Novel LPIN2 mutations in Majeed syndrome induce NF-κB pathway activation
Edited by Zhiyu Wang and Lishao Guo.
Graphical Abstract
Mutations in LPIN2, located on the short arm of chromosome 18, can lead to Majeed syndrome, a rare autosomal recessive disorder characterized by fever, chronic relapsing multifocal osteomyelitis, congenital erythropoietic dysplasia, and markedly elevated markers of blood inflammation.1-3 The high variability of the Majeed syndrome phenotype and the unclear correlation between genotype and phenotype present the challenge for the early diagnosis and treatment of the disease. Here, we reported a Chinese patient with Majeed syndrome due to a novel compound heterozygous variant in LPIN2, c.2534delG (p.G845Efs*9)/c.1966A>G (p.N656D), and systematically summarized and analyzed the patient's clinical phenotype and the genetic characteristics of the mutation locus. Subsequently, we suggested that the mutations might be involved in the interleukin (IL)-1β-mediated inflammatory response in Majeed syndrome by activating the nuclear factor-kappaB (NF-κB) signaling pathway, which would provide a molecular basis for the treatment of the disease.
The proband, a 7-year-old boy from Shandong Province, was admitted at 18 months of age because of the inability to walk on his own and to cry when walking with assistance. He was readmitted at the ages of 3, 4, and 6 years, with major clinical symptoms of intermittent osteoarticular pain in both legs, hypotonia, mild anemia, and growth retardation. The patient complained of pain in the left leg and dorsum of the left foot, frequently after prolonged periods of stillness. After administration of amoxicillin and dexamethasone, vitamin D and calcium, the patient's pain was temporarily relieved, and the temperature decreased to normal. Ultrasonography revealed a visible effusion in the right knee. The patient had been injected with Etanercept (recombinant human type 2 tumor necrosis factor receptor) 3 years ago and had pain relief for 1 month, but on the 7th injection, the pain could not be eliminated. Laboratory tests showed hemoglobin level of 108 g/L, white blood cell count of 10.5 × 109/L, C-reactive protein level of 44.6 mg/L, and serum IL-6 level of 63.79 pg/mL (normal range: ≤5.40 pg/mL). The results of the Child Neurointellectual Development Scale showed that the patient's intelligence was below normal. The patient's parents were non-consanguineous and denied family heredity.
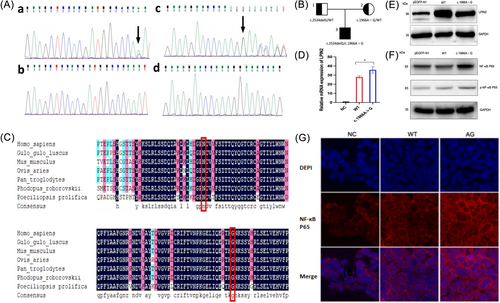
Whole Exome Sequencing (WES) revealed the presence of a compound heterozygous variants of LPIN2 (NM_014646.2) in this patient, c.2534delG (p.G845Efs*9) and c.1966A>G(p.N656D), a deletion mutation located in exon 19 and a missense mutation located in exon 15. Sanger sequencing showed that the father of the proband has a c.2534delG (p.G845Efs*9) heterozygous mutation and the mother was a heterozygous carrier of c.1966A>G (p.N656D), and the two mutations in the proband were from his mother and father, respectively, in accordance with an autosomal recessive pattern of inheritance (Figure 1A,B). The above mutations were not detected in 200 healthy control individuals, and the normal population genetic databases (gnomeAD, 1000Genome, ESP6500, and ExAC) showed that both variants had a frequency of 0 in the population, suggesting that the variants were co-segregating in the family. To initially determine the effect of c.2534delG (p.G845Efs*9)/c.1966A>G (p.N656D) on the patient, we firstly analyzed the pathogenicity of the two mutations. Mutation Taster (http://www.mutationtaster.org/) predicted these two mutations to be pathogenic with a score of 1. Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph2/) analysis predicted that the amino acid substitution due to c.1966A>G (p.N656D) may be pathogenic with a score of 1. Subsequently, we performed sequence comparison of LPIN2 protein from several species including Homo sapiens, Mus musculus, Ovis aries, and Pan troglodytes obtained from the National Center for Biotechnology Information website, and the results showed that G845 and N656 are both located in the conserved structural domain of LIPIN2 protein (Figure 1C).
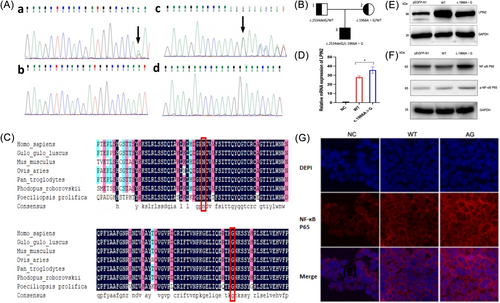
To investigate the effect of mutation c.1966A>G on the expression level of LPIN2, we transfected HEK293T cells with wild-type LPIN2 plasmid (WT) and mutation plasmid (c.1966A>G), respectively. Real-time quantitative polymerase chain reaction results showed that the mRNA expression of LPIN2 in the WT group was significantly lower than that of the c.1966A>G group (p < 0.05), but the Western blot results showed that the protein expression of LPIN2 in the WT group was higher than that of the c.1966A>G group (Figure 1D,E). This suggested that mutation c.1966A>G caused an abnormal increase in mRNA expression of LPIN2 during transcription, but the spatial structure of LPIN2 may have been altered during translation, resulting in a decrease in protein expression.
Studies have shown that IL-1β plays an important role in the pathogenesis of Majeed syndrome and that the associated inflammatory response is closely linked to the NF-κB signaling pathway.4, 5 Therefore, we investigated the effect of mutation c.1966A>G on the NF-κB signaling pathway under the stimulation of IL-1β with the aim of exploring the potential molecular mechanisms of Majeed syndrome. We found that the expression of NF-κB P65 and phosphorylated-NF-κB P65 (p-NF-κB P65) were significantly increased in the c.1966A>G group after HEK293T cells were treated with IL-1β (Figure 1F). In addition, immunofluorescence results showed that a nuclear translocation of NF-κB P65 occurred in the c.1966A>G group. This suggested that mutation c.1966A>G participates in IL-1β-mediated inflammatory response through activating the NF-κB pathway (Figure 1G). However, the validation of a single cell line and the lack of in vivo experiments make this hypothesis limited.
In summary, this study characterized the clinical manifestations of a Chinese patient with Majeed syndrome and identified novel compound heterozygous variants in LPIN2, which can help to further assess the genotype-phenotype correlations of the disease. In addition, cytological experiments explored the effect of the c.1966A>G mutation on the expression level of LPIN2 and its molecular mechanism involved in the inflammatory response, providing new ideas for finding molecular therapeutic targets for the disease.
AUTHOR CONTRIBUTIONS
Yibing Han and Yuanxuan Ma drafted the work. Renmei Cai revised it critically for important intellectual content. Shiguo Liu and Bin Liu did substantial contributions to the conception or design of the work.
ACKNOWLEDGMENTS
I would like to express my heartfelt gratitude to all the teachers and students who have helped me. This study was financially supported by the National Natural Science Foundation of China, Grant/Award Number: 30971586.
CONFLICT OF INTEREST STATEMENT
The authors of the above-mentioned paper have no affiliations or associations with any organization/entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
ETHICS STATEMENT
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 27300).
Open Research
DATA AVAILABILITY STATEMENT
Readers can obtain all research data from this article upon reasonable request.