Non-esterified fatty acid-associated ability of follicular fluid to support porcine oocyte maturation and development
Funding information
This study was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science JSPS for Scientific Research C (KAKENHI grant no. 16K07996) and a Grant-in-Aid for JSPS Fellows (KAKENHI grant no. JP16J07329), as well as from the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Abstract
Purpose
The effect of supplementing maturation medium with follicular fluid (FF) was examined according to its non-esterified fatty acid (NEFA) content or with a fatty acid mixture (FA-Mix) on the developmental competence of oocytes, as well as the mitochondrial quality and quantity in the oocytes and cumulus cells.
Method
Porcine oocytes from a slaughterhouse were used.
Results
The FF or FA-Mix in maturation medium increased the lipid content in both the oocytes and the cumulus cells, but the adenosine triphosphate content was differentially affected. The FF supplementation increased the mitochondrial DNA copy number, survival of cumulus cells, and rate of oocyte development to the blastocyst stage, whereas the FA-Mix supplementation did not show these effects. The expression levels of GPC4, PFKP, PRDX3, and TFAM in the cumulus cells increased after FF supplementation, but the expression of GJA1 decreased, compared with the cells that were cultured without FF.
Conclusion
Adding FF and FA-Mix to the maturation medium increased the lipid content in the oocytes and cumulus cells. The effects of FF on the cumulus cells and oocytes were not observed after FA-Mix supplementation, indicating that the concentration of the NEFAs in the FF are closely associated with an ability to support oocyte maturation and the metabolism of cumulus cells and oocytes.
1 INTRODUCTION
Follicular fluid (FF) is the sole environment for oocyte growth and maturation. Non-esterified fatty acids (NEFAs) are a major component of FF and the composition and concentration of NEFAs depend on the maternal physiological conditions.1, 2 High NEFA levels in FF are believed to be toxic for oocytes, as a high NEFA concentration (150 μ mol L−1 palmitic acid + 200 μ mol L−1 oleic acid + 75 μ mol L−1 stearic acid) adversely affects bovine oocyte developmental ability through endoplasmic reticulum (ER) stress.3 In addition, one study showed that high NEFA levels in the maturation medium reduced bovine oocyte development, whereas inhibiting beta oxidation restored oocyte maturation.4 Most of these findings were obtained from studies in which a single, or a combination of major fatty acids, was added to oocyte culture media5-7 and the significance of either intrinsic NEFA in the FF or properties of the FF that are associated with the NEFA content has not yet been clarified. One interesting study reported a relationship between the ability of FF to support oocyte maturation and its fatty acid content.8 In that report, lipid-rich human FF (eighth-highest lipid-containing FF out of 64 samples) and poor lipid FF (lowest eighth FF of 64 samples) was added to the maturation medium of mouse oocytes at a concentration of 50%. It was observed that the lipid-rich FF induced lipid accumulation and ER stress in the oocytes and reduced oocyte maturation. The researchers cautioned that the significance of the follicular lipid content should be examined among different species. This is because during meiotic maturation, the oocytes enhance lipid usage through beta oxidation and the extent of this effect is species-dependent; inhibiting beta oxidation during maturation profoundly reduces pig oocyte maturation, whereas lipid-poor mouse oocytes only exhibit slightly decreased maturation.9
It was shown that supplementing the culture medium with FF enhances the fertilization ability of porcine oocytes.10 In addition, one study11 suggested that, in bovine oocytes, FF enhances the adenosine triphosphate (ATP) content, mitochondrial distribution, and improves oocyte fertilization ability through cumulus cells.12 Based on these reports, it is speculated that certain beneficial components in FF support oocyte maturation and the significance of FF NEFA concentrations regarding oocyte maturation is unclear.
In the present study, porcine FF was categorized based on its NEFA concentration and added to the maturation medium of porcine oocytes. Subsequently, oocyte developmental ability, as well as the mitochondrial number and function in the oocytes and cumulus cells, was examined. Furthermore, to distinguish the effects of FF from those of major fatty acids, the maturation medium was supplemented with a fatty acid mixture (FA-Mix) resembling FF and then the oocyte developmental ability and mitochondrial number and function in oocytes and cumulus cells were examined.
2 MATERIALS AND METHODS
2.1 Chemicals and media
All the chemicals were purchased from Nacalai Tesque (Kyoto, Japan), unless otherwise indicated. The medium that was used for in vitro maturation (IVM) was porcine oocyte medium that was supplemented with 3 mg/mL polyvinyl alcohol,13 0.5 m mol L−1 L-cysteine, 10 ng/mL epidermal growth factor (Sigma-Aldrich, St. Louis, MO, USA), 10 IU/mL equine chorionic gonadotropin (ASKA Pharma Company, Ltd, Tokyo, Japan), and 10 IU/mL human chorionic gonadotropin (Fuji Pharma Company, Ltd, Tokyo, Japan). The FF was collected from antrum follicles (3-6 mm in diameter), centrifuged (10 000 g for 5 min), and stored at −30°C.
2.2 Ovary collection
Ovaries from gilts were collected from a local slaughterhouse (Kanagawa Meat Center, Atsugi, Japan), placed in phosphate-buffered saline (PBS), containing 10 IU/mL of penicillin G potassium and 0.1 mg/mL of streptomycin sulfate, and then transported to the laboratory within 1 hour. During transport, the temperature of the ovaries was maintained at 37°C.
2.3 In vitro maturation, activation, and in vitro culture
During the forty-four hour maturation period, cumulus–oocyte complexes (COCs) were cultured in maturation medium. The IVM medium was supplemented with either FF or FA-Mix, according to the experimental design. In order to determine the ability of the oocytes to develop to the blastocyst stage, after IVM the oocytes were activated with a single electrical pulse of 60 V and 0.1 ms by NEPA21 (Nepa Gene Company, Ltd, Chiba, Japan), followed by culturing in a medium containing 10 μg/mL cytochalasin B and cycloheximide for 6 hours. After activation, the embryos were cultured for 7 days in culture medium and the blastulation rate and total blastocyst cell number were examined. In order to determine the maturation rate and total blastocyst cell number, the embryos were fixed in 4% paraformaldehyde, mounted on glass slides by using an antifade reagent containing 4’,6-diamidino-2-phenylindole (DAPI) (ProLong Gold antifade reagent with DAPI; Invitrogen, Carlsbad, CA, USA), and observed by using a fluorescence digital microscope (BZ-8000; Keyence, Tokyo, Japan). The IVM was performed at 38.5°C in an atmosphere containing 5% CO2 and 95% air. In vitro embryo culturing was performed at 38.5°C in an atmosphere containing 5% O2, 5% CO2, and 90% N2.
2.4 Measurement of the mitochondrial DNA copy number
The mitochondrial (mt)DNA copy number in the cumulus cells was obtained by dividing the mtDNA copy number by the cell number of the samples and the mtDNA copy number also was determined in individual oocytes. The mtDNA number in the oocytes and cumulus cells was determined by real-time polymerase chain reaction (RT-PCR). After IVM, the oocytes were denuded from the cumulus cells, after which individual oocytes were transferred to a PCR tube and the cumulus cell suspensions were centrifuged to obtained cellular pellets. The DNA was extracted from individual oocytes or cumulus cell pellets by using an extraction buffer (20 m mol L−1 Tris-HCl, 0.9% Nonidet-40 and Tween 20 and 0.4 mg/mL proteinase K) and by heating at 55°C for 30 min, followed by 98°C for 5 min. The PCR was performed with a Rotor-Gene 6500 real-time rotary analyzer (Qiagen GmbH, Hilden, Germany) with a primer that was set to target the mtDNA sequence and the one copy gene and Ssofast-TM EvaGreen Supermix (Bio-Rad, Hercules, CA, USA). The primers that were used for the mitochondrial genome and one copy gene are listed in Table 1. They were designed by using Primer3Plus (http://sourceforge.net/projects/primer3/) and the National Center for Biotechnology Information database (porcine mitochondrion gene, NC_000845.1, and GCG glucagon, NC_010457). The PCR was performed with an initial denaturation at 95°C for 1 minute, followed by 40 cycles at 98°C for five-seconds and 60°C for 10 seconds. A standard curve was generated for each run by using 10-fold serial dilutions that represented the copy number of the external standard. The external standard was the PCR product of the corresponding gene that was cloned into a vector by using the Zero Blunt TOPO PCR cloning kit (Invitrogen, Carlsbad, CA, USA) and the PCR product was sequenced for confirmation before use. The amplification efficiencies of all the trial runs were >1.9.
| Gene | Primer | Length | |
| Mitochondria | F | 5’- cgagagagcactttccaagg-3’ | 152 bp |
| (COX2) | R | 5’- ctaattcgggtgttggtgct -3’ | |
| GCG glucagon | F | 5’-agcagaatcaacaccatcggt-3’ | 154 bp |
| (1 copy gene) | R | 5’-tggctccacccatagaatgc-3’ | |
| β-Actin | F | 5’-atcgtgcgggacatcaagga-3’ | 179 bp |
| R | 5’-aggaaggagggctggaagag-3’ | ||
| TFAM | F | 5’-ggcagactggcaggtgta-3’ | 164 bp |
| R | 5’-cgaggtctttttggttttcca-3 | ||
| GJA1 | F | 5’-ggtgtctctcgccttgaaca-3’ | 126 bp |
| R | 5’-cagtctttggaggggctcag-3’ | ||
| INHA | F | 5’-ttcaagtacgagacggtgcc-3’ | 116 bp |
| R | 5’-gtgggaactctgccttcctc-3’ | ||
| PRDX3 | F | 5’-tatccgacatgtgagtgccg-3’ | 105 bp |
| R | 5’-ccacagcacacttgtcaagc-3’ | ||
| GPC4 | F | 5’-cccagtaccacttcaccgac-’3 | 183 bp |
| R | 5’-tgaccacggagactttgctc-’3 | ||
| PFKP | F | 5’-cccaaagaccaactgcaacg-3’ | 121 bp |
| R | 5’-cacggcgaacatcttgtgtc-3’ | ||
- F, forward; GJA1, gap junction alpha-1 protein; GPC4. glypican-4; INHA, inhibin A; TFAM, mitochondrial transcription factor A; PRDX3, phosphofructokinase platelet; R, reverse; PFKP, thioredoxin-dependent peroxide reductase.
2.5 Adenosine triphosphate content
In vitro-matured oocytes were denuded from the cumulus cells and individually transferred to PCR tubes containing distilled water. The cellular suspension was divided equally into two groups that were centrifuged to obtain cellular pellets. The pellets were resuspended in water, followed by placing them in liquid nitrogen in order to determine the ATP content afterward, while the other cohort of pellets was used for DNA extraction in order to determine the cellular number by RT-PCR targeting the one copy gene. The ATP level in the samples was determined by measuring the luminescence that was generated in an ATP-dependent luciferin–luciferase bioluminescence assay (ATP assay kit; Toyo-Inc., Tokyo, Japan), as described previously.14 The values were divided by the cellular number in each sample.
2.6 Survival rate and the number of cumulus cells
After maturation, the COCs were vortexed to separate the oocytes and the cumulus cells and the cumulus cell suspensions were centrifuged in order to obtain cellular pellets that were resuspended in PBS. The cellular number was determined by the volume of the cellular suspension and the cellular concentration was determined by a hemocytometer. In order to obtain the cellular survival rate, the cells were stained with 10 μg/mL Hoechst 33342 for all cells and propidium iodide was used for dead cell exclusion.
2.7 Measurement of the lipid content in the oocytes and cumulus cells
After maturation, the COCs were vortexed in order to separate the oocyte and the cumulus cells and the cumulus cell suspensions were centrifuged to obtain cellular pellets. The oocytes and cumulus cells were incubated in PBS containing 10 μg/mL Nile Red (Wako, Osaka, Japan) and 10 μg/mL Hoechst 33342 for 5 minutes, followed by observation under a fluorescence microscope (Keyence). In order to determine the oocyte lipid content, the fluorescence intensity of Nile Red was captured. To obtain the lipid content in the cumulus cells, the fluorescence intensity of Nile Red of ~200 cells was divided by the intensity of Hoechst staining.
2.8 Real-time polymerase chain reaction
RNA was extracted from cumulus cells by using a RNA isolation kit (RNAqueous-Micro; Ambion, Austin, TX, USA), according to the manufacturer's instructions. Fifty randomly selected COCs were used to obtain cellular pellets for RNA extraction. The extracted RNA was reverse-transcribed to cDNA by using the Thermo script RT-PCR system (Invitrogen), according to the manufacturer's instructions. The primer that was used for the RT-PCR was the oligo (dT) 20 primer that is included in the kit. Quantification of the cDNA then was performed by RT-PCR using the Rotor-Gene 6500 system (Qiagen). The primer sets that were used for quantifying β-Actin, mitochondrial transcription factor A (TFAM), gap junction alpha-1 protein (GJA1), inhibin A (INHA), thioredoxin-dependent peroxide reductase (PRDX3), glypican-4 (GPC4), and phosphofructokinase platelet (PFKP) were designed by using the DNA Data Bank of Japan (http://arsa.ddbj.nig.ac.jp/top-j.html) and Primer3 (http://frodo.wi.mit.edu/primer3/); the primers are listed in Table 1. The PCR was performed with an initial denaturation step of 95°C for 1 minute, followed by 40 cycles of 98°C for five-seconds and 60°C for 11 seconds. The SYBR Green fluorescence was measured at the end of each extension step. A melting curve was analyzed to determine the specificity of the PCR products and agarose gel electrophoresis was carried out to determine the resulting product sizes. The relative expression levels of each gene of interest were calculated by normalizing to the expression levels of the endogenous control, β-Actin. The reactions were run in duplicate and the experiments were repeated four times with different oocyte series. A standard curve was generated for each run by using 10-fold serial dilutions, representing the copy number of the external standard. The external standard was the PCR product of the corresponding gene that was cloned into a vector using the Zero Blunt TOPO PCR cloning kit (Invitrogen) and the PCR product was sequenced for confirmation before use. The amplification efficiencies of all the trial runs were >1.9.
2.9 Determination of the fatty acid content in the follicular fluid
The FF was collected from antral follicles (3-5 mm in diameter) of 80 gilts and the NEFA concentration in each FF sample was determined by using a Lab Assay TM NEFA (Wako), according to the manufacturer's protocol. The FF with the top five, middle five, and bottom five NEFA concentrations was selected and each category of FF was equally mixed to create high-NEFA FF, middle-NEFA FF, and low-NEFA FF. In the present study, five batches were prepared by using 400 gilts (Figure 1A). Before the experiment, a small volume of FF (200 μL) was collected from all the batches and mixed equally to create high-NEFA, middle-NEFA, and low-NEFA FF mixtures that were subjected to gas chromatography analysis (Toray Research Center, Inc., Tokyo, Japan). As seen in Figure 1B, C14:0–C24:1 fatty lipids were detected in the FF, with palmitic, stearic, and oleic acids as the most frequent components. For experiment 1, high-NEFA FF and low-NEFA FF were added at a concentration of 10% or 30% to the IVM medium. In experiment 2, a FA-Mix was made according to the fatty acid concentrations of the media containing 30% high-NEFA FF or 10% low-NEFA FF, that were then termed “H-FA” and “L-FA” media, respectively (Figure 2). The control media were supplemented with the same concentration of bovine serum albumin (BSA).
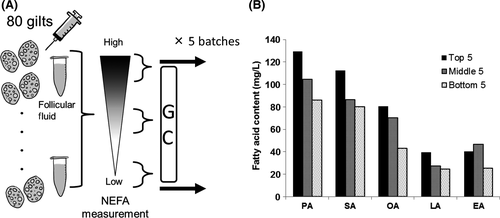
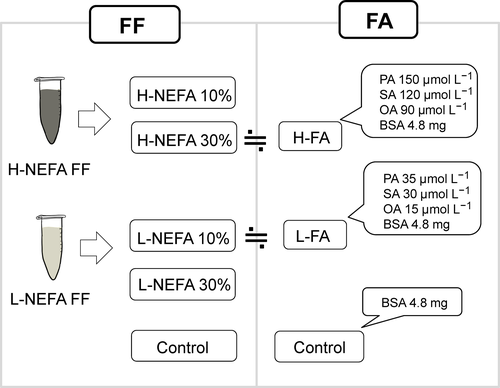
2.10 Experimental design
2.10.1 Effect of the follicular fluid on the cumulus–oocyte complexes
The oocyte-and-granulosa cell complexes were incubated in a maturation medium that was supplemented with 10% or 30% of either low-NEFA FF or high-NEFA FF. The oocyte developmental competence (maturation and blastulation rate) then was examined. At the end of the maturation period, the lipid content, ATP content, mtDNA copy number in the oocytes and cumulus cells, and the number and survival rate of the cumulus cells were determined. Furthermore, the cumulus cells were used to examine the expression levels of the genes that were reported to be associated with oocyte developmental competence (TFAM, GJA1, INHA, PRDX3, GPC4, and PFKP).
2.10.2 Effect of the fatty acids on the cumulus–oocyte complexes
The COCs were incubated in a control (with BSA), H-FA, or L-FA medium. The developmental ability of the oocytes, lipid content, ATP content, mtDNA number of both the oocytes and the cumulus cells, as well as the number and survival rate of the cumulus cells at the end of IVM, were determined, as described for experiment 1 in Section 2.9.
2.11 Statistical analysis
All the data were analyzed by using an ANOVA, followed by a post hoc Tukey's test. The percentages were arcsine-transformed before the analysis. The correlation between the FF NEFA content and the FF concentration was evaluated by a two-way ANOVA. The analysis was conducted by using IBM SPSS Statistics for Windows software (v. 21; IBM Corporation, Armonk, NY, USA). P-values that were <.05 were considered to be statistically significant.
3 RESULTS
3.1 Follicular fluid exposure improved the oocyte's developmental ability, whereas fatty acids alone had no effect
Supplementing the maturation medium with FF did not affect the oocyte maturation rate, whereas supplementation with 30% low-NEFA FF or 10% high-NEFA FF resulted in a significantly higher rate of development to the blastocyst stage, compared with the rate observed on culturing without FF supplementation (Table 2). The highest developmental rate was observed for the 10% high-NEFA FF groups, whereas supplementing with 30% high-NEFA FF yielded a low developmental rate that was similar to the rate of the control group. In addition, there was a significant correlation between the FF NEFA content and the FF concentration regarding the rate of development to the blastocyst stage. In the case of the FA-Mix supplementation, neither the maturation rate nor the rate of development to the blastocyst stage was affected (Table 3).
| Group | Oocytes | Cumulus cells | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of trials | No. of oocytes | Maturation rate (%) | No. of oocytes | Developmental rate (%) | No. of trials | Cell no. | Survival rate (%) | |
| Control (%) | 5 | 106 | 90.9 ± 5.5 | 200 | 3.1± 1.5a | 4 | 1576 ± 172a | 76.60 ± 1.3a |
| L-NEFA (10) | 5 | 105 | 89.0± 3.1 | 200 | 8.1 ± 1.0abc | 4 | 2406 ± 86ab | 84.50 ± 1.1b |
| L-NEFA (30) | 5 | 110 | 90.0 ± 5.8 | 200 | 9.5 ± 1.2bc | 4 | 3347 ± 76c | 88.00 ± .4bc |
| H-NEFA (10) | 5 | 104 | 89.2± 5.1 | 200 | 11.4 ± 1.2b | 4 | 3128 ± 393bc | 87.00 ± 1.0bc |
| H-NEFA (30) | 5 | 110 | 87.0 ± 1.5 | 200 | 5.8 ± 1.2ac | 4 | 3861 ± 183c | 88.50 ± .3c |
- H, high; L, low; NEFA, non-esterified fatty acid. a–c, P < .05.
| Group | Oocytes | Cumulus cells | ||||||
|---|---|---|---|---|---|---|---|---|
| No of trials | No. of oocytes | Maturation rate (%) | No. of oocytes | Developmental rate (%) | No. of trials | Cell no. | Survival rate (%) | |
| Control | 5 | 180 | 88.4 ± 4.3 | 173 | 13.7 ± 2.8 | 5 | 1523 ± 312 | 64.8 ± 4.6 |
| L-FA | 5 | 169 | 81.7 ± 2.0 | 157 | 7.4 ± 2.1 | 5 | 1535 ± 245 | 68.7 ± 5.2 |
| H-FA | 5 | 179 | 86.7 ± 1.6 | 171 | 9.6 ± 2.7 | 5 | 1621 ± 350 | 65.2 ± 6.0 |
- FA, fatty acid; H, high; L, low.
3.2 Follicular fluid exposure improved the cellular survival and proliferation of the cumulus cells, whereas fatty acid exposure did not
At the end of the maturation periods, the FF increased the cumulus cell number and higher concentrations of FF enhanced this increase (Table 2). In addition, supplementing the culture medium with FF improved the cumulus cell survival rate, irrespective of the NEFA concentration. Supplementation with the FA-Mix did not affect the number or survival rate of the granulosa cells (Table 3).
3.3 Follicular fluid and fatty acid exposure induced lipid accumulation in both the cumulus cells and oocytes
The NEFA concentrations of both the FF and FA-Mix in the culture medium closely reflected the lipid content in the cumulus cells, but this was not the case for the oocytes (Table 4 and 5). Supplementing the culture medium with either FA-Mix or FF increased the lipid content in the oocytes, except for 30% high-NEFA FF, which did not increase lipid accumulation. However, there was a significant correlation between the categories and concentration of FF (Tables 4 and 5).
| Group | Oocytes | Cumulus cells | |||
|---|---|---|---|---|---|
| Trial no. | No. of oocytes | Lipids | Trial no. | Lipids | |
| Control (%) | 5 | 82 | 1.00 ± .01a | 4 | 1.00 ± .06a |
| L-NEFA (10) | 5 | 82 | 1.12 ± .02c | 4 | 3.14 ± .05b |
| L-NEFA (30) | 5 | 82 | 1.26 ± .04b | 4 | 6.35 ± .30c |
| H-NEFA (10) | 5 | 82 | 1.21 ± .02b | 4 | 4.97± .14d |
| H-NEFA (30) | 5 | 82 | 1.00 ± .02ac | 4 | 7.34 ± .07e |
- H, high; L, low; NEFA, non-esterified fatty acid. a–e, P < .05.
| Group | Oocytes | Cumulus cells | |||
|---|---|---|---|---|---|
| Trial no. | No. of oocytes | Lipids | Trial no. | Lipids | |
| Control | 5 | 105 | 1.00 ± .01a | 5 | 1.00 ± .01a |
| L-FA | 5 | 105 | 1.17 ± .02b | 5 | 1.43 ± .04b |
| H-FA | 5 | 105 | 1.19 ± .02b | 5 | 2.19 ± .17c |
- FA, fatty acid; H, high; L, low. a–c, P < .05.
3.4 Follicular fluid and fatty acids differentially affected the mitochondrial number and function of the cumulus cells and oocytes
The FF increased the mtDNA copy number, showing a significant correlation between the NEFA and FF concentrations regarding the mtDNA copy number in both the oocytes and the cumulus cells (Table 6). On the contrary, adding fatty acids to the culture medium did not affect the mtDNA copy number in either the oocytes or the cumulus cells (Table 7). Irrespective of the NEFA concentration, the FF increased the ATP content in the oocytes to a similar extent, whereas only 30% low-NEFA FF significantly increased the ATP content in the cumulus cells (Table 6). Supplementing with H-FA increased the ATP content in the oocytes but had no effect on the cumulus cells (Table 7).
| Group | Oocytes | Cumulus cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of trials | No. of oocytes | Mitochondrial no. | No. of trials | No. of oocytes | ATP (pmol) | No. of trials | Mitochondrial no. | ATP (pmol) | |
| Control (%) | 2 | 23 | 108 560 ± 9743a | 2 | 31 | 1.82 ± .04a | 4 | 241 ± 11a | .11 ± .01a |
| L-NEFA (10) | 2 | 22 | 151 001 ± 13 928ab | 2 | 31 | 2.30 ± .03b | 4 | 368 ± 7b | .13 ± .02a |
| L-NEFA (30) | 2 | 24 | 141 784 ± 9233ab | 2 | 31 | 2.26 ± .02b | 4 | 411 ± 9c | .22 ± .02b |
| H-NEFA (10) | 2 | 23 | 122 722 ± 7658ab | 2 | 31 | 2.37 ± .06b | 4 | 331 ± 11b | .13 ± .02a |
| H-NEFA (30) | 2 | 23 | 155 275 ± 13 156b | 2 | 31 | 2.41 ± .04b | 4 | 422 ± 10c | .17 ± .02ab |
- H, high; L, low; NEFA, non-esterified fatty acid. a–c, P < .05.
| Group | Oocytes | Cumulus cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of trials | No. of oocytes | mtDNA copy no. | No. of trials | No.of oocytes | ATP (pmol) | No. of trials | mtDNA copy no. | ATP (pmol) | |
| Control | 2 | 27 | 112 713 ± 6865 | 3 | 48 | 2.12 ± .09a | 5 | 179 ± 19 | .08 ± .01 |
| L-FA | 2 | 25 | 125 327 ± 6069 | 3 | 48 | 2.31 ± .10a | 5 | 167 ± 8 | .07 ± .01 |
| H-FA | 2 | 27 | 126 523 ± 5965 | 3 | 48 | 2.73 ± .10b | 5 | 176 ± 35 | .08 ± .03 |
- FA, fatty acid; H, high; L, low. a–b, P < .05.
3.5 Follicular fluid exposure affects the expression levels of developmental competence marker genes in the cumulus cells
Supplementing the maturation medium with FF increased the expression levels of TFAM, PRDX3, PFKP, INHA, and GPC4, but decreased the levels of GJA1 in the cumulus cells (Figure 3). There was a significant correlation between the effect of the NEFA and FF concentrations on the expression levels of INHA, GJA1, PRDX3, PFKP, and GPC4.
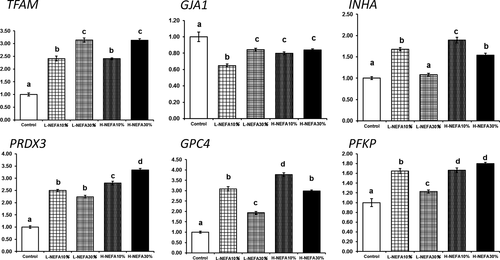
4 DISCUSSION
The present study observed a large variation of NEFA concentrations in porcine FF and revealed that the ability of FF to support oocyte maturation was associated with the NEFA concentration. Overall, supplementing the culture medium with FF or a mixture of NEFA induced lipid accumulation. Furthermore, the FF improved the viability of the cumulus cells and the ability of the oocytes to develop to the blastocyst stage, whereas the FA-Mix did not affect these parameters.
It has been shown that exogenous fatty acids and FF increase the lipid content in oocytes and cumulus cells.6, 8 Consistent with this finding, culturing in a medium containing either FF or FA-Mix increased the lipid content in both oocytes and cumulus cells. In addition, the extent of lipid accumulation in the cumulus cells reflects the NEFA content in the medium. In line with this study's results, it was reported that cumulus cells are more sensitive to lipids in the culture milieu.1 One exception was found, however, as 30% high-NEFA FF did not affect the lipid content in the oocytes, with a low rate of development to the blastocyst stage. As a high NEFA concentration is detrimental to oocytes,8 it was hypothesized that high NEFA concentrations in the FF adversely affected the cumulus cells and oocytes.
The lipid content in the oocytes and embryos alters energy metabolism, resulting in the low use of glucose and pyruvate.5, 15 A study16 found that active lipid metabolism in cumulus cells contributes to oocyte maturation and another study12 reported that supplementing the culture medium with FF increases the oocytes’ ATP content and changes the distribution of the mitochondria. Furthermore, the lipid content is closely related to a high ATP content in pig oocytes.17 These studies support the notion that the lipid content in cells and oocytes alters ATP generation and energy metabolism. Consistent with these findings, the present study showed that the ATP content in the oocytes increased after supplementation with either the FF or FA-Mix. However, the ATP content significantly increased in the cumulus cells only when the COCs were incubated in a medium containing 30% low-NEFA FF. This result could be related to complex interactions of the follicular contents or the low contribution of mitochondria toward ATP generation in cumulus cells because granulosa and cumulus cells mostly depend on glycolysis for ATP production.18
Follicular fluid supports oocyte growth and maturation. Accumulating evidence indicates that supplementing the maturation medium with FF increases oocyte maturation and the fertilization ability of pigs and cows.11, 12, 19 Consistent with these findings, this study found that the FF enhanced cumulus cell viability and proliferation, as well as oocyte developmental competence. However, exposure to a NEFA mixture resembling FF could not reproduce the beneficial effects of the FF. This indicates that the NEFA concentrations of FF are associated with other factors, such as linolenic acid, proteins, and microRNAs, which play key roles in oocyte maturation.6, 20-23 The NEFA is an important component of FF that reflects the physical condition of the donor animal. For example, fasting cows with a negative energy balance have high NEFA concentrations in their FF1, 24 and fatty acid composition depends on the Body Mass Index of women.2 In line with this, the researchers observed a significant correlation between an increased NEFA and FF concentration on an increased developmental rate, lipid content in the oocytes, and survival rate of the cumulus cells, indicating that the NEFA concentrations interact with crucial factors required for oocyte maturation. However, the present study did not determine the factors that are associated with the NEFA in the FF of gilts.
Interestingly, it was found that supplementing the culture media with FF, but not FA-Mix, significantly increased the mtDNA amounts in the cumulus cells, but only slightly in the oocytes. Mitochondrial biogenesis is regulated by TFAM expression,25 and accordingly, higher expression levels of TFAM were observed in the cumulus cells that had been cultured in the FF medium, compared with those that had been cultured without FF. Adding the FA-Mix to the culture medium did not affect the mtDNA copy number in either the oocytes or the granulosa cells. To the authors’ knowledge, this study is the first to report that FF impacts mitochondrial biogenesis in cumulus cells, but molecular events underlying the increase in biogenesis is a subject for further experiments. Nevertheless, studies have shown that the mtDNA copy number in cumulus cells reflects a high yield of good embryos in humans.26, 27 Thus, a beneficial effect of FF on oocyte developmental competence is associated with an increased mtDNA in cumulus cells.
The expression levels of several genes in cumulus cells are associated with oocyte developmental competence.28-31 In the present study, the effect of FF on the cumulus cells was evaluated by determining the expression of marker genes. GPC4 encodes a heparin sulfate proteoglycan that is associated with the cell surface and is reported in cow as a cumulus cell marker for oocyte developmental competence.31 Furthermore, the expression levels of the PFKP and PRDX3 genes in cumulus cells are associated with good-quality embryos in humans29; this study's results showed that the expression levels of these genes were higher in the cumulus cells that had been cultured in FF medium. GJA1 encodes connexin 43, a gap junction protein, and low expression levels of GJA1 in cumulus cells are associated with oocyte maturity.30 In addition, low expression levels of GJA1 in cumulus cells are associated with high developmental competence in human oocytes.28 In line with this, culturing COCs in a medium containing FF resulted in low GJA1 expression levels. These findings suggest that culturing COCs in a medium containing FF is beneficial for oocyte maturation. Furthermore, a clear correlation between the FF NEFA content and the FF concentration in the medium was observed, suggesting that NEFA could be a FF component that is responsible for oocyte maturation.
The present study revealed that fatty acids and FF induced lipid accumulation in oocytes and cumulus cells and affected energy metabolism in oocytes. In addition, the FF improved oocyte maturation and developmental ability and the NEFAs in FF were found to be closely linked with factors that regulate the mitochondrial number and survival rate of cumulus cells.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent: This article does not contain any study with human participants that was performed by any of the authors. Animal studies: In this study, the porcine ovaries were collected from a slaughterhouse. The ovaries were discarded without any use in edible meat; therefore, this study was approved by the Ethical Committee for Animal Experiments of the Tokyo University of Agriculture, Tokyo, Japan.




