Systemic lupus erythematosus caused acquired hemophilia B without evidence of factor IX inhibitors
Edited by Zhiyu Wang and Lishao Guo.
Lifang Wang and Yan Ding contributed equally to this study.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multisystem involvement. In addition to butterfly erythema, photosensitivity, arthritis, and other clinical manifestations, SLE can also result in hematological complications, such as coagulopathy. Acquired hemophilia has been documented in patients with SLE, attributed to the presence of inhibitors against coagulation factors in the circulating blood.1 Furthermore, deficiency of factor XII (FXII) has been reported in patient with SLE in the absence of FXII inhibitors.2, 3 However, there have been no reports of a deficiency of FIX without the presence of FIX inhibitors in patients with SLE. In this report, we present a case of a patient with SLE who exhibited recurrent mucosal bleeding attributed to reduced activity of FIX without detectable inhibitors.
A 56-year-old Chinese female patient presented to Peking University Shougang Hospital with hematuria, accompanied by low back pain and fever in June 2023. Enhanced abdominal computerized tomography (CT) revealed thickening and irregularity of the wall of the right renal pelvis and abdominal segment of the ureter, suggesting possible inflammatory lesions or lymphoma. Laboratory tests indicated anemia (hemoglobin [Hb]: 63 g/L), hypocomplementemia (C3: 0.52 g/L), and prolonged activated partial thromboplastin time (APTT: 84.6 s). Tests for lupus anticoagulant (LA), anticardiolipin (aCL) antibody, and anti-beta 2-glycoprotein I (β2-GPI) antibodies were all negative. The patient was diagnosed with dermatomyositis (DM) 6 years ago, due to swollen and painful joints, muscle pain and weakness, pulmonary fibrosis showed by chest CT, elevated creatine kinase (CK) and creatine kinase isoenzymes (CKMB), along with positive antinuclear antibodies (ANA), anti-Mi2 antibodies, and anti-Ro52 antibodies. Symptoms significantly improved following treatment with glucocorticoids and cyclophosphamide. However, the patient discontinued the medication on her own in 2021.
To clarify the cause of ureteral thickening, ureteroscopy was performed. The ureteroscopy revealed multiple patchy ecchymoses in the right renal pelvis, calyces, and right ureter, with no evidence of tumors. Consequently, the hematuria might be associated with the prolonged APTT. Given the significant prolongation of APTT, further APTT mixing study were conducted. The results showed that the prolonged APTT could be partially corrected by APTT 1:1 mix, but over 2 h incubation at 37°C, the APTT mix rise 2.1 s (Supporting Information: Table S1). Therefore, we proceeded to evaluate the activity of coagulation factors. The results demonstrated that the activities of FVIII, FIX, and FXII were 184.9% (normal range: 50%–150%), 40.3% (normal range: 65%–150%) and 36.6% (normal range: 50%–150%), respectively, and the inhibitors of FIX and FXII were negative using Nijmegen Bethesda assay (Siemens).
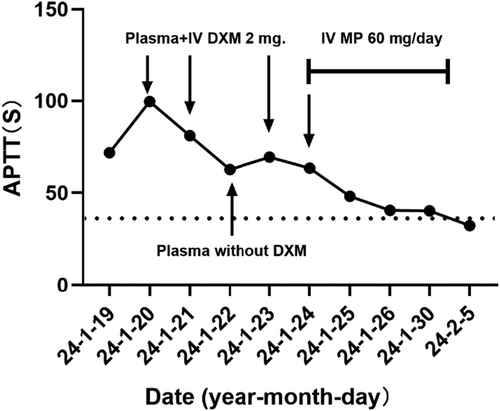
Interestingly, we observed that the prolonged APTT could be partially ameliorated following the red blood cell transfusion during hospitalization. Dexamethasone at a dosage of 5 mg was administered intravenously before transfusion. Considering the patient's diagnosis of autoimmune diseases, we hypothesized that glucocorticoids might play a pivotal role in mitigating the prolongation of APTT. To confirm our hypothesis, we prescribed methylprednisolone at a dosage of 40 mg/day intravenously for the patient. After treatment, the patient's hematuria improved and gradually resolved, while the prolonged APTT returned to 29.2 s.
After improvement of the condition and subsequent discharge from the hospital, the patient independently discontinued medication once again. Three months after cessation of the medication, the patient presented with recurrent epistaxis and skin ecchymosis of the right upper arm. Laboratory examinations showed thrombocytopenia (platelets [PLT]: 66 × 109/L, hypocomplementemia (C3: 0.75 g/L), and a prolonged APTT (42.2 s). Upon readmission, the APTT further prolonged to 99.6 s, and the PLT count decreased to 50 × 109/L. APTT mixing study was performed in another institution, and the results were similar to the first hospitalization (Supporting Information: Table S1). Tests for LA, aCL, and β2-GPI antibodies remained negative. APTT mixing study demonstrated that the prolonged APTT could be partially corrected (Supporting Information: Table S1). Evaluation of coagulation factor activity was repeated in another institution: FXII 27.4% (normal range: 50%–150%), FIX 16.6% (normal range: 65%–150%). Both FXII and FIX activities were extremely low, but inhibitors of coagulation factors remained negative using Nijmegen Bethesda assay.
The patient was subsequently diagnosed with SLE based on the presence of alopecia, joint pain, hypocomplementemia, thrombocytopenia, and positive results for ANA and anti-rRNP antibodies, according to the 1997 American College of Rheumatology and 2012 Systemic Lupus International Clinical Collaboration classification criteria for SLE. Consequently, intravenous methylprednisolone at a dosage of 60 mg/day was administered to the patient. One week later, the APTT significantly decreased to 40.3 s, and coagulation factor activity returned to normal levels: FIX 65.0%, FX II 30%. After discharge, the patient received prednisone at a dose of 50 mg/day for sequential treatment. The observed coagulation dysfunction may be attributed to SLE, and mycophenolate mofetil was prescribed to tapper prednisone. The changes in patient's APTT during hospitalization were illustrated in Figure 1. Two months after her discharge, the APTT was 23.3 s and the PLT count was 128 × 109/L. We followed up with the patient for 6 months, during which no further mucosal bleeding was reported. In addition, the condition of SLE remained stable with a Systemic Lupus Erythematosus Disease Activity Index score of 0.
Actually, the patient could be diagnosed with overlap syndrome (SLE and DM) characterized by a prolonged APTT attributed decreased activity of FIX. Notably, the DM was stable, leading us to conclude that the current manifestations were primarily related to SLE. In addition, our present report of SLE inducing low activity of FIX is distinct from those acquired hemophilia B with inhibitors against FIX. This rare case suggests that SLE can cause reduced FIX activity or FIX deficiency in the absence of detectable FIX inhibitors.
To mitigate the risk of false-negative results in FIX inhibitor detection, coagulation factor inhibitor assessments of the present case were conducted using the modified Nijmegen Bethesda assay. The modified Nijmegen Bethesda approach demonstrated improved specificity and cost-effectiveness compared to the classical Bethesda assay.4 However, there is still a limitation for the present result of negative FIX inhibitors. As plasma samples with endogenous coagulation factor activity exceeding 5% could be heated to 56°C for 30 min before testing to eliminate residual factor activity, thereby minimizing interference and optimizing the assay's diagnostic accuracy and sensitivity for inhibitor detection.5 In the present case, despite measurable FIX activity exceeding the 5% threshold in the patient's plasma, the recommended thermal pretreatment protocol was omitted. This methodological deviation may have compromised the assay's sensitivity, potentially contributing to the observed false-negative inhibitor result. The patient's APTT mixing study demonstrated immediate correction upon mixing with normal plasma, followed by a prolonged APTT after a 2-h incubation (exceeding the reference range). Given the normalization of APTT following glucocorticoid therapy, we hypothesize the presence of undetectable autoantibodies against FIX in the plasma of this SLE patient, leading to acquired FIX deficiency. Although no inhibitors were detected using conventional assays, the antibodies may have been undetectable due to extremely low titers or currently unidentified mechanisms, as we did not conduct the thermal inactivation method to validate the influence of endogenous FIX in this patient.
The patient clinically meets the criteria for acquired hemophilia, but due to the limitations of the testing methods, no inhibitors were detected. In the future, when encountering similar patients, it is important to pay attention to the testing methods and conduct thermal inactivation method to mitigate false-negative results and investigate potential novel or unidentified pathogenic mechanisms.
AUTHOR CONTRIBUTIONS
Lifang Wang wrote the manuscript. Lianjie Shi, Yan Ding, and Lei Wang contributed to the discussion and edited the manuscript. Lianjie Shi and Lifang Wang collected the clinical data and participated the follow-up. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the patient who made this case report possible. This study was supported by a grant from Peking University Shougang Hospital Research Funds, Grant/Award Number: SGYYZ202403.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Obtained patient's consent for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.