Belimumab shows promise in neuropsychiatric lupus: Another challenge to find the offender
Abstract
Introduction
Agents that can be used for the treatment of neuropsychiatric lupus (NPSLE) are lacking in the therapeutic armamentarium. Belimumab is a monoclonal antibody targeting the B-cell activating factor (BAFF) and is approved by the US Food and Drug Administration as an additional treatment for systemic lupus erythematosus patients with persistent disease activity and lupus nephritis (LN); however, severe active central nervous system manifestations were excluded.
Case Report
We report on a treatment-naïve LN patient with refractory NPSLE complicated with progressive posterior reversible encephalopathy syndrome (PRES) who was successfully treated via the combination of mycophenolate and belimumab, resulting in reversal of persistent headache and neuroradiologic manifestations.
Conclusion
Research on this topic could be relevant for identifying a possible correlation between BAFF and psychiatric NPSLE manifestations.
Key points
-
The nephrotic state is a specific risk factor for posterior reversible encephalopathy syndrome (PRES) in patients with lupus renal flares.
-
Belimumab showed efficacy and safety in a lupus nephritis patient with PRES.
1 INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome characterized by a rapid onset of headache, cortical blindness, altered mental state, generalized seizure, hypertension, and recognizable radiological features. Although PRES has not been included in the American College of Rheumatology (ACR) nomenclature, it has been described as an emerging neurological manifestation with a prevalence of 0.7%–1.4% among patients with systemic lupus erythematosus (SLE).1 The nephrotic state poses a unique risk for PRES, along with known associations including cyclophosphamide, cyclosporin A, or FK-506 neurotoxic syndrome.2 Here, we report on a treatment-naïve lupus nephritis (LN) patient with refractory neuropsychiatric SLE (NPSLE) complicated with progressive PRES who was successfully treated using belimumab, with subsequent reversal of persistent headache and neuroradiologic manifestations.
2 CASE REPORT
A 37-year-old Chinese female patient was first diagnosed with NPSLE in July 2018, complaining of a persistent migraine headache but refusing active treatment. Brain T2 magnetic resonance imaging (MRI) demonstrated bilateral asymmetric hyperintensity in the basal ganglia (Figure 1A). In November 2020, she visited the Emergency Department with a severe headache, hypertension (230/161 mmHg), fever, nausea, vomiting, and abdominal pain. Initial labs revealed lymphocytopenia, markedly elevated creatinine (4.667 mg/dL), low serum complement level, high titer of an antinuclear antibody (ANA) (×320, homogenous and speckled pattern), and high titers of anti-dsDNA, anti-Sm, anti-SSA, anti-Ro-52, and antiribosomal P antibodies; a negative test result was indicated for antiphospholipid antibodies. Urine analysis showed active sediments with nephrotic range proteinuria (3 g/24 h). Cerebrospinal fluid pressure was 225 mm H2O, with no signs of intracranial infection. The SLE disease activity index score at PRES was 40, indicating very active disease. MRI demonstrated patchy cortical and subcortical edema in posterior lobes suggestive of PRES (Figure 1B). Therefore, the diagnosis of highly active NPSLE and LN complicated with PRES was confirmed.
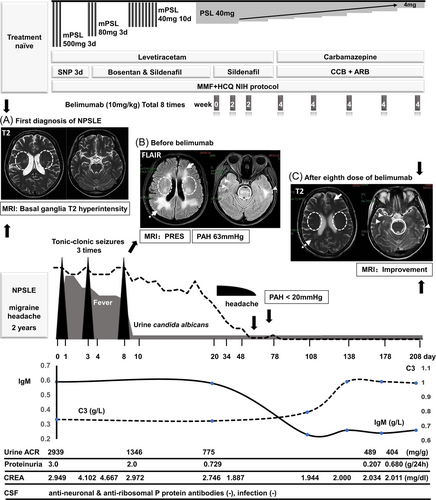
According to the European League Against Rheumatism (EULAR) recommendations,3 a pulse of intravenous methylprednisolone (500 mg/day × 3 days) combined with mycophenolate mofetil and hydroxychloroquine was used. Blood pressure was under control without antihypertensives by the fourth day; however, despite aggressive treatment, renal function deteriorated rapidly (glomerular filtration rate < 20 mL/min) and remained at this low level thereafter. Simultaneously, neuropsychiatric symptoms developed progressively, with tonic–clonic seizures in three instances. The patient received intravenous diazepam and oral levetiracetam therapies as rescue therapy. Twenty days after admission, she was initiated on intravenous belimumab therapy at 10 mg/kg × 3 doses every 2 weeks, then continued on maintenance therapy with 10 mg/kg intravenously every 4 weeks until Week 24. Two weeks after initiating belimumab, when she visited our outpatient care center to receive the third infusion, the headache had alleviated significantly. After the eighth dose of belimumab, this patient showed complete remission of proteinuria with a substantially reduced glucocorticoid dose. Vasodilator therapy with bosentan and sildenafil had normalized her right ventricular enlargement with mild tricuspid valve regurgitation (pulmonary artery hypertension < 20 mmHg). MRI showed that the typical PRES pattern, posterior parietal and occipital lobe lesion, was reversed; perhaps most importantly, the basal ganglia lesions were absent compared with 2 years ago (Figure 1C).
3 DISCUSSION
Belimumab, a monoclonal antibody against B-cell activating factor (BAFF), is the first specific drug approved for the treatment of both SLE4, 5 and LN.6 The literature describing the effects of belimumab in patients with refractory NPSLE remains scarce.7-9 While the results of further prospective clinical trials are eagerly awaited, a post hoc analysis of the available phase 3 trials of lupus patients with a headache at baseline concluded in favor of belimumab10; two further studies and our case are consistent with this finding.7, 8 Additionally, MRI findings stabilized over time. Elevated levels of BAFF in patients with NPSLE may be locally produced by astrocytes or immigrant immune cells, or it could derive from systemic cytokine production degrading the blood–brain barrier via an invasion of autoantibodies into the brain, then progressing to tissue damage.11 PRES frequently occurs during active SLE. The nephrotic state is a specific risk for PRES, and may involve multiple factors; these patients are often hypertensive, receiving high-dose corticosteroids or cytotoxic therapy with cyclophosphamide, may have severe hypoproteinemia, and may have massive edema with fluid retention and associated increased vascular permeability.12 However, in this case, PRES occurred in a treatment-naïve patient, which might be explained by endothelial dysfunction resulting from disease activity playing an important pathogenetic role.13 Emerging evidence has shown that PRES in patients with SLE may be influenced by inflammatory mechanisms.14 Therapy with belimumab could promote the normalization of serologic activity and reduce BAFF-dependent B-cell subsets in serologically and clinically active SLE. However, it is unclear whether belimumab itself was the cause of the resolution of PRES in this patient: the patient's PRES may have improved with antihypertensive therapy alone or some aspect of the combination of mycophenolate and belimumab may have been responsible for the improvement. Currently, there is a paucity of high-quality trial data to guide firm recommendations. Research on this topic could be relevant for identifying a possible correlation between BAFF and psychiatric NPSLE manifestations.
AUTHOR CONTRIBUTIONS
Shuilian Yu, Zhiping Liu, Qilin Yang, and Wenhui Huang examined the patient as the attending physician. Wenhui Huang performed MRI examinations. Shuilian Yu and Zhiping Liu wrote the manuscript. Ling Zhong and Wenhui Huang supervised the study. All authors contributed to the discussion and approved the final version.
ACKNOWLEDGMENTS
The authors thank all the Department of Rheumatology clinical staff at the Second Hospital of Guangzhou Medical University, Guangzhou, China.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the article. The patient understands that her name and initial will not be published and due efforts will be made to conceal the identity of the patient, although anonymity cannot be guaranteed. The information will be published without the patient's personal information and every attempt will be made to ensure anonymity.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.