Effectiveness of high-dose i.v. immunoglobulin therapy for pregnant women with aspirin–heparin-resistant secondary antiphospholipid syndrome
Abstract
Purpose
This study aimed to assess the efficacy of high-dose i.v. immunoglobulin (HIVIg) therapy in pregnant women with antiphospholipid syndrome (APS) secondary to systemic lupus erythematosus with a history of pregnancy failure, despite receiving low-dose aspirin plus unfractionated heparin therapy, of which condition being designated as “aspirin–heparin-resistant APS” (AHRAPS).
Methods
The HIVIg therapy (20 g/d, 5 days) was performed for the pregnancies of five women with AHRAPS.
Results
Five of the eight pregnancies ended in live births. The gestational ages of delivery in four of the five pregnancies were extended, compared with previous pregnancies. The HIVIg therapy was considered to be successful for these four pregnancies. Excluding one pregnancy that ended in miscarriage with an abnormal chromosome karyotype of the villi, the HIVIg therapy was considered to be successful in four (57.1%) of the seven pregnancies of the women with AHRAPS. Although all the live newborns were prematurely delivered, no adverse effect of the HIVIg therapy was observed.
Conclusions
The HIVIg therapy might be beneficial as an immune modifier for pregnant women with AHRAPS. However, the precise indication of which women with AHRAPS who should receive HIVIg therapy remains unknown.
1 INTRODUCTION
Antiphospholipid syndrome (APS), advocated by Hughes in 1983, is an acquired autoimmune disorder that manifests clinically as recurrent venous or arterial thrombosis and causes obstetrical complications in women.1, 2 Pregnant women with APS have an increased risk of obstetrical complications and poor pregnancy outcomes, including fetal loss, recurrent miscarriage (RM), preeclampsia, and placental insufficiency.3 As randomized controlled studies have demonstrated that low-dose aspirin (LDA) plus unfractionated or low-molecular-weight heparin therapy improves the live birth rate in pregnant women with APS who have a history of RM,4, 5 LDA plus heparin have been used as a standard therapeutic modality worldwide. However, 20%–30% of pregnancies in women with APS who have a history of RM result in fetal loss again, despite receiving LDA plus heparin therapy.6 Such cases have been designated as aspirin–heparin-resistant antiphospholipid syndrome (AHRAPS).7 Some case reports have shown that high-dose i.v. immunoglobulin (HIVIg) therapy leads to a live birth for pregnant women with AHRAPS successfully.7-9 The present study reports on the experience of HIVIg therapy in eight pregnancies from five women with AHRAPS.
2 MATERIALS AND METHODS
This prospective study was approved by the institutional ethical boards of Kobe University Hospital, Kobe, Japan (No. 260004) and informed consent was obtained from all the participants. Between April 2009 and December 2016, women with APS were enrolled into this study if they met all of the following inclusion criteria: (i) APS based on the updated Sydney classification criteria10; (ii) the presence of a history of premature delivery of a morphologically normal newborn before 34 gestational weeks (GWs) due to eclampsia, severe preeclampsia, or placental insufficiency, an unexplained miscarriage without fetal abnormality at 10 GWs or later, or three or more RMs; (iii) the presence of a history of miscarriage, stillbirth, or premature birth before 34 GWs despite LDA plus heparin therapy; and (iv) obtained written informed consent. Women who had an allergy to immunoglobulin or immunoglobulin A deficiency were excluded.
The laboratory criteria that were used in the present study included repeated positive tests for lupus anticoagulant (LA), immunoglobulin G (IgG)/immunoglobulin M (IgM) anticardiolipin antibody (aCL), and IgG β2 glycoprotein I-dependent anti-aCL (aCLβ2GPI). A dilute Russell's viper venom time-based test (Gradipore LA Screen and LA Confirm; Gradipore, Ltd., Frenchs Forest, NSW, Australia) was used for the LA measurements. A screen clotting time/confirm clotting time ratio of 1.3 was defined as the cut off value of LA. The IgG, IgM, and aCL were measured by using an enzyme-linked immunosorbent assay (MESACUP cardiolipin test IgG/IgM; MBL Company, Ltd., Nagoya, Japan), based on the methods described in one of the studies:11 10 U/mL of IgG and 8 U/mL of IgM were defined as the cut-off values. The IgG aCLβ2GPI was measured by using an enzyme immunoassay (Anti-CLβ2GPI Yamasa EIA Kit; Yamasa Company, Ltd., Tokyo, Japan): 3.5 U/mL was defined as the cut off value. In the present study, IgG aCLß2GPI was measured for IgG aβ2GPI. The IgM aCLβ2GPI measurement is not commercially available in Japan. The IgG anti-phosphatidylserine/prothrombin antibody (aPS/PT) was measured by using an enzyme-linked immunosorbent assay (aPS/PT ELISA Kit; MBL Company, Ltd.): 12 U/mL was defined as the cut-off value.
The pregnant women received HIVIg therapy (intact-type immunoglobulin 20 g daily over a course of 5 days; a total dose of 100 g) in addition to LDA plus heparin therapy. The HIVIg therapy was commenced after a gestational sac in the uterus was detected by transvaginal ultrasound at an earlier gestational age than that at which previous fetal losses had occurred. The peripheral natural killer (NK) cell activity was measured by using a 51chromium release assay before HIVIg and 1 week after HIVIg therapy. Prednisolone was administered to those women who had a high level of disease activity or an exacerbation of autoimmune disease. If the index pregnancy with HIVIg therapy ended in a live birth at a gestational age later than that of previous pregnancies, HIVIg therapy was considered to have been successful. When the index pregnancy ended in miscarriage, the chromosome karyotype of the villi was analyzed.
3 RESULTS
The HIVIg therapy was given for eight pregnancies for five women with AHRAPS. Table 1 shows the clinical backgrounds and outcomes of these eight pregnancies, which were complicated by systemic lupus erythematosus (SLE). SLE was controlled at conception in the index pregnancy, as well as previous pregnancies. Seven of the eight pregnancies had no hypocomplementemia at conception, while one (case 4) had mild hypocomplementemia (CH50: 18.2 U/mL; C3: 53 mg/dL; C4: 7.3 mg/dL). A SLE flare during pregnancy was observed in only one case (case 1-2).
| Case no. | Complications | Positive antiphospholipid antibody | Pregnancy history | Therapy for previous pregnancies | At conception of pregnancy index | Therapy for the index pregnancy | GW of HIVIg | Pregnancy outcome | Neonate | Success or failure of HIVIg therapy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Activity of SLE | ||||||||||
| 1-1 | SLE |
IgG aCL (>120 U/mL) IgG aCLβ2GPI (>125 U/mL) LA (2.24) |
5 Gs 5 MSs (7-8 GWs) |
PSL (5 mg) + LDA for 2nd and 3rd pregnancies PSL (5 mg) + LDA + H for 4th and 5th pregnancies |
31 | Remission with 5 mg PSL( (without hypocomplementemia) |
5 mg PSL LDA + H HIVIg |
6 | MS with abnormal chromosome karyotype (9 GWs) | N/A | N/A |
| 1-2 |
7 Gs 7 MSs (6-8 GWs) |
PSL (5 mg) + LDA for 7th pregnancy | 31 |
Remission with 7 mg PSL (without hypocomplementemia) |
7-30 mg PSL LDA + H HIVIg |
6 and 29 |
Live birth (31 GWs), SLE flare, oligohydramnios, NRFS |
1570 g (–0.2 SD) | Success | ||
| 2 | SLE, Sjögren's syndrome, Evans syndrome |
LA (1.5) IgG aPS/PT (129 U/mL) |
3 Gs One live birth (29 GWs with FGR, HELLP syndrome) 2 MSs (21 GWs with FGR and 7 GWs) |
PSL (10-20 mg) + LDA + H for 2nd pregnancy, which ended in a live birth | 32 |
Remission with 7.5 mg PSL (without hypocomplementemia) |
7.5-20 mg PSL LDA + H HIVIg |
15 |
Live birth (26 GWs), HDP, FGR, liver and renal dysfunction |
750 g (–1.3 SD) |
Failure |
| 3 | SLE |
IgG aCL (15 U/mL) IgG aCLβ2GPI (9.9 U/mL) LA (1.3) IgG aPS/PT (24 U/mL) |
6 Gs One stillbirth (22 GW with FGR, HDP) 4 MSs (6-19 GWs) 1IA |
PSL (10-20 mg) + LDA + H for 4th and 5th pregnancies PSL (10 mg) + LDA for 6th pregnancy |
36 |
Remission with 4 mg PSL (without hypocomplementemia) |
4-10 mg PSL LDA + H HIVIg |
11 |
Live birth (29 GWs), HDP, FGR, oligohydramnios, NRFS |
936 g (–1.9 SD); rupture of the stomach |
Success |
| 4 | SLE |
IgG aCL (26 U/mL) IgG aCLβ2GPI (3.7 U/mL) LA (2.5) IgG aPS/PT (24 U/mL) |
2 Gs One live birth (33 GWs with CA-BSI) 1 MS (16 GWs) |
PSL (0-20 mg) + LDA + H for 2nd pregnancy | 37 |
Remission with 15 mg PSL (with mild hypocomplementemia: CH50: 18.2 U/mL C3: 53 mg/dL C4: 7.3 mg/dL) |
15-20 mg PSL LDA + H HIVIg |
9 | Live birth (36 GWs) |
2434 g (–0.1 SD) |
Success |
| 5-1 | SLE, a history of myocardial infarction |
IgG aCL (45 U/mL) IgG aCLβ2GPI (27 U/mL) LA (2.5) IgG aPS/PT (28 U/mL) |
1 G One stillbirth (22 GWs with HDP, HELLP, eclampsia) |
LDA + H for 1st pregnancy | 32 | Remission without PSL(without hypocomplementemia) |
LDA + H HIVIg |
5 | MS with unknown chromosome karyotype (6 GWs) | N/A | Failure |
| 5-2 |
2 Gs One stillbirth (22 GWs) 1 MS (6 GWs) |
LDA + H + HIVIg for 2nd pregnancy | 32 | Remission without PSL(without hypocomplementemia) |
LDA + H HIVIg |
5 | MS with normal chromosome karyotype (17 GWs) | N/A | Failure | ||
| 5-3 |
3 Gs One stillbirth (22 GWs) 2 MSs (6 and 17 GWs) |
LDA + H + HIVIg for 3rd pregnancy | 33 | Remission without PSL)(without hypocomplementemia) |
10 mg PSL LDA + H HIVIg |
5 and 13 |
Live birth (23 GWs), FGR, HDP, NRFS |
320 g (–3.7 SD) |
Success | ||
- aCL, anticardiolipin antibody; aCLβ2GPI, anticardiolipin β2-glycoprotein I; aPS/PT, antiphosphatidylserine/prothrombin antibody; CA-BSI, catheter-associated blood stream infections; FGR, fetal growth restriction; G, gravidity; GW, gestational weeks; H, unfractionated heparin; HDP, hypertensive disorder of pregnancy; HELLP syndrome, hemolysis, elevated liver enzymes, and low platelet count; IA, induced abortion; IgG, immunoglobulin G; LA, lupus anticoagulant; LDA, low-dose aspirin; MS, miscarriage; N/A, not applicable; NRFS, non-reassuring fetal status; PSL, prednisolone; SD, standard deviation; SLE, systemic lupus erythematosus.
Three of the eight pregnancies ended in miscarriages (cases 1-1, 5-1, and 5-2). One miscarriage had an abnormal chromosome karyotype of the villi (case 1-1). In another miscarriage, the chromosome karyotype of the villi was unknown because of the failure of the cell culture (case 5-1). The other miscarriage occurred at 17 GWs and had a normal chromosome karyotype without a fetal anomaly or abnormal pathology of the placenta (case 5-2).
Five of the eight pregnancies ended in live births (cases 1-2, 2, 3, 4, and 5-3). The gestational ages of delivery in four of the five pregnancies were extended, compared with previous pregnancies (cases 1-2, 3, 4, and 5-3). Therefore, HIVIg therapy was considered to be successful for these four pregnancies. Excluding one pregnancy that ended in miscarriage with an abnormal chromosome karyotype of the villi, HIVIg therapy was considered to be successful in four (57.1%) of the seven pregnancies in the women with AHRAPS, yielding a live birth rate of 71.4% (5/7) in the present study. Although all the live newborns were prematurely delivered, no adverse effect of the HIVIg therapy was observed.
One woman (case 1-2) received HIVIg therapy at 6 GWs and developed thrombocytopenia (platelet count: 90,000/μL) at 29 GWs. The HIVIg therapy was provided additionally for the idiopathic thrombocytopenia and her platelet count increased successfully to normal levels. Another woman (case 5-3) received HIVIg therapy at 13 GWs in addition to 5 GWs because a previous miscarriage had happened at 17 GWs after HIVIg therapy at 5 GWs. In terms of obstetrical complications, all five live newborns were prematurely delivered: three pregnancies were complicated by fetal growth restriction (FGR) of ≤−1.3 standard deviations; three had hypertensive disorder of pregnancy (HDP); three had non-reassuring fetal status (NRFS); and two had oligohydramnios.
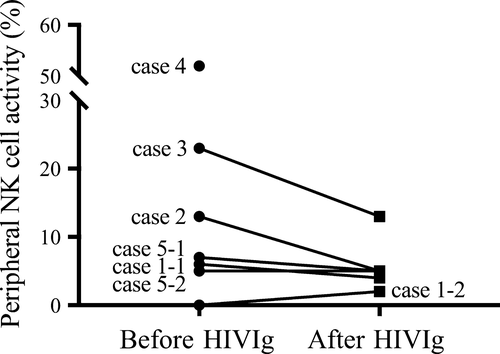
Peripheral natural killer (NK) cell activity before and after HIVIg therapy is shown in Figure 1. No significant change in the NK cell activity was found using Wilcoxon's matched pairs test. However, the NK cell activity was suppressed by HIVIg in case 2 and 3, who had higher levels of NK cell activity before HIVIg, while the NK cell activity did not change after HIVIg in cases 1 and 5, who had lower levels of NK cell activity before HIVIg.
4 DISCUSSION
Randomized controlled studies have shown that LDA plus heparin therapy is effective in improving the live birth rate4, 5 and reducing morbidity12, 13 in pregnant women with APS who have a history of RM. Although LDA plus heparin is recommended as a standard therapy,4 high rates of low birthweight newborns,14 FGR, and premature delivery15 are observed in pregnant women with APS and LDA plus heparin therapy. Unfortunately, 20%–30% of pregnancies in women with APS who have a history of RM result in fetal loss again, despite receiving LDA plus heparin therapy.6 Such cases are designated as AHRAPS.7
In the present study, HIVIg therapy was successful in four (57.1%) of the seven pregnancies, yielding a live birth rate of 71.4% (5/7) in the women with AHRAPS. These relatively high rates of success and live births using HIVIg therapy imply the possible efficacy of this therapeutic modality for pregnant women with AHRAPS, which is the most severe manifestation of APS. Obstetrical complications, such as premature delivery, FGR, HDP, NRFS, and oligohydramnios, were observed frequently in this study. Therefore, careful perinatal management is necessary during pregnancies in women with AHRAPS who receive HIVIg therapy.
High-dose i.v. immunoglobulin therapy is known to be practically effective and this therapeutic modality has long been applied to a wide variety of immune-mediated diseases, including idiopathic thrombocytopenic purpura, Guillain-Barré syndrome, Kawasaki's disease, and myasthenia gravis.16, 17 The HIVIg therapy (20 g/d, five consecutive days, total 100 g) for the women who had a history of four or more consecutive spontaneous abortions of unexplained etiology has been developed by the authors’ group, yielding an 89.8% live birth rate.18 One study first reported successful HIVIg therapy (400 mg/kg/d, five consecutive days at 17 GWs, and two consecutive days at 22 and 27 GWs) in a pregnant woman with APS who had a history of nine RMs.19 A randomized controlled trial that compared LDA plus heparin plus HIVIg (1 g/kg/d, two consecutive days, monthly) with LDA plus heparin therapy in 16 women with APS failed to show differences in their efficacy.20 One study reported that LDA plus low-molecular-weight heparin yielded a higher birth rate (84%) than that of i.v. immunoglobulin (400 mg/kg/d, two consecutive days and 400 mg/kg/d, monthly) alone (57%) in women with RM with aCLβ2GPI.21 Thereafter, they demonstrated successful i.v. immunoglobulin therapy (1 g/kg, monthly) in eight of 10 women with AHRAPS.22 Recent case reports also have shown that HIVIg therapy led to a live birth to pregnant women with AHRAPS successfully.7-9 Therefore, HIVIg therapy might serve as an effective immune modifier in pregnant women with a pathophysiology underlying AHRAPS. However, the precise indication of women with AHRAPS who should receive HIVIg therapy remains unknown. Similar to previous reports of IVIg therapy in women with RM,23 the suppression of NK cell activity in pregnant women who had higher levels of NK cell activity before HIVIg was observed in this study. But, it is unclear whether the suppression of NK cell activity is associated with a better pregnancy outcome in women with AHRAPS.
The medical cost of HIVIg is much higher than that of LDA plus heparin therapy. The results of the present study included several uncertainties. The enrollment of the participants in the study design was not randomized nor placebo-controlled. In order to prove the efficacy of HIVIg therapy in pregnant women with AHRAPS and a history of RM, a randomized placebo-controlled study is necessary in the future.
ACKNOWLEDGEMENTS
We are grateful for the involvement of the participants and the care that was provided by the staff members at Kobe University Hospital, Kobe, Japan. We thank the clinical and laboratory personnel who supported this study at Kobe University Hospital. This work was supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science (No. 26462489 and 25462593).
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. This study was approved by the institutional ethical boards of Kobe University Hospital (No. 260004). Informed consent or a substitute for it to be included in the study was obtained from all the patients.




