodents (Table 2).39-63 Most of these markers are expressed in progenitor SSCs, including As spermatogonia and undifferentiated spermatogonia (Apr and Aal spermatogonia). Traditionally, As spermatogonia have been included in the SSC population that self-renews in order to maintain a foundational stem cell pool and the transition to Apr spermatogonia represents the initial step of spermatogenesis.1, 6 Recent findings show that the SSC population is not limited to the As spermatogonia population.64 Some progenitor SSCs also exhibit stem cell behavior.
| Molecular marker | Species | ||||||
|---|---|---|---|---|---|---|---|
| Human | Mouse | Cattle | Pig | Sheep | Goat | Buffalo | |
| VASA/DDX4 | ND |
+ (Sakai, Noce, Yamashina39) |
+ (Fujihara, Kim, Minami, Yamada, Imai40) |
ND |
+ (Borjigin, Davey, Hutton, Herrid41) |
ND |
+ (Goel, Reddy, Mandal, Fujihara, Kim, Imai42) |
| UCHL1 |
+ (He, Kokkinaki, Jiang, Dobrinski, Dym43) |
+ (Kwon, Kikuchi, Setsuie, Ishii, Kyuwa, Yoshikawa44) |
+ (Herrid, Davey, Hill45) |
+ (Luo, Megee, Rathi, Dobrinski46) |
+ (Rodriguez-Sosa, Dobson, Hahnel47) |
+ (Heidari, Rahmati-ahmadabadi48) |
+ (Goel, Reddy, Mandal, Fujihara, Kim, Imai42) |
| DBA | ND | ND |
+ (Izaydar49) |
+ (Goel, Sugimoto, Minami, Yamada, Kume, Imai50) |
+ (Borjigin, Davey, Hutton, Herrid41) |
ND |
+ (Goel, Reddy, Mandal, Fujihara, Kim, Imai42) |
| PLZF | ND |
+ (Buaas, Kirsh, Sharma, et al51) |
+ (Reding, Stepnoski, Cloninger, Oatley52) |
+ (Goel, Sugimoto, Minami, Yamada, Kume, Imai50) |
+ (Borjigin, Davey, Hutton, Herrid41) |
ND | ND |
| THY1 |
+ (He, Kokkinaki, Jiang, Dobrinski, Dym43) |
+ (Kubota, Avarbock, Brinster53) |
+ (Reding, Stepnoski, Cloninger, Oatley52) |
ND | ND |
+ (Abbasi, Tahmoorespur, Morteza, Nasiri54) |
+ (Rafeeqi, Kaul55) |
| POUF1 | ND |
+ (Pesce, Wang, Wolgemuth, Schöler56) |
+ (Fujihara, Kim, Minami, Yamada, Imai40) |
+ (Goel, Sugimoto, Minami, Yamada, Kume, Imai50) |
ND | ND |
+ (Goel, Reddy, Mandal, Fujihara, Kim, Imai42) |
| NANOG | ND | ND | ND | ND | ND | ND | ND |
| GFRα1 |
+ (He, Kokkinaki, Jiang, Dobrinski, Dym43) |
+ (Naughton, Jain, Strickland, Gupta, Milbrandt57) |
+ (Sahare, Kim, Otomo, et al58) |
+ (Lee, Park, Lee, et al59) |
ND | ND | ND |
| GFR125 | ND |
+ (Seandel, James, Shmelkov, et al60) |
ND | ND | ND | ND | ND |
| RET | ND |
+ (Naughton, Jain, Strickland, Gupta, Milbrandt57) |
ND | ND | ND | ND | ND |
| ID4 | ND |
+ (Oatley, Brinster61) |
ND | ND | ND | ND | ND |
| ITGA6 | ND |
+ (Shinohara, Avarbock, Brinster62) |
+ (de Barros, Worst, Saurin, Mendes, Assumpção, Visintin63) |
ND | ND | ND | ND |
| ITGB1 | ND |
+ (Shinohara, Avarbock, Brinster62) |
ND | ND | ND | ND | ND |
- +, expression of the protein in undifferentiated SSCs; ND, not determined.
Some of these markers are identified as SSC markers in domestic animals (Table 2) and are conserved among mammalian species. The markers, GPR125, GFR1, THY1, ZBTB16, SSEA-4, and PLZF, that have been identified for SSCs in rodents have also been characterized in human spermatogonia and more differentiated GCs.43, 65, 66
5 IN VITRO CULTURE OF THE SPERMATOGONIAL STEM CELLS
5.1 Isolation and enrichment of the spermatogonial stem cells
The isolation and enrichment of SSCs is the first step towards establishing GS cell lines. The isolation of SSCs is challenging because of their limited number in the testis. A two-step enzymatic digestion was first proposed by Davis and Schuetz,67 which is the most widely used technique for the isolation of SSCs in rodents. For further enrichment of SSCs, different approaches, such as differential plating,68 percoll gradient,23 magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS) have been used independently or in combination. In livestock species, SSC isolation and enrichment methods have progressed during the last few years. Differential plating is one feasible method for the enrichment of SSCs, along with MACS and FACS for bovine SSCs.69
5.2 Establishment of a culture system for germ cell lines
The limited number of SSCs in the testis4 hampers studies that elucidate biological characteristics and for applying SSCs. One approach to solve this problem is to develop a culture system that supports the self-renewal of SSCs and maintains their GC and stem-cell potentials. Glial cell-derived neurotrophic factor was shown to be the first molecule that regulates the self-renewal and differentiation of mouse SSCs.70 Glial cell-derived neurotrophic factor signals act through the multicomponent receptor complex that is composed of GFRα-1 and RET tyrosine kinases in various cell types.71 The GFRα-1 and RET also have been recognized as spermatogonial markers that are expressed in gonocytes, SSCs, and differentiated spermatogonia.72 These coreceptors of GDNF-mediated signaling have been shown to be necessary for the self-renewal of GCs in rodents.57 Subsequently, Nagano, Ryu, Brinster, Avarbock, and Brinster developed a short-term culture system that is supplemented with GDNF that improves the survival of GCs.15 These cells complete spermatogenesis after transplant into the testis of immunodeficient mice. The long-term culture of SSCs is achieved by adding other growth factors and hormones in addition to GDNF.73 These cells proliferate over a 2 year period (>1085-fold) in the presence of GDNF, while maintaining stable genetic and epigenetic properties and restoring spermatogenesis following transplant into the seminiferous tubules of infertile recipient mice. However, the growth factor requirements for the proliferation of GCs is strain-specific: in mice, the C57BL/6 and 129/Sv strains require fibroblast growth factor (FGF) and GDNF,74 while strain DBA requires FGF, GDNF, and epidermal growth factor.75 By using species-specific culture components, culture systems and GC lines have been established in rats,76, 77 hamsters,78 and rabbits.79
Spermatogonial stem cells under appropriate culture conditions acquire embryonic stem (ES) cell-like characteristics called “multipotent GCs,” which were first generated from GCs in the neonatal mouse testis without the introduction of any exogenous reprogramming factor.80 These cell populations failed to form colonies following testicular transplants, which shows that they are devoid of GC potential and have the ability to differentiate into three germ layers. Later, successful evidence of the generation of a multipotent GS cell line was shown for adult mice.60, 81
The successful translation of an in vitro culture of SSCs in rodents led to the establishment of a culture system for human SSCs from prepubertal and adult testes.82, 83 In humans, multipotent stem cell lines have been developed from SSCs by exposing the cells to ES cell culture conditions.84, 85 These cell lines can form a teratoma after they are injected into immunodeficient mice. These findings provide an important foundation for developing methods for the generation of autologous stem cell lines from human SSCs that have been collected from patients with cancer before the initiation of cancer treatment and the subsequent autologous transplant after cancer treatment could be a means for preserving the fertility of male patients with cancer.86
5.3 Spermatogonial stem cell culture in livestock species
In livestock species, long-term culture systems for GCs and the establishment of multipotent GC lines could reduce the time and costs for producing transgenic animals and to preserve endangered species. These systems also could be an alternative for pronuclear microinjection and somatic cell cloning.87 Although several attempts have been made to develop a culture system for livestock species, as shown in Table 3,40, 58, 88-94 most of these studies achieved only short-term SSC cultures. The culture system for bovine SSCs has been demonstrated in the pre-pubertal testis29, 49, 89-91 and the neonatal testis.40 In pigs, cultured SSCs cannot survive more than 1 week50, 92. In these studies, serum was used as an important component in the culture medium for the survival and self-renewal of SSCs. Some undefined factors in the serum induce cell differentiation, whereas others have detrimental effects on ES cells and GC survival in the culture.53, 95 In order to overcome this problem, serum-free culture systems have been developed for long-term cultures of SSCs in mice74, 96 and rats.77 However, no long-term culture system for livestock species has been developed. In the authors’ laboratory, growth factors, matrix substrates for culture dishes, and serum-free supplements have been examined in order to develop a defined system for culturing primitive GCs (gonocytes) from the neonatal bovine testis.58, 97, 98 Poly-L-lysine is a suitable substrate for the selective inhibition of the growth of somatic cells and makes it possible to maintain gonocytes. Among the serum-free supplements that were tested, knockout serum replacement (KSR) in the culture medium supports the proliferation and survival of the gonocytes after sequential passages of the colonies. Under these optimized culture conditions that consist of 15% KSR on poly-L-lysine-coated dishes, the stem cell and GC potentials of cultured gonocytes can be maintained for more than 2 months. Subsequently, also developed was a culture system to maintain the SSCs from immature and adult testis in cattle.99 H The SSCs from the immature testis are cultured under serum-free conditions in the presence of GDNF and bovine leukemia inhibitory factor-conditioning media. Established cell lines resemble ES-like cell properties and express both pluripotent and GC markers. However, the SSCs from the adult bovine testis are cultured in a low-serum concentration media that is supplemented with 6-bromoindirubin-3’-oxime, which is a small-molecule inhibitor of glycogen synthase kinase-3α that leads to the activation of the wingless-type (Wnt)/β-catenin signaling pathway.100 The established cell lines can be maintained under in vitro culture conditions for more than 3 months. This cell line has a normal karyotype and botryoidal morphology that is similar to the male GC lines from mouse SSCs. Taken together, this new finding provides a promising strategy to conserve GCs from livestock species at different stages of animal development.
| Reference | Culture conditions | Age of donor | Culture term |
|---|---|---|---|
| Cat | |||
| Izadyar, Den Ouden, Stout, et al88 | Compare MEM and KSOM medium 0%-10% FCS | 5 mo | MEM+2.5% FCS is effective for germ cell survival than KSOM, no expansion, showing differentiation during 150 days culture |
| Oatley, Reeves, McLean89 | DMEMF + 10% FBS + GDNF | 1-2 mo | 2 wk |
| Aponte, Soda, van de Kant90 | MEM +2.5% FCS + GDNF | 4-6 mo | 25 d, no passage, differentiation |
| Aponte, Soda, Teerds, Mizrak, van de Kant91 | StemPro-SFM + GDNF, EGF, and FF | 4-6 mo | 25 d, no appearance of colonies after passage |
| Fujihara, Kim, Minami, Yamada, Imai40 | DMEMF12 + 10% FCS | 1-10 d | 1.5 mo |
| Sahare, Kim, Otomo, et al58 | DMEMF12 + 15% KSR on poly-L-lysine-coated dishes | 1-10 d | >2 mo |
| Pig | |||
| Dirami, Ravindranath, Pursel, Dym92 | DMEMF12 + 10% FCS | 2 mo | 1 wk |
| Goel, Fujihara, Tsuchiya, et al93 | DMEMF12 + 10% FCS | 1-10 d | 3 wk, reduction of germ cells every passage |
| Goel, Fujihara, Tsuchiya, et al94 | StemPro SFM + GDNF, EGF, and FF | 3-4 d | 9 passages (30 d), reduction of germ cells every passage |
- DMEMF, Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; EGF, epidermal growth factor; FCS, fetal calf serum; FF, feeder-free; GDNF, glial cell-derived neurotrophic factor; KSOM, potassium simplex optimized medium; KSR, knockout serum replacement; MEM, minimum essential medium; SFM, serum- and feeder-free medium.
6 CONCLUSION
Recently, GCs with a GC lineage have been derived from ES cells101, 102 and induced pluripotent stem cells in mice.103-105 The molecules that are involved in GC commitment, such as BMP4 and Wnt3, have been identified102, 106 and PGCs are induced from pluripotent stem cells under the control of these molecules and other cell differentiation-inducing factors.102, 106 In humans, PGCs also are induced in similar culture conditions.107-109 The induced mouse PGCs can be maintained in a normal manner and differentiated into spermatozoa and oocytes with the ability to develop to term.110, 111 At this time, GC formation for the spermatozoa and oocytes was achieved under ex vivo conditions, in which somatic cells that were associated with spermatogenesis or oogenesis were cocultured and aggregated with the indicated PGC population.111 Therefore, although additional studies are necessary in order to maintain and induce GCs in vitro, GS cell lines that have been established in some mammalian species might be candidates to produce spermatozoa and oocytes in vitro. These technologies in the near future will be helpful for the retention of the fertility of patients before cancer therapy, the production of transgenic animals for human disease models, domestic animal improvement, and the conservation of endangered species.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent: This article does not contain any studies with human subjects performed by any of the authors. Animal studies: The protocol for the research project, including the animal participants, was approved by a suitably constituted ethics committee.




