Efficacy and safety of anifrolumab across organ domains of systemic lupus erythematosus: A systematic review and meta-analysis
Edited by Zhiyu Wang and Lishao Guo.
Abstract
Background
Type I interferons are associated with autoantibody-mediated pathology in systemic lupus erythematosus (SLE). Anifrolumab is a monoclonal antibody antagonist to type I interferons. Our objective was to consolidate data on anifrolumab's efficacy and safety across organ-specific outcomes in SLE.
Methods
PubMed, Scopus, Cochrane Library, Google Scholar, and ClinicalTrials.gov were searched for clinical trials investigating anifrolumab's efficacy and safety on September 10, 2023 and repeated on March 25, 2025. A meta-analysis was conducted using risk ratios (RR) or mean difference (MD) with corresponding 95% confidence intervals (CI). Composite lupus scores, C3, C4, flare rates, cutaneous lupus, joint disease, and adverse events were meta-analyzed.
Results
Fourteen included studies yielded 1322 patients; five studies were meta-analyzed. Compared to placebo, anifrolumab 300 mg increased the proportion of patients with response across composite lupus disease measures and a ≥50% reduction in both cutaneous lupus scores (RR = 1.82, 95% CI: 1.29, 2.57, p = 0.0006) and in tender and swollen joint count (RR = 1.31, 95% CI: 1.04, 1.65, p = 0.02). Anifrolumab 300 mg treatment was associated with lower flare rates (RR = 0.75, 95% CI: 0.60, 0.93, p = 0.01) and increased C3 (MD = 0.08, 95% CI: 0.04, 0.11, p < 0.0001). In active renal disease, treatment with anifrolumab did not meet the primary endpoint but a higher dose regimen is under further investigation. Anifrolumab 300 mg did not increase serious adverse events, but higher incidence of herpes zoster (RR = 3.17, 95% CI: 1.92, 5.23, p < 0.00001) and numerically higher death rate were observed (0.88% vs. 0.19% in placebo).
Conclusions
Anifrolumab 300 mg reduces various SLE manifestations but add-on usage benefits in lupus nephritis remain under investigation. Anifrolumab is relatively tolerated, but reported pneumonia-related deaths may warrant caution in the prescription of anifrolumab to those at high risk of infections.
Key points
-
Anifrolumab was approved in 2021 based on evidence that has since grown.
-
Anifrolumab improves cutaneous manifestations, joint disease, C3 levels, and reduces flares in systemic lupus erythematosus.
-
Potential for add-on use in active lupus nephritis remains uncertain.
-
Anifrolumab significantly increases herpes zoster rates.
-
Deaths, mainly due to pneumonia, were numerically increased.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by widespread inflammation and tissue damage affecting multiple organ domains including the skin, joints, kidneys, and central nervous system. The pathogenesis of SLE involves a combination of genetic, environmental, and immunological factors.1 Type I interferons play an important role in the innate immune defense against various pathogens;2 however, this pathway plays a dysfunctional role in the autoantibody-mediated immune responses characteristic of SLE.1 Although the pathogenicity of type I interferons in SLE has been established, their correlation to disease severity and progression is heterogenous.1, 3
Despite the presence of several standard of care therapies for SLE, adequate control and tolerability are not always possible.4 In recent years, monoclonal antibodies have become a focus of pharmaceutical research.5 Anifrolumab is a fully human monoclonal IgG1κ antibody that targets signaling by all type I interferon receptors.6 Successful phase 3 clinical trials have led to the approval of anifrolumab, in its intravenous mode of administration and at a recommended dose of 300 mg, to treat moderate to severe disease.7-9
A previous 2020 meta-analysis on anifrolumab suggested its efficacy in SLE, analyzing composite lupus disease outcomes and cutaneous lupus activity.10 Flares, joint activity, and serology data were not consolidated, and initial exploratory data on the subcutaneous dose and renal SLE involvement were not yet available.11, 12 Subsequently, a meta-analysis was conducted focusing only on safety of anifrolumab, suggesting its safety and low serious adverse events (SAEs) rate,13 but evidence from long-term open-label and placebo-controlled trials has since emerged.14, 15 The growing body of evidence surrounding anifrolumab warrants further evaluation to characterize the evolving picture of the efficacy and safety of the drug.
Our systematic review and meta-analysis investigates the efficacy and safety of anifrolumab across multiple organ domains in SLE, incorporating recently published data, with the aim to contextualize its role in the current clinical management of multiple organ involvement in SLE and provide insight for future research.
2 METHODS
2.1 Protocol
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 The protocol was registered on PROSPERO (CRD42023461606).
2.2 Search strategy
An electronic search was carried out on 5 databases: PubMed, Scopus, Cochrane Library, Google Scholar, and ClinicalTrials.gov on September 10, 2023 and repeated on March 25, 2025. The Medical Subheadings (MeSH) used for the population were (“Systemic lupus erythematosus” OR “Lupus nephritis” OR “Lupus” OR “Lupus vasculitis” OR “Cutaneous lupus OR Lupus Erythematosus Disseminatus”); for the intervention: (“Anifrolumab” OR “type I IFN-alpha receptor” OR “38RL9AE51Q” OR “Anti-Interferon-α Receptor Monoclonal Antibody” OR “Type 1 Interferon inhibitor” OR “Type 1 Interferon inhibition”) and for the outcome it was (“BICLA” OR “SRI(4)” OR “Healing”). All search terms were combined using Boolean operators as seen in Supporting Information: Table S1.
2.3 Eligibility criteria and study selection
2.3.1 Inclusion criteria
The review's inclusion criteria were (1) clinical trials (randomized controlled trials [RCTs] and open-label studies) (2) investigating the efficacy and safety of anifrolumab on SLE patients. Full-texts had to be available for all clinical trials; however, abstracts providing supplementary data to previously published full-texts of a clinical trial were included.
2.3.2 Exclusion criteria
Studies were excluded if they were (1) observational in design such as case series and cohort studies, (2) not published in English, (3) focusing on pharmacokinetics and pharmacodynamics with no efficacy data, (4) secondary studies such as reviews and commentaries, and (5) preliminary trials on healthy patients.
2.3.3 Study selection
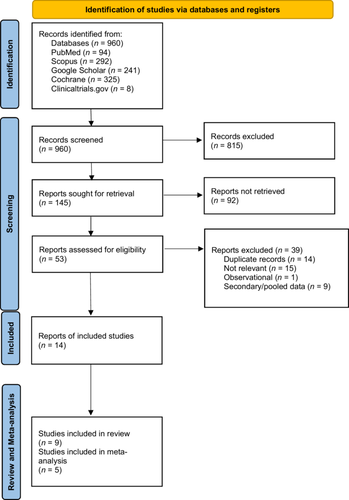
Our search yielded 960 results across all databases, which were combined using Microsoft Excel (version 2504). Next, a total of four reviewers (M.R., A.H., H.M., M.S.) screened the records such that each title, abstract, and full text was screened twice independently. The total included full-texts and abstracts were 14, 5 of which were suitable for quantitative synthesis via meta-analysis (five RCTs). Subsequently, a manual search was done through similar articles and references of all included studies with no additional results (Figure 1). The database search was repeated on March 25, 2025, and articles published from 2023 to 2025 were screened, with no additional studies found meeting the inclusion criteria.
2.4 Data extraction
Data extraction for each study was done by 2 independent reviewers and was revised by a 3rd reviewer for differences (M.E., Y.S., S.S., D.R.). Graphical data was extracted with the aid of PlotDigitizer (https://plotdigitizer.com/). The data extraction form included 13 parts: (1) baseline study characteristics, (2) anifrolumab dose and regimen of administration, the composite lupus disease measures (3) British Isles Lupus Assessment Group (BILAG)–based Composite Lupus Assessment (BICLA),17 (4) Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-2K),18 and (5) Systemic Lupus Erythematosus Responder Index (SRI),19 (7) Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)20 response, (8) participants attaining ≥50% reduction in active (swollen and tender) joints, (9) annualized flare rates, (10) glucocorticoid tapering, (11) Complement component 3 (C3), Complement component 4 (C4), (12) anti-dsDNA, and (13) safety parameters including herpes zoster, SAEs and death outcomes.
For renal SLE: study characteristics were collected in addition to mean 24-h Urine Protein Creatinine Ratio (UPCR), cumulative UPCR, complete renal response, and safety outcomes.
For dichotomous data (BICLA, SRI, CLASI, joint disease, glucocorticoid tapering, herpes zoster infection, adverse events, death outcomes), count of participants achieving the outcome and total number was collected. For continuous data (age, SLEDAI-2K, C3, C4, anti-dsDNA), mean/median change and standard deviation/interquartile range of each outcome were collected. For annualized flare rates, rate ratios with the corresponding 95% confidence interval (CI) were collected.
2.5 Risk of bias assessment
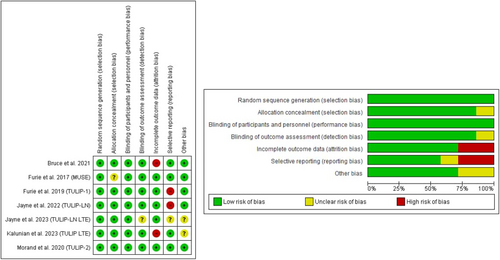
The RCTs included were assessed using the Cochrane risk of bias (ROB) 1 tool.21 This included assessment of randomization methods, allocation, blinding, attrition bias, omission of data, and selective reporting. Open-label studies were evaluated utilizing the National Institute of Health risk of bias tool.22 The risk assessment was conducted by two independent reviewers (M.R., Y.M.), and disagreements were resolved by discussion (Figure 2).
2.6 Data synthesis
Dichotomous data were analyzed using a fixed effect Mantel–Haenszel model with the effect measure being risk ratio (RR) and a corresponding 95% CI, except for death outcomes, which were presented as a total percentage due to its relative rarity. Continuous data were analyzed using a fixed effect model with mean difference (MD) as the effect measure and a 95% CI. Data presented as median and interquartile range were converted to mean using (Q1 + median + Q3)/3 and standard deviation (SD) using (Q3 − Q1)/1.35, whilst data presented as median and range were converted to mean ± SD using the formulas provided by Hozo et al.23 For annualized flare rates, rate ratios were analyzed using the inverse variance method. A p-value of <0.05 was considered statistically significant.
Heterogeneity was assessed using I2 and Cochrane Q statistics and interpretation was done by visual inspection and the leave-one-out method.24 Heterogeneity was addressed by switching to a random effects model, and then, if unresolved, the relevant study was excluded from meta-analysis. Review Manager version 5.4 was used for data synthesis. Data synthesis was conducted by five authors (Y.M., M.R., M.S., D.R., Y.S.).
All evaluated outcomes contained ≤4 studies; therefore, statistical analysis of publication bias using funnel plot asymmetry was not possible and would give invalid interpretations according to Egger et al.25
3 RESULTS
3.1 Search results
Figure 1 shows the study flow diagram. From five databases, 960 records were identified, of which 14 relevant studies were included in the review. Seven were RCTs4, 6, 11, 12, 14, 26, 27 and two were one-arm clinical trials.15, 28 Five additional reports were included, which provided supplementary data from the included clinical trials.29-33 Out of the 14 included studies, 54, 6, 12, 14, 26 were suitable for meta-analysis, with all being included in qualitative synthesis. More information about the studies not included in meta-analysis is presented in Supporting Information: Table S2.
For the open-label clinical trials, Chatham et al.15 was judged to have a low risk of bias, whilst Tanaka et al.28 was determined to have a high risk of bias due to the high dropout rate and small sample size (Supporting Information: Figure S1). Open-label studies were not included in meta-analysis due to a lack of placebo group.
3.2 Characteristics of included studies
The 14 studies comprised data from 9 anifrolumab clinical trials and 1322 SLE patients with a mean age of (40.40 ± 11.65) years. The majority were female (n = 1213, 91.8%). All included studies were funded by AstraZeneca. Participants received anifrolumab or placebo on top of the standard of care (Table 1).
| Study | Total sample size (n) | Groups | Sample size (n) | Age (years) | Female | Follow-up period (weeks) | Characteristics of participants |
|---|---|---|---|---|---|---|---|
Furie, 2017 (MUSE)4 With additional published data from: Casey, 202033 |
305 | Anifrolumab 300 mg intravenous injection | 99 | 39.1 ± 11.9 | 93 (93.9) | 52 | Adult patients (18–65 years) diagnosed with SLE according to the ACR 1997 classification. Disease activity had to be moderate-to-severe according to SLEDAI-2K scores. |
| Anifrolumab 1000 mg intravenous injection | 104 | 40.8 ± 11.6 | 99 (95.2) | ||||
| Placebo | 102 | 39.3 ± 12.9 | 93 (91.2) | ||||
| Furie, 2019 (TULIP-1)26 | 457 | Anifrolumab 150 mg intravenous injection | 93 | 40.8 ± 12.0 | 86 (92.5) | 52 | Same as above but adult patients (18–70 years). |
| Anifrolumab 300 mg intravenous injection | 180 | 42.0 ± 12.0 | 165 (91.7) | ||||
| Placebo | 184 | 41.0 ± 12.3 | 171 (92.9) | ||||
| Morand, 2020 (TULIP-2)6 | 362 | Anifrolumab 300 mg intravenous injection | 180 | 43.1 ± 12.0 | 168 (93.3) | 52 | |
| Placebo | 182 | 41.1 ± 11.5 | 170 (93.4) | ||||
Additional published pooled TULIP-1 and TULIP-2 data: |
726 | Anifrolumab 300 mg intravenous injection | 360 | — | — | — | — |
| Placebo | 366 | — | — | — | — | ||
| Tanaka, 202028 | 17 | Anifrolumab 100 mg intravenous injection | 6 | 43.0 ± 12.0 | 6 (100) | 156 | Japanese adult (18–65 years) with moderate-to-severe SLE according to ACR criteria. |
| Anifrolumab 300 mg intravenous injection | 5 | 34.0 ± 11.0 | 4 (80.0) | ||||
| Anifrolumab 1000 mg intravenous injection | 6 | 37.0 ± 10.0 | 6 (100) | ||||
| Bruce, 202111 | 36 | Anifrolumab 150 mg SC | 14 | 46.3 ± 9.1 | 12 (85.7) | 52 | 18–70 years old fulfilling SLE 1997 ACR classification criteria. Patients had to have at least CLASI ≥ 10. |
| Anifrolumab 300 mg SC | 13 | 41.5 ± 9.2 | 12 (92.3) | ||||
| Placebo | 9 | 47.8 ± 14.2 | 8 (88.9) | ||||
| Chatham, 202115 | 218 | Initially, Anifrolumab 1000 mg IV, which was then switched to Anifrolumab 300 mg | 218 | 40.8 ± 12.2 | 203 (93.1) | 156 | Adult patients (18–65) who completed the MUSE trial. |
Kalunian, 2023 (TULIP-LTE)14 With additional published data from: Vital, 202332 |
369 | Anifrolumab 300 mg intravenous injection | 257 | 43.4 ± 12.0 | 237 (92.2) | 208 | Patients completing the TULIP trials and reconsenting to participate in the LTE study. |
| Placebo | 112 | 41.4 ± 11.5 | 103 (92.0) | ||||
| Renal SLE studies | |||||||
| Jayne, 2022 (TULIP-LN)12 | 145 | Anifrolumab BR (300 mg intravenous injection) | 45 | 34.0 ± 12.0 | 37 (82.2) | 52 | Adult patients 18-70 with a diagnosis of Class III or IV lupus nephritis according to the International Society of Nephrology criteria and biopsy confirmation. |
| Anifrolumab IR (900 mg intravenous injection × 3 followed by 300 mg intravenous injection) | 51 | 35.0 ± 11.8 | 45 (88.2) | ||||
| Placebo | 49 | 32.0 ± 10.0 | 38 (77.6) | ||||
| Jayne, 2023 (TULIP-LN second year extension)27 | 75 | Anifrolumab BR | 23 | 40.0 ± 12.0 | 22 (95.7) | 104 | Same as above but having completed year 1 of treatment and achieving at least a partial renal response. |
| Anifrolumab IR | 29 | 39.0 ± 10.25 | 29 (100) | ||||
| Placebo | 23 | 37.5 ± 9.0 | 15 (65.2) | ||||
| Morand, 202131 (Pooled data from TULIP-1 and TULIP-2 renal patients) | 99 | Anifrolumab 300 mg intravenous injection | 45 | 37.8 | 85 (85.9) | 52 | Adult patients with renal SLE involvement according to: SLEDAI-2K renal scores or BILAG-2004 renal score or UPCR > 0.5 mg/mg. |
| Placebo | 54 | ||||||
- Note: Data presented as n (%) or mean ± standard deviation.
- Abbreviations: ACR, American College of Rheumatology; BILAG, British Isles Lupus Assessment Group; BR, basal regimen; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; LN, lupus nephritis; LTE, long-term extension; IR, intensified regiment; SC, subcutaneous; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index; UPCR, Urine Protein Creatinine Ratio.
3.3 Composite lupus disease scores
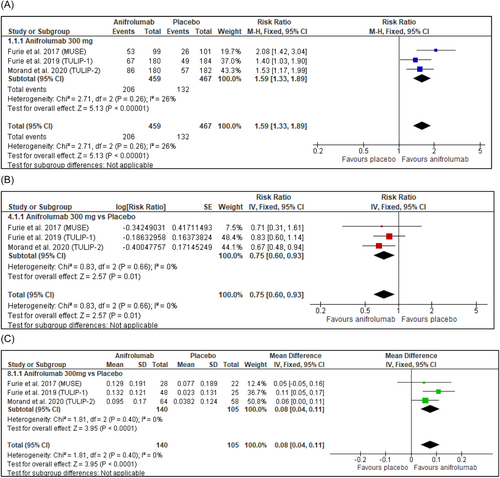
Anifrolumab 300 mg intravenous injection treatment resulted in a significantly (p < 0.00001) greater proportion of BICLA responders compared to placebo (Figure 3A). Glucocorticoid tapering was achieved significantly more in anifrolumab 300 mg-treated patients (RR = 1.60, 95% CI: 1.27, 2.01, p < 0.0001) (Table 2 and Supporting Information: Figure S2). Patients treated with anifrolumab 300 mg were significantly (p < 0.0001) more likely to be SRI(4) responders (Table 2 and Supporting Information: Figure S3). TULIP-126 was excluded only from the meta-analysis of SRI(4) due to statistically significant heterogeneity. SRI(8) response was assessed including TULIP-1,26 and anifrolumab 300 mg intravenous injection was associated with significantly (p = 0.0004) greater SRI(8) responders (Table 2 and Supporting Information: Figure S4).
| Effect measure | RR (95% CI) | p-Value | Heterogeneity (I2, %) |
|---|---|---|---|
| BICLAa | 1.59 (1.33, 1.89) | <0.0001 | 26 |
| SRI (4) (Excluding TULIP-1)b | 1.51 (1.27, 1.81) | <0.0001 | 0 |
| SRI (8)c | 1.59 (1.23, 2.05) | 0.0004 | 0 |
| CLASId | 1.82 (1.29, 2.57) | 0.0006 | 0 |
| Joint diseasee | 1.31 (1.04, 1.65) | 0.0200 | 0 |
| Flaresf | 0.75 (0.60, 0.93) | 0.0100 | 0 |
| Glucocorticoid taperingg | 1.60 (1.27, 2.01) | <0.0001 | 37 |
- Abbreviations: BICLA, British Isles Lupus Assessment Group (BILAG)–based Composite Lupus Assessment (BICLA); CI, confidence interval; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; MD, mean difference; RR, risk ratios; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index; SRI, Systemic Lupus Erythematosus Responder Index.
- a Reduction in lupus disease activity according to BILAG-2004 A and BILAG-2004 B criteria.17
- b ≥4 points reduction in SLEDAI-2K score.26
- c ≥ 8 points reduction in SLEDAI-2K score.26
- d ≥50% reduction in CLASI activity score.20
- e ≥ 50% Reduction in Swollen and Tender Joint Count in patients with ≥8 joint involvement at baseline.26
- f Annualized flare rate.
- g Glucocorticoid tapering to ≤7.5 mg/day in patients with baseline glucocorticoid dose ≥ 10 mg/day.26
Flare rates, shown in Figure 3B, were significantly (p = 0.01) reduced in anifrolumab 300 mg-treated patients compared to placebo at 52 weeks. In addition, Kalunian et al.,14 a long-term extension study, showed a lower annualized flare rate of 0.1 in the anifrolumab 300 mg group versus 0.2 in the placebo group.
3.4 Serology
In patients with consumed complement at baseline, anifrolumab-treated patients did not have a statistically significant increase in C4 (Supporting Information: Figure S5) levels, however, increases in C3 were significant (p < 0.0001) (Figure 3C). Anifrolumab 300 mg did not significantly decrease anti-dsDNA in patients with elevated anti-dsDNA at baseline (Table 3 and Supporting Information: Figure S6). Anifrolumab modulated hematological parameters in a long-term trial, with lymphocytes, hemoglobin, platelet count, and complement levels being reportedly improved relative to placebo.32 In addition, serological markers related to cardiovascular risk such as GlycA and circulating endothelial cells were shown to be improved by anifrolumab treatment according to Casey et al.33
| Effect measure | MD (95% CI) | p-Value | Heterogeneity (I2, %) |
|---|---|---|---|
| SLEDAI-2Ka | −0.86 (−1.65, −0.06) | 0.0300 | 0 |
| C3 | 0.08 (0.04, 0.11) | <0.0001 | 0 |
| C4 | 0.01 (−0.00, 0.01) | 0.1100 | 0 |
| Anti-dsDNA | −42.25 (−112.71, 28.21) | 0.2400 | 0 |
- Abbreviations: CI, confidence interval; MD, mean difference; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index.
- a Systemic Lupus Erythematosus Disease Activity Index 2000.18
3.5 Cutaneous and joint disease
Anifrolumab 300 mg-treated patients were 1.82 times more likely to achieve ≥50% reduction in CLASI activity scores compared to patients receiving placebo (Figure 4A). Particularly, anifrolumab treatment was associated with improvement in erythema and scale symptoms with no worsening in alopecia and mucosal ulceration compared to placebo, according to Werth et al.30 Moreover, in an exploratory study on the subcutaneous administration of anifrolumab, Bruce et al.11 reported that 80% (8/10) of those receiving subcutaneous anifrolumab 150 mg and 91% (10/11) of those receiving anifrolumab 300 mg achieved CLASI response in contrast to 44% (4/9) receiving placebo.
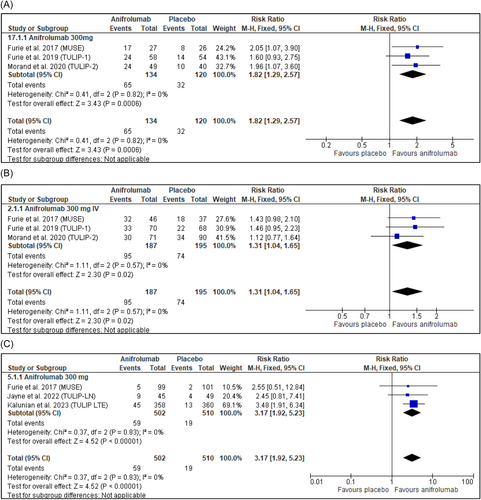
Further, anifrolumab 300 mg-treated patients were 1.31 times more likely to achieve ≥50% reduction in swollen and tender joint count compared to placebo, as seen in Table 2 and Figure 4B.
3.6 Renal disease
In lupus nephritis (LN) patients, the phase II TULIP-LN trial and its extension year12, 27 showed no statistically significant reduction in 24-h UPCR (the primary endpoint) in patients receiving any regimen of anifrolumab compared to placebo. However, Jayne et al.12 reported that patients treated with the anifrolumab intensified regimen (IR) had a higher proportion of response across secondary complete renal response definitions requiring ≤0.5 mg/mg UPCR, inactive urinary sediment, and glucocorticoid tapering. The anifrolumab (basic regimen [BR]/Anifrolumab 300 mg) was not found to be effective and was associated with lower pharmacokinetic exposure due to urinary loss. Mean non-renal SLEDAI-2K (Supporting Information: Figure S7) and cumulative UPCR were reportedly numerically reduced in patients receiving anifrolumab in TULIP-LN,12, 27 particularly in those administered the anifrolumab IR. Further, in pooled data of mild renal involvement in TULIP-1 and TULIP-2, cumulative UPCR was numerically decreased in those receiving anifrolumab.12, 31
3.7 Adverse events
The common adverse events reported in participants receiving anifrolumab across phase 2 and 3 and open-label trials were headache, nasopharyngitis, upper respiratory tract infections, infusion-related reactions, bronchitis, influenza, herpes zoster, and urinary tract infections.4, 6, 11, 12, 14, 15, 26-28 Common adverse events did not differ in the subcutaneous route from the intravenous.11 Meta-analysis showed that the incidence of SAE was approximately equal in the anifrolumab 300 mg group compared to placebo with a risk ratio of 1.01 (95% CI: 0.81, 1.27, p = 0.9000) (Supporting Information: Figure S8). Notably, patients treated with anifrolumab 300 mg were more than three times more likely than placebo-treated patients to experience herpes zoster as a side effect (RR = 3.17, 95% CI: 1.92, 5.23, p < 0.00001) (Figure 4C).
Death outcomes were higher in the anifrolumab group with seven deaths compared to one in placebo, resulting in a death rate of 0.88% (7/796) and 0.19% (1/526), respectively. Five of seven were due to pneumonia, one due to acute colitis, and one due to coronavirus disease 2019. The death rate was 1.15% (6/522) in anifrolumab 300 mg groups specifically. One death in the placebo group occurred due to an acute myocardial infarction.4, 6, 11, 12, 14, 15, 26-28 An additional death was reported in the placebo group due to encephalitis, but it was not formally counted in TULIP-1 due its occurrence outside the 28-day window period for treatment-related adverse events.26 Regarding psychiatric risk, Manzi et al.29 reported no increase in depression or suicidality in SLE patients across the TULIP trials, nor in the rate of antidepressant medication initiation.
4 DISCUSSION
Anifrolumab is the first drug to be Food and Drug Administration-approved for the treatment of SLE in over a decade.7 This study strengthens previous meta-analytic evidence of anifrolumab 300 mg's efficacy in treating SLE with analysis of several disease outcome measures.13 Analyses of anifrolumab clinical trials have shown that a discordance in response rate exists between the composite lupus disease outcomes BICLA and SRI(4).34 This meta-analysis demonstrated anifrolumab's efficacy across both measures, in addition to the more stringent SRI(8), which requires an ≥8 points reduction in SLEDAI-2K. In the meta-analysis of SRI(4), TULIP-126 was excluded due to heterogeneity. Furie et al.26 suggest that TULIP-1's SRI(4) results did not attain statistical significance due to the classification of nonsteroidal anti-inflammatory drug users as non-responders in the prespecified analysis and due to SRI(4) measuring only complete resolutions and not partial changes. On the contrary, BICLA response was homogenous across all phase 2 and 3 trials (Figure 3A). Lupus trials should continue to utilize several disease scoring systems to grant more confidence in a drug's efficacy.
Anifrolumab 300 mg appears effective in the cutaneous and musculoskeletal domains. This is consistent with a previous meta-analysis10 and is echoed in real-world case reports and observational studies showing marked improvement in skin rash and disfigurement after anifrolumab treatment, including in refractory cutaneous lupus not responding to belimumab and other therapies.35-37 Anifrolumab can be considered a first-choice add-on therapy for patients suffering from refractory cutaneous manifestations. In addition, although findings are limited by a small sample size, both the anifrolumab 150 and 300 mg subcutaneous doses may be promising for cutaneous lupus. The study investigators elaborate that subcutaneous anifrolumab has a high bioavailability, with similar exposure to the intravenous.11 Joint disease outcomes were not previously meta-analyzed10, 38 despite discordant results: the most recent phase 3 trial, TULIP-2, showed anifrolumab treatment to not be associated with significant joint improvement6 in contrast with its predecessors MUSE and TULIP-1.4, 26 This meta-analysis, which included all 3 trials, showed greater joint improvement in patients treated with anifrolumab 300 mg, albeit with a smaller effect size than for cutaneous lupus (Figure 4B).
Anifrolumab improved C3 levels significantly (p < 0.0001), but not C4 or anti-dsDNA. It is unclear why clinical improvement by type I interferon inhibition would be associated with increased C3 but not improved C4 or anti-dsDNA; this warrants further exploration. Hypocomplementemia is associated with lupus disease activity including renal lupus and fever and can be of prognostic value, but serological markers do not correlate strictly with clinical severity or improvement.39, 40 Serum markers related to cardiovascular risk were reportedly improved by anifrolumab,33 but a long-term trial has shown similar rates of cardiovascular events in anifrolumab-treated patients compared to placebo.14
For renal SLE involvement, anifrolumab's benefit is yet uncertain. Of note, data from TULIP-LN indicates lower exposure to anifrolumab 300 mg in renal patients due to proteinuria-related urinary clearance of the drug.12 However, the higher dose regimen, anifrolumab IR, is under further investigation in the phase III IRIS trial.27 The planned primary endpoint of IRIS is binary complete renal response instead of 24-h UPCR, which was not met in its predecessor.12 This is consistent with the AURORA trial, which led to approval of voclosporin, a calcineurin inhibitor, for lupus nephritis.41
Anifrolumab treatment had a similar SAEs rate to placebo in this meta-analysis, which included the longest placebo-controlled SLE study to date.14 This contrasts with a previous meta-analysis, which reported that rates of SAEs are lower compared to placebo.13 Herpes zoster rates were markedly increased by anifrolumab, therefore, herpes zoster vaccines may be of benefit in those initiating anifrolumab therapy. The American College of Rheumatology recommends the administration of the recombinant herpes zoster vaccine in rheumatic patients receiving immunosuppressants.42 Depression and suicidal ideation does not appear to be a side effect of anifrolumab,29 unlike other monoclonal antibody immunosuppressants including belimumab, the only other monoclonal antibody previously receiving Food and Drug Administration approval for SLE.43 Thus, for depressed SLE patients, anifrolumab is probably preferrable to belimumab.
No formal statistical analysis of death rates was done as many trials had zero events, however, there is a higher death rate in anifrolumab-treated patients compared to placebo (0.88% vs. 0.19%, respectively). Most deaths were due to pneumonia, a disease in which type I interferon signaling reportedly plays a protective role.44, 45 A risk-benefit assessment comparing anifrolumab and belimumab suggested that anifrolumab's broad blockage of type I interferons impairs the immune response to infections, particularly viral, more than belimumab.46 Kirou et al.46 suggest that anifrolumab should be administered cautiously to patients with a high risk of infection, such as those with a history of recurrent and chronic infections.
This study's strengths include consideration of anifrolumab's efficacy across several organ domains and lupus disease scoring systems. Weaknesses include limited conclusions on renal patients and the subcutaneous administration due to the data being exploratory, which was not sufficient for meta-analysis. Particularly, subcutaneous administration may improve the compliance and convenience of the treatment, pending support from larger trials. As both placebo and anifrolumab were taken on top of the standard of care, background medication could affect the outcomes. Whilst meta-analysis shows that anifrolumab treatment is associated with glucocorticoid tapering, effects on other background therapy are unclear. Exploration of anifrolumab versus standard of care, as opposed to on top of standard of care could be a focus of future clinical trials. All RCTs excluded neuropsychiatric SLE, so the potential for use in this domain could not be considered in this review. Finally, the evaluation of anifrolumab's efficacy and safety in pediatric, geriatric, and pregnant populations is needed.
5 CONCLUSION
This study found anifrolumab 300 mg to be effective in treating SLE across various scoring systems like BICLA, SRI(4), musculocutaneous disease, disease flares, and C3. Anifrolumab's effectiveness in the context of renal manifestations remains uncertain. Larger phase 3 trials are needed for the renal subgroup and subcutaneous administration as well as drug surveillance for long term survival outcomes.
AUTHOR CONTRIBUTIONS
Yomna W. Mahmoud: Conceptualization; writing—original draft; writing—review and editing; formal analysis. Mohamed Ramadan S.: Writing—original draft; data curation; formal analysis; methodology. Doaa Radwan G.: Writing—original draft; data curation; formal analysis; methodology. Mariam S. Abdulhafiz: Writing—original draft; data curation; formal analysis; methodology. Yassin M. El-Shnawy: Writing—original draft; data curation; formal analysis. Alaa Alaa H.: Methodology; writing—original draft. Sarah Nasir Sebie: Data curation; writing—original draft. Maryam A. Elashmawi: Data curation; methodology. Husni Zahi M.: Data curation; methodology. Mohammad T. Abuawwad: Writing—review and editing; data curation; planning and supervision. Mohammad J. Taha: Writing—review and editing; data curation; planning and supervision. Abdulqadir J. Nashwan: Writing—review and editing; data curation; planning and supervision. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge and thank Prof. Dr. Hanan Kotb of rheumatology and rehabilitation for her incredibly helpful comments, advice, and encouragement and Prof. Dr. Soha ElMorsy of clinical pharmacology for her support and encouragement.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This systematic review was registered with PROSPERO (ID: CRD42023461606). Ethical approval was not required as no primary data were collected.
Open Research
DATA AVAILABILITY STATEMENT
All underlying data is available publicly and as part of this article, and no additional data is required.