A case of Sjogren's syndrome evolving into multicentric reticulohistiocytosis
Abstract
Introduction
Multicentric reticulohistiocytosis (MRH) is a rare disease that is known to affect the skin and joints, primarily. It is considered a rare form of non-Langerhans cell histiocytosis (Group C) that can cause destructive inflammatory arthritis involving both the small and large joints. Cutaneous eruptions of periungual, “coral beads” and nodules appearing over the distal fingers are considered pathognomonic clues for identifying this disorder. Histology evaluation of the cutaneous papules typically shows infiltrative histiocytes and multinucleated giant cells. Although no well-established therapies exist to date, a variety of immunosuppressants have been used with varying degrees of success.
Case Description
A 53-year-old Caucasian female patient with a family history of rheumatoid arthritis and a personal history of Sjogren's syndrome presented to the rheumatology clinic complaining of pain in her bilateral hands and fingers. There were several small, papulo-nodular lesions ranging from 1 to 2 mm in size noted at the base of her nails. A 4 mm punch biopsy of one of the papules from the neck showed dermal infiltration of eosinophilic mononucleated and multinucleated giant cells with “ground glass” appearing cytoplasm consistent with MRH. X-ray of her hands showed periarticular demineralization and erosions surrounding several bilateral proximal and distal interphalangeal joints, and thus tofacitinib in addition to methotrexate, hydroxychloroquine, and dexamethasone (dosed weekly) was started to help control any further articular damage.
Conclusion
Our aim is to further support the relation of MRH with autoimmune diseases, including Sjogren's syndrome. Autoimmune diseases have been reported in association with MRH, although a clear association has yet to be made.
Key points
-
Multicentric reticulohistiocytosis (MRH) is a rare, multisystemic disease that can be distinctly characterized by the presence of papulonodular lesions appearing over the distal fingers and periungual area, typically described as having a “coral beads” pattern.
-
There are no diagnostic laboratory tests for MRH, although the diagnosis is typically made by the presence of the pathognomonic papulonodular lesions with aggressive erosive arthritis seen predominantly in the distal interphalangeal joints, as well as a skin and/or synovial biopsy.
-
Methotrexate has been considered as the most effective initial disease-modifying antirheumatic drug to use, although other options have also shown success including leflunomide, azathioprine, tumor necrosis factor inhibitors, thalidomide, interleukin (IL)-6 inhibitors, and IL-1 inhibitors.
1 INTRODUCTION
Multicentric reticulohistiocytosis (MRH) is a multisystem cutaneous (Group C) non-Langerhans cell granulomatous disease that can cause a very aggressive, erosive, inflammatory arthritis with the potential to progress to arthritis mutilans in approximately 45% of cases, compared with ~5% in rheumatoid and psoriatic arthritis.1-10 It mainly affects middle-aged women, aged 40–50 years. Papulo-nodular skin eruptions, typically occurring in the upper extremities and phalanges, are a common presentation associated with the erosive arthritis.8 These skin lesions can be commonly seen grouped together, giving a “cobblestone” or “coral bead” appearance, typically involving the periungal area.8
The arthritic changes seen can mimic rheumatoid arthritis (RA), however, the distal interphalangeal joints (DIPs) are the most frequently affected (nearly 75% of cases) and considered one of the identifying features of the disease.7, 11, 12 The arthritis was shown to precede the onset of rash in about 40%–60% cases, however, early cutaneous involvement occurs in <20% of patients.12 For these reasons, MRH can be frequently misdiagnosed as RA or dermatomyositis. Another key distinguishing feature of this disease is the very rapid progression of erosive joint disease with a disproportionate amount of changes seen radiographically when compared to clinically.7
MRH may be associated in approximately 5%–20% of cases with other autoimmune conditions although the mechanism of this association is not well known.6, 11 Systemic involvement has been reported and includes pleural effusions, congestive heart failure, mesenteric lymphadenopathy, and urogenital lesions.11 To date and our knowledge, there are only six reported cases of MRH associated with Sjogren's syndrome.1-6
2 CASE REPORT
A 53-year-old Caucasian female patient with a family history of RA presented to the rheumatology clinic complaining of pain in her bilateral hands and fingers. She was diagnosed with Sjogren's syndrome over 10 years prior. The patient reported a previous history of xerostomia and keratoconjunctivitis sicca when she was first evaluated over 10 years ago. Serologic labs at that time showed the following: positive antinuclear antibody (ANA) > 1:1280 speckled pattern along with a positive anti-Ro, La, ribonucleoprotein, and rheumatoid factor (RF) of 53 (N < 20). Anticyclic ctirullinated, double-stranded DNA, and Smith antibodies were all negative. Complements, C3 (83, N > 86) and C4 (17, N > 20), were both mildly reduced, and C-reactive protein and serum gamma globulin were slightly elevated. There was no mention of any biopsy done. She was subsequently started on hydroxychloroquine daily for Sjogren's syndrome as well as oral methotrexate 15 mg weekly (with daily folate) for inflammatory polyarthritis which was attributed to RA at that time. She had no rashes or papules noted.
At her most recent visit, the patient reported persistent and intermittent worsening pain in both her hands and fingers with continued xerostomia and keratoconjunctivitis. Her physical exam was noteworthy for tenderness, warmth, and erythema at multiple proximal interphalangeal (PIP), DIP, and metacarpophalangeal joints bilaterally. Due to her worsening joint pain in her hands, the methotrexate dose was increased to 20 mg weekly while continuing hydroxychloroquine and folate daily. Pilocarpine was also recommended to help treat her xerostomia.
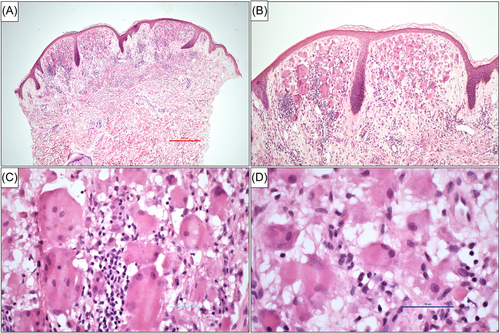
A few months later, the patient began developing a progressive erythematous rash on her face that spread to her hands, neck, and ears, as well as new, small nodules on her fingers. There were several small, papulo-nodular lesions ranging from 1 to 2 mm in size noted at the base of her nails (Figure 1). She mentioned that the nodules had bled intermittently. A 4 mm punch biopsy of one of the papules from the neck showed dermal infiltration of eosinophilic mononucleated and multinucleated giant cells with “ground glass” appearing cytoplasm consistent with MRH (Figure 2).
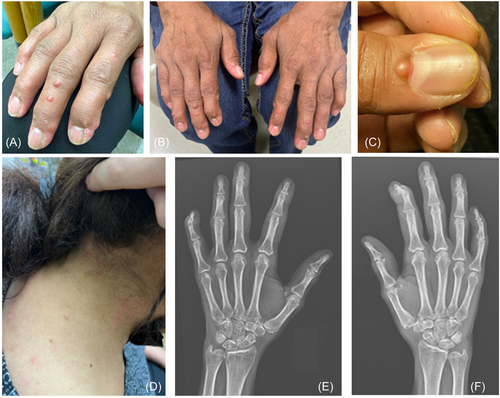
Since her diagnosis of MRH, a variety of immunosuppressants have been used including methotrexate, tocilizumab, adalimumab, and cyclosporine. A computed tomography (CT) of the abdomen/pelvis, which resulted normal, was done to rule out any possible occult malignancy. A follow-up X-ray of her hands showed periarticular demineralization and erosions surrounding several bilateral PIPs and DIPs, and thus tofacitinib in addition to methotrexate, hydroxychloroquine, and dexamethasone (dosed weekly) was started to help control any further articular damage. At a recent follow-up, the patient reported clinical improvement with decreased synovitis on exam.
3 DISCUSSION
MRH is a rare, multisystemic disease that can be distinctly characterized by the presence of papulonodular lesions appearing over the distal fingers and periungual area, typically described as having a “coral beads” pattern.7, 11 It is classified as a (Group C) non-Langerhans cutaneous cell histiocytosis that predominantly affects middle-aged Caucasian females with a higher incidence reported in western countries and Japan.11 There are no diagnostic laboratory tests for MRH, although the diagnosis is typically made by the presence of the pathognomonic papulonodular lesions with aggressive erosive arthritis seen predominantly in the DIPs, as well as a skin and/or synovial biopsy. Histopathological findings typically show a lymphohistiocytic infiltrate with multinucleated histiocytes and multinucleated giant cells with a ground-glass appearing eosinophilic cytoplasm.13
MRH has been reported to present as a paraneoplastic disease, including squamous cell carcinoma of lung, pancreatic adenocarcinoma, and melanoma.14 Autoimmune diseases have also been reported in association with MRH, although a clear association has yet to be made.6 It has been hypothesized that MRH could be induced by an autoimmune disease with cellular and/or cytokine autoimmune mechanisms that may induce the activity of histiocytic cells.6 In the most recent, single-center case series of MRH patients (performed by the Mayo Clinic between 1984 and 2017), one-third of the patients had confirmed concomitant autoimmune disorders, 44% had a positive ANA, 28% had a positive RF, 44% had a positive anti-CCP, and 75% had a positive anti-Ro antibody.11
Given the aggressive nature of the erosive arthritis known to occur in patients with MRH, prompt recognition and timely immunomodulatory initiation is warranted to prevent irreversible articular damage. Although no well-established therapies exist to date, a variety of immunosuppressants have been used with varying degrees of success. Methotrexate has been considered as the most effective initial disease-modifying antirheumatic drug to use, although other options have also shown success including leflunomide, azathioprine, tumor necrosis factor inhibitors, thalidomide, interleukin (IL)-6 inhibitors, and IL-1 inhibitors.2, 8, 15 Only two prior case reports discussed clinical improvement with Janus kinase (JAK) inhibitors, and given previously described elevated IL-6 in serum and tissue samples in MRH, targeting JAK-1 pathway and thus IL-6 signaling may modify inflammatory responses in MRH.16, 17 Bisphosphonates have also been speculated to be effective (potentially as “add-on” therapy) given their effect on preventing focal bone resorption and decreasing bone erosions.6, 8 Although next-generation sequencing for our patient was not performed, identifying potential driver mutations such as MRH RAS-MAPK pathway could further elucidate targeted therapies for MRH.18
AUTHOR CONTRIBUTIONS
Conceptualization: Daniel Gonzalez. Writing—original draft: Daniel Gonzalez, Enrique Medina, Rachelle Gietzen, and Vinh Lu. Writing—review and editing: All authors.
ACKNOWLEDGMENTS
We thank Ty Bohrnstedt for his expertise and assistance with technical and image editing for this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Signed consent from the patient was obtained for publication.
Open Research
Data sharing is not applicable to this article as no new data were created or analyzed in this study.