Mitochondrial dynamics in autoimmune diseases
Ruicong Ma, Cheng Zhang, and Jiaqing Liu contributed equally to this study.
Edited by Zhiyu Wang and Lishao Guo.
Abstract
Mitochondria, as the “energy factories” of cells, are vital for maintaining cell life and function. Mitochondria are highly mobile organelles within cells, constantly changing their morphology through fusion and fission processes to achieve dynamic transitions between interconnected network structures and fragmented states. This phenomenon is known as mitochondrial dynamics. Disorders in mitochondrial dynamics contribute to the onset of autoimmune conditions such as rheumatoid arthritis, multiple sclerosis, myasthenia gravis, and systemic lupus erythematosus, among others. This article aims to review the roles of mitochondrial dynamics disorders in autoimmune diseases and small molecule drugs targeting mitochondrial dynamic proteins, with the ultimate goal of aiding the development of new clinical treatments.
Key points
-
Introduced small molecule drugs targeting mitochondrial dynamic proteins.
-
Discussed the roles of mitochondrial dynamics disorders in autoimmune diseases.
-
Explored the application prospects of small molecule drugs targeting mitochondrial dynamic proteins in autoimmune diseases.
1 INTRODUCTION
Mitochondria have been recognized as the cell “energy factory,” with 95% of the energy required for cellular life activities coming from them.1 Mitochondria constantly engage in processes like fusion, fission, and autophagy, which maintain their structure, number, distribution, and prevent cellular damage—a process known as mitochondrial dynamics.2 It is now widely recognized that mitochondria regulate various cellular processes, including autophagy, apoptosis, differentiation, stem cell maintenance, cell migration, innate immune response, inflammation, and cellular metabolism by altering their morphology through mitochondrial dynamics.3 The imbalance between mitochondrial fusion and fission may cause mitochondrial dysfunction, exacerbate cellular disorders, and ultimately lead to the rapid progression of diseases.4 Previous studies have found that the imbalance of mitochondrial division and fusion can induce immune cell dysfunction, but there is still a lack of direct evidence to indicate whether mitochondrial dynamics abnormalities are the cause or secondary phenomenon of autoimmune diseases. In addition, the roles of mitochondrial dynamics in different immune cells or tissues may be opposite, requiring more detailed cell-type-specific studies. Moreover, small molecule drugs targeting mitochondrial dynamic proteins have not yet achieved clinical translation.
In this review, we discussed the molecular biology basis of mitochondrial dynamics, small molecule drugs targeting mitochondrial motor proteins, and the roles of mitochondrial dynamics in autoimmune diseases.
2 MITOCHONDRIAL FISSION AND FUSION
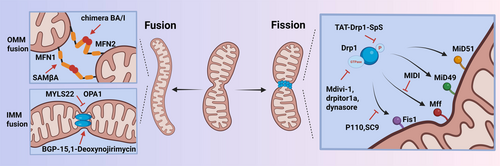
Mitochondrial fission and fusion proteins have been widely studied. Mitochondrial fission proteins are composed of dynamin-related protein 1 (Drp1), mitochondrial fission protein 1 (Fis1), mitochondrial fission factor (Mff), mitochondrial dynamics proteins of 49,000 and 51,000 (MiD49, MiD51). The proteins responsible for mitochondrial fusion include mitofusin 1 (MFN1), mitofusin 2 (MFN2), and optic atrophy factor 1 (OPA1).2 The dynamic balance of mitochondrial fission and fusion is crucial for maintaining mitochondrial homeostasis and organelle operation. Additionally, there has been a gradual development of small molecule drugs targeting mitochondrial dynamics as illustrated in Table 1 and Figure 1.
| Drugs | Mechanism | Application in autoimmune diseases |
|---|---|---|
| Mitochondrial fission | ||
| Mdivi-1 | Inhibit the GTPase activity of Drp15 | RA,6 EAE,7 T1D,8 AIH9 |
| Drpitor1a | Inhibit the GTPase activity of Drp110 | - |
| Dynasore | Inhibit the GTPase activity of Drp111 | - |
| TAT-Drp1-SpS | Block GSK3β-induced phosphorylation of Drp112 | - |
| P110 | Block the interaction between Drp1 and Fis113 | MS14 |
| SC9 | Block the interaction between Drp1 and Fis115 | - |
| MIDI | Block the interaction between Drp1 and Mff16 | - |
| Mitochondrial fusion | ||
| MYLS22 | Inhibit the activation of OPA117 | - |
| BGP-15 | Activate OPA118 | - |
| 1-Deoxynojirimycin | Targeting OPA1 to promote its oligomerization19 | - |
| SAMβA | Inhibit the interaction between MFN1 and βIIPKC.20 | - |
| Chimera BA/I | Activate MFN221 | - |
- Abbreviations: AIH, autoimmune hepatitis; βIIPKC, β II protein kinase C; Drp1, dynamin-related protein 1; EAE, experimental autoimmune encephalomyelitis; Fis 1, fission protein 1; GSK, glycogen synthase kinase; GTPase, guanosine triphosphatase; Mdivi-1, Mitochondrial division inhibitor 1; Mff, mitochondrial fission factor; MFN, mitochondrial fusion include mitofusin; MS, multiple sclerosis; OPA1, optic atrophy factor 1; RA, rheumatoid arthritis; T1D, type 1 diabetes.
2.1 Mitochondrial fission-related proteins
Drp1
Drp1 belongs to the family of guanosine triphosphatases (GTPases) related to dynamin-like proteins. Drp1 consists of four different domains, including the N-terminal GTPase domain, the middle domain, the variable domain, and the C-terminal effector domain.22 Drp1 is distributed in the cytoplasm and plays a crucial role in mitochondrial fission, but it cannot perform fission independently. Drp1 requires receptors to recruit it from the cytoplasm to the outer mitochondrial membrane (OMM). These outer membrane receptors include MiD49, MiD51, Mff, and Fis1. At the onset of mitochondrial fission, Drp1 is recruited by the receptor to OMM, forming a circular structure that mediates mitochondrial contraction, and this process can be enhanced by GTPases. Subsequently, Dynamin 2 (Dnm2) was recruited to the Drp1-mediated mitochondrial contraction site and terminated membrane separation, forming two daughter mitochondria.23 Additionally, phosphorylation is the main mode of Drp1 activation, and serine (ser) 616 and ser637 are its two key phosphorylation sites. Ser616 phosphorylation and ser637 dephosphorylation can enhance Drp1 activity and promote mitochondrial division, while ser616 dephosphorylation and ser637 phosphorylation weaken Drp1 activity and inhibit mitochondrial division.24
Recently, small molecule drugs targeting Drp1 have also been developed, including mitochondrial division inhibitor 1 (Mdivi-1), Drpitor1a, Dynasore, and TAT-Drp1-SpS. Mdivi-1 is a quinolone compound that can cross the cell membrane and effectively block the GTPase activity of Dnm1.25 The study also confirmed that Mdivi-1 has a protective effect on various diseases, including cancer, cardiovascular disease, autoimmune diseases, etc.5 Drpitor1a is a novel specific Drp1 GTPase inhibitor that significantly inhibits mitochondrial fission in pulmonary artery smooth muscle cells.10 In addition, Dynasore can competitively inhibit the GTPase activity of Drp1, and Dynasore is applied in diseases such as Parkinson's disease.11 TAT-Drp1-SpS is a novel artificial peptide that can specifically block glycogen synthase kinase (GSK)3β-induced phosphorylation of Drp1.12
Fis1
Fis1 is a transmembrane protein that participates in mitochondrial fission by regulating the correct assembly of Drp1 complexes and activating the fission activity of Drp1 complexes in the later stages of the process. Overexpression of human Fis1 contributes to extensive mitochondrial fission, while the knockdown of Fis1 leads to mitochondrial elongation.26 P110 is a peptide inhibitor that blocks the interaction between Drp1 and Fis1, reducing the pathological function of many diseases without blocking the physiological function of Drp1.13 Similarly, SC9 can selectively inhibit the pathological Drp1-Fis1 interaction and has the same mechanism of action as the peptide inhibitor P110.15
Mff
The consistent recruitment of Drp1 depends on Mff, operating independently from Fis1. Mff has a putative Drp1 binding domain at its N-terminus. Overexpression of Mff causes mitochondrial fragmentation, while the deletion of Mff gene induces mitochondrial elongation and significantly reduces the recruitment of Drp1 in mitochondria.27 MIDI is a newly developed mitochondrial fission inhibitor that disrupts the interaction between Drp1 and Mff.16
MiD49 and MiD51
The MiD49 protein is a product of the Smith–Magenis syndrome chromosome region candidate gene 7 (SMCR7) gene, also known as mitochondrial extension factor 2. Similarly, MiD51 protein is the product of the SMCR7L gene, also known as mitochondrial elongation factor 1.28 Drp1 exists in the cytoplasm in the form of dimers or tetramers. Unlike Mff and Fis1, adapter MiD49 and MiD51 specifically recruit Drp1 to the mitochondrion. After fission, guanosine diphosphate production leads to oligomeric Drp1 disassembly, disrupting the interaction between MiD49, MiD51, and Drp1. Ultimately, Drp1 is released from the membrane and relocated to the cytosol to act freely in another mitochondrial fission cycle.29 In recent years, research on MiD51 has gradually emerged and confirmed its important role in hyperproliferative diseases. The previous research of the research group also found that the increased expression of MiD49 and MiD51 in endothelial cells was closely linked to the progression of atherosclerosis. The microRNA-107-hypoxia inducible factor-1α-MiD51 pathway may provide a new therapeutic target for atherosclerosis.30
2.2 Mitochondrial fusion-related proteins
OPA1
The fusion of the mitochondrial inner membrane is mainly regulated by OPA1. OPA1 proteins are located in the intermembrane space and interact with both the inner membrane and outer membrane. During fusion, L-OPA1 forms homotypic interactions on the inner membrane, which facilitates contact between adjacent mitochondria. L-OPA1 can form higher-order oligomeric structures that may help bring the inner membranes of neighboring mitochondria closer together, facilitating their fusion.31 OPA1 interacts with the mitochondrial outer membrane fusion proteins MFN1/2, coordinating the fusion of the inner and outer membranes. MFN1/2 first promotes outer membrane fusion, after which OPA1 mediates inner membrane fusion. Mitochondrial dynamics are also involved in T cell differentiation. The increased mitochondrial fusion mediated by OPA1 can transform effector T cells into memory T cell phenotypes.17 MYLS22, as an inhibitor of OPA1, can inhibit the proliferation and migration of tumor cells.32 Furthermore, small molecule BGP-15 (a hydroxylamine derivative) regulates the GTPase activity of OPA1 and alleviates lung injury.18 1-Deoxynojirimycin (DNJ) can improve mitochondrial function by targeting OPA1 to alleviate mouse hypertrophic cardiomyopathy.19
MFN1 and MFN2
The MFN gene stands for mitochondrial fusion protein, which is primarily located in the OMM and exists in two main isoforms: MFN1 and MFN2. MFNs have a conserved molecular structure consisting of one N-terminal GTPase domain, two hydrophobic repetitive helical regions (HR), and two transmembrane domains.33 The trans interaction between HR2 and GTPase regions of MFNs initiates mitochondrial outer membrane fusion and increases surface contact between adjacent mitochondria. GTP hydrolysis leads to conformational changes in MFNs, promotes mitochondrial docking, and increases membrane contact sites, thereby mediating OMM fusion.20 The novel small peptide SAMβA inhibits the interaction between MFN1 and β II protein kinase C (βIIPKC), thereby alleviating brain edema in rats with subarachnoid hemorrhage.21 In addition, chimera BA/I (MFN2 agonist) can activate MFN2, thereby restoring mitochondrial morphology and improving neurological deficits.34
3 ROLES OF MITOCHONDRIAL DYNAMICS IN AUTOIMMUNE DISEASES
Mitochondrial dynamics is the foundation of cellular functional operation, and the maintenance of immune cell function also relies on the involvement of mitochondria. The inflammatory response in autoimmune diseases is often out of control, leading to immune system attacks and damage to healthy cells and tissues. Dysfunction of mitochondria within immune cells may result in elevated production of reactive oxygen species, impairment of mitochondrial autophagy, and alterations in energy metabolism, ultimately causing immune system dysfunction and inflammation. In this section, we focused on the association between mitochondrial dynamics and common autoimmune diseases as shown in Table 2.
| Autoimmune diseases | Gene | Cell/Tissue | Typical features |
|---|---|---|---|
| Rheumatoid arthritis | Drp1, Fis1 | RA-FLSs |
|
| CD4+ T cells | |||
| Systemic lupus erythematosus | Drp1, Mff | T cell, Podocytes, PBMCs |
|
| Sjögren's Syndrome | Fis1, Mff, Drp1 | PBMCs, Salivary glands | The expression levels of Mff and Drp1 genes in patients with Sjogren's syndrome were increased in PBMCs.39, 40 |
| The expression levels Drp1 gene was upregulated in salivary glands in patients with Sjogren's syndrome, while the expression levels of MFN1, MFN2 and OPA1 genes were downregulated.41 | |||
| MFN1, MFN2, OPA1 | |||
| Multiple sclerosis | OPA1 | Oligodendrocytes, Microglia, neuronal cells |
|
| Myasthenia gravis | Mfn1/2, OPA1, Drp1, Fis1, Mff | PBMCs, Gastrocnemius | Compared with healthy control group patients, the expression of Drp1 and Fis1 in PBMCs of MG group patients was increased, while the expression of OPA1 and MFN1/2 was lower.45 |
| In the gastrocnemius of MG animal models, the decreased expression of MFN1/2, OPA1, Drp1, Fis1 and Mff resulted in abnormal energy metabolism.46 | |||
| Type I diabetes | OPA1, Drp1 | MCECs, heart tissue, retinal pericytes, pancreatic β cells | The expression of OPA1 in endothelial cells is decreased, while the expression of Drp1 is increased.47 |
| The expression of Drp1 in cardiac tissue is increased.48 | |||
| The expression level of Drp1 was upregulated in retinal pericytes.8 | |||
| Knocking down Drp1 can inhibit mitochondrial excessive division and restore pancreatic β cell function.49 | |||
| Psoriasis | Drp1 | Skin tissue, HaCaT cells, macrophage | The expression of Drp1 is upregulated in skin tissue and HaCaT cells.50 |
| The inhibition of Drp1 can reduce macrophage infiltration and inflammatory cytokine release.51 | |||
| Autoimmune hepatitis | Drp1 | Liver tissue, liver cells | The expression of Drp1 is upregulated in liver tissue and liver cells.9 |
- Abbreviations: Drp1, dynamin-related protein 1; FIS1, fission protein 1; HaCaT cells, human immortalized keratinocytes cells; MFF, mitochondrial fission factor; MFN, mitochondrial fusion include mitofusin; MG, Myasthenia gravis; MiD49, mitochondrial dynamics proteins of 49,000; MiD51, mitochondrial dynamics proteins of 51,000; MS, Multiple sclerosis; OLs, oligodendrocytes; OPA1, optic atrophy factor 1; PBMCs, peripheral blood mononuclear cells; RA-FLS, rheumatoid arthritis fibroblast-like synoviocytes; SLE, Systemic lupus erythematosus.
3.1 Rheumatoid arthritis (RA)
RA is an autoimmune disease based on synovial hyperplasia and inflammation, which may ultimately lead to joint deformities.52 Fibroblast-like synoviocytes (FLS) in the inner layer of synovial membrane plays an important role in maintaining joint function. RA-FLS exhibits high invasiveness and excessive proliferation, indicating mitochondrial dysfunction in RA-FLS.53 It has been found that the expression of Drp1 in the synovium of RA patients is increased compared to non-RA patients. Knocking down Drp1 in FLS can reduce FLS proliferation and invasion function. Furthermore, Mdivi-1 can alleviate joint inflammation in collagen-induced arthritis (CIA) mice by reducing levels of inflammatory cytokines and ROS, which is related to the inhibition of classical inflammatory signaling pathways.6 Therefore, excessive mitochondrial fission exists in FLS and can exacerbate inflammatory reactions. Irisin is a hormone-like factor with anti-inflammatory and antiproliferative properties. Another study also found that it can inhibit the proliferation, inflammation, and invasion ability of RA-FLS by suppressing the yes-associated protein (YAP)-Drp1 signaling pathway.54 In addition, adenosine monophosphate-activated protein kinase signaling plays an indispensable role in promoting the dynamic balance of mitochondrial fission and fusion. The study identified AMPK as a therapeutic target for Osthole through network pharmacology and molecular docking. Mechanistically, it has been found that Osthole improves mitochondrial dynamics imbalance and reduces levels of inflammatory factors by activating AMPK. Osthole inhibited synovial inflammation both in vivo and in vitro.55 Therefore, inhibiting RA-FLS mitochondrial fission may be an effective measure for treating RA.
Additionally, it is worth noting that activated CD4+T cells also play an important role in the pathogenesis of RA. Research has found that intraperitoneal injection of Flavonoid Naringenin at a dose of 50 mg/kg in CIA rats can alleviate joint pain and inflammation levels, inhibit polarization of CD4+T cells in the spleen, and suppress mitochondrial fission proteins Drp1 and Fis1. In vitro experiments have also confirmed that Flavonoid Naringenin reverses C-X-C motif chemokine ligand 12 (CXCL12) induced polarization and migration of synovial CD4+T lymphocytes by reducing mitochondrial fission.35 In addition, our research team has found that MiD49 and MiD51, as novel mitochondrial fission proteins, are highly expressed in RA-FLS and promote the excessive proliferation and invasive function of RA-FLS. We will continue to explore the roles of novel mitochondrial dynamic proteins in FLS and immune cells in RA.
3.2 Systemic lupus erythematosus (SLE)
SLE is a chronic systemic autoimmune disease characterized by multiple system damage and the presence of a large number of auto-antibodies in the body.56 Early studies found that the expression of Drp1 in peripheral blood lymphocytes of SLE patients was significantly reduced compared to healthy controls. Similar phenomena were also observed in the SLE mouse model. Previous studies have found that human T-cell leukemia virus-related endogenous sequence-1 (HRES)-1/Rab4 is highly expressed in lupus T cells, accompanied by abnormal mitochondrial dynamics. Rab geranylgeranyl transferase inhibitor 3-PEHPC can increase the expression of Drp1 and reduce mitochondrial mass in T cells, and thus delays disease development in lupus-prone mice.36
Podocytes are important structures for maintaining glomerular filtration barrier function, and mitochondrial dynamics abnormalities in podocytes can lead to the occurrence of lupus nephritis. Research has found that Drp1S616 phosphorylation is enhanced in podocytes, inducing mitochondrial division and exacerbating cell damage. Inhibition of Drp1 in vivo and in vitro can significantly improve podocyte damage and mitochondrial fission, thereby alleviating lupus nephritis. In addition, researchers found that the expression of C5a receptor 1 (C5aR1) was significantly upregulated in podocytes of lupus nephritis. C5aR1 inhibitors can significantly inhibit Drp1 phosphorylation and improve podocyte function. This study confirmed that the C5a-C5aR1-Drp1 axis was a new mechanism leading to podocyte damage in lupus nephritis.37 Additionally, the expression of Mff gene in peripheral blood mononuclear cells (PBMCs) of SLE patients was reduced in contrast to healthy controls.38 The changes in mitochondrial motor proteins in different cells of SLE may vary, and targeted therapy for mitochondrial motor proteins in different cells is also crucial.
3.3 Sjögren's syndrome (SS)
SS is a common chronic inflammatory autoimmune disease that often affects the salivary and lacrimal glands. The levels of Drp1 and phosphorylated Drp1 increased in PBMCs of SS patients. And the expression of Drp1 in the submandibular gland tissue of SS mice also increased, with aggravated mitochondrial fission. Huoxue Jiedu Recipe can improve submandibular gland inflammation infiltration in SS mice by inhibiting mitochondrial fission.39 In addition, the expression level of Mff gene in patients with SS is increased in PBMCs in contrast to healthy controls.40 The expression levels of MFN1, MFN2, and OPA1 genes are downregulated in salivary glands in SS patients.41 Further exploration is needed on the mechanism of mitochondrial dynamics in salivary gland epithelial cells and immune cells among SS patients.
3.4 Multiple sclerosis (MS)
MS is an autoimmune disease characterized by central nervous system white matter inflammatory demyelinating lesions. The core function of oligodendrocytes (OLs) is to produce myelin, form myelin sheaths to wrap axons, improve nerve conduction rate, and play an important role in central nervous system injury repair. Overactivation of Drp1 in OLs was observed in both in vitro inflammatory stimulation and MS mouse models. P110 can significantly inhibit Drp1 mediated mitochondrial division, reduce OLs death, and decrease the number of activated microglia and astrocytes, thereby improving disease progression in MS mice.14
Microglia are key immune effectors in inflammatory lesions. Chronic inflammation of microglia can damage myelin and axons. Mdivi-1 inhibits the phosphorylation of Drp1 in microglia and promotes the transformation of microglia into an anti-inflammatory M2 phenotype. The mechanism may be related to the inhibition of the classical nuclear factor kappa-B (NF-κB) signaling pathway.42 During the course of MS, persistent inflammation erodes neurons, exacerbating neurodegeneration and dysfunction. Peroxynitrite (ONOO-) induces mitochondrial division mediated by Drp1, leading to axonal degeneration and neuronal cell death.43 On this basis, another study found that Acteoside reduced neuronal cell death and inhibited inflammatory responses. The mechanism is related to the inhibition of excessive mitochondrial autophagy and Drp1-mediated excessive division.44 The Drp1/PTEN-induced putative kinase 1 (PINK1)/Parkin signaling pathway may be an important therapeutic target for MS.57 In addition, Mdivi-1 can alleviate the progression of experimental autoimmune encephalomyelitis by regulating the balance of Th1, Th17, and Treg cells.7
3.5 Myasthenia gravis (MG)
MG is an autoimmune disease characterized by a neural muscular junction transmission disorder mediated by autoantibodies. A study found that compared with healthy control group patients, the expression of Drp1 and Fis1 in PBMCs of MG group patients was increased, while the expression of OPA1 and MFN1/2 was lower. Mitochondrial fission and fusion proteins were proposed as potential biomarkers for diagnosing MG.45 In addition, researchers constructed a rat model of MG and Qiangji Jianli decoction was used to evaluate the therapeutic effect. The results showed that MFN1/2, OPA1, Drp1, and Fis1 mRNA and proteins expression were significantly decreased in MG rats in contrast to normal rats.
These changes lead to abnormal mitochondrial function. However, the imbalance of mitochondrial dynamics in the rat gastrocnemius muscle tissue was significantly improved, and muscle tissue function was also restored after drug treatment.46
3.6 Type 1 diabetes (T1D)
T1D is an autoimmune disease caused by the immune system attacking pancreatic beta cells, and T1D is often accompanied by a variety of complications. Cardiovascular complications are the main cause of death in T1D. Hyperglycemia often leads to endothelial dysfunction, which is the initial step of vascular injury. Compared with normal mice, coronary endothelial cells in T1D mice showed mitochondrial fragmentation. The expression of OPA1 in endothelial cells decreased, while the expression of Drp1 increased. Superoxide anion scavengers can reduce mitochondrial fission in endothelial cells and restore mitochondrial morphology and function.47 Researchers injected apigenin into T1D rats and found a decrease in blood lipids, blood glucose, and myocardial injury markers, as well as an improvement in cardiac structure. Moreover, the expression of Drp1 in cardiac tissue decreased after treatment, and the levels of Drp1 were significantly correlated with various parameters, suggesting that apigenin may alleviate cardiovascular complications in T1D rats by improving mitochondrial dynamics.48 Ischemic retinopathy is also a serious complication of T1D. The phosphorylation levels at positions S616 and S637 of Drp1 in the retina of T1D mice were significantly increased, resulting in mitochondrial swelling, fragmentation, and functional impairment.8 The degree of retinal pericyte loss and capillary degeneration is reduced in Epac1 gene-deficient mice. Knockout of Epac1 gene can inhibit high glucose-induced phosphorylation of Drp1 and production of ROS in retinal pericytes.58
The death of pancreatic islet β cells leads to the loss of functional pancreatic β cells and induces T1D. Hypoxia induces excessive mitochondrial division in pancreatic β cells, leading to cell death, while knocking down Drp1 and administering Mdivi-1 can inhibit mitochondrial excessive division and restore cell function.49 Additionally, Acetate and Butyrate stress can improve pancreatic cell dysfunction induced by streptozotocin. Acetate and Butyrate stress can improve pancreatic cell dysfunction induced by streptozotocin, reduce the expression of Drp1 and Fis1, and inhibit mitochondrial division.59
In addition, several immunosuppressive drugs have shown partial efficacy by preserving β-cell function, but cannot achieve persistent immune tolerance or maintain insulin sensitivity. The latest research has found that the activation of different Drp1 phosphorylation sites can affect the interconversion between subgroups of terminally exhausted T cells. Moreover, blocking mitochondrial fission with Mdivi-1 can enhance the effectiveness of anti-CD3 mAb immunotherapy by promoting the generation of more terminally exhausted T cells.60 Therefore, mitochondrial motor proteins play an important role in T1D, and the mechanisms of other mitochondrial fission and fusion proteins in T1D need further exploration.
3.7 Other autoimmune diseases
Psoriasis is characterized by proliferation of keratinocytes and chronic inflammation. A study found that the expression of Drp1 gene in the skin of psoriasis patients was significantly increased and positively correlated with the severity of the disease. And knocking down Drp1 in human immortalized keratinocytes (HaCaT) cells reduced cell proliferation ability and the release of inflammatory mediators.50 In addition, some studies have shown that macrophages also play an important role in psoriasis. IMQ induced ganglioside-induced differentiation-associated protein 1 like 1 (GDAP1L1)-dependent Drp1 S616 phosphorylation and caused mitochondrial fission, which induced the release of inflammatory factors.61 Antrodia cinnamomea exhibits anti-inflammatory activities. 2,4-dimethoxy-6-methylbenzene-1,3-diol (DMD) is the active ingredient of Antrodia cinnamomea. It has been found that DMD can reduce macrophage infiltration and release of inflammatory factors. Mechanistically, DMD inhibit MAPK/NF-κB Phosphorylation and GDAP1L1/Drp1 translocation from the cytoplasm to mitochondria.51
Autoimmune hepatitis (AIH) is a chronic progressive liver inflammatory disease mediated by autoimmune response, with a high mortality rate.62 Research found that the expression of Drp1 and phosphoglycerate mutase 5 (PGAM5) was increased in liver biopsy tissues of AIH patients compared to healthy individuals. PGAM5 was also highly expressed in liver cells of AIH patients and AIH mouse tissues. PGAM5 deficiency protected mice from liver damage. Meanwhile, it has been confirmed that downstream of PGAM5, Drp1-mediated mitochondrial fission is an essential step in driving liver necrosis and tissue damage.9
4 CONCLUSIONS AND FUTURE PROSPECTS
Mitochondrial dynamics imbalance was observed in various autoimmune diseases, including RA, SLE, MG, etc. However, there is still a significant vacuum in the understanding of B cell and T cell mitochondrial dynamics in autoimmune diseases. Small molecule drugs targeting mitochondrial fission and fusion proteins may provide new opportunities for autoimmune diseases.
AUTHOR CONTRIBUTIONS
Ruicong Ma, Cheng Zhang, and Jiaqing Liu wrote the original manuscript. Jinyi Ren searched for relevant references. The manuscript was reviewed and edited by Xia Li and Ying Zhao. All authors have approved the final version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (82071834, 82271839), Application Basic Research Plan of Liaoning Provincial Department of Science and Technology (2022JH2/101300082), and Liaoning Provincial Education Department Basic Research Project (LJ212410161034). The authors would like to acknowledge all lab members for insightful discussions. The authors would like to thank BioRender for providing the icons. Figures were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
Xia Li is a member of the Rheumatology & Autoimmunity editorial board and is not involved in the peer-review process of this article. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
This article does not involve research data. Data sharing is not applicable as no new data were generated.