Effect of human umbilical cord mesenchymal stem cells on ovarian function in lupus mice
Abstract
Background
Patients with systemic lupus erythematosus (SLE) suffer from a high incidence of premature ovarian failure, which might be due to cyclophosphamide gonadal toxicity, immune abnormalities, or other reasons. This study aimed to investigate whether the transplantation of human umbilical cord mesenchymal stem cells (HUC-MSCs) can improve ovarian reserve function in lupus mice.
Methods
We used MRL/lpr mice to observe changes in ovarian structure and secretory function in SLE. Lupus mice and controls were injected with HUC-MSCs at Weeks 12 and 16. We detected serum concentrations of the sex hormones estradiol (E2), follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH), using enzyme-linked immunosorbent assays. Hematoxylin and eosin staining showed ovarian tissue structure and enabled the counting of follicles. Hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1) expression in ovarian tissue was observed by immunohistochemistry.
Results
Ovarian function in lupus mice was abnormal, as indicated by decreased serum E2 and AMH concentrations, and increased FSH concentrations. HUC-MSC transplantation caused significant upregulation of serum E2 and AMH and downregulation of FSH (all p < 0.05). Ovarian structure improved and the follicle number increased after HUC-MSC transplantation. Multiple infusions of HUC-MSCs at Weeks 12 and 16 resulted in a significantly higher number of primordial follicles than infusions of HUC-MSCs at only Week 12 (p < 0.05). Immunohistochemistry showed that IGF-1 and HGF expression increased after HUC-MSC transplantation, but this was not significant.
Conclusions
HUC-MSCs transplantation restores disturbed hormone secretion and folliculogenesis in lupus mice. HUC-MSC transplantation should be repeated for the best treatment effect.
Key points
-
Ovarian function in lupus mice was abnormal with disturbed hormone secretion and folliculogenesis and human umbilical cord mesenchymal stem cell transplantation could recover ovarian function in lupus mice.
-
Systemic lupus erythematosus (SLE) itself may have an abnormal ovarian function. We also suggested novel strategies to treat patients with SLE with premature ovarian failure.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that occurs in women of childbearing age. The incidence of premature ovarian failure (POF), which seriously affects reproductive function and consequently mental health, is significantly higher in women with SLE than in healthy women.1 Therefore, the protection of ovarian function in patients with SLE is of great clinical interest. Gonadal toxicity of cyclophosphamide treatment is more likely to be associated with POF in patients with SLE;2 however, immune abnormalities could also be involved.3 The involvement and role of abnormal immune function in POF remain unclear. Mesenchymal stem cells (MSCs) have shown considerable safety and efficacy in the treatment of POF in animal models.4 However, the use of MSCs to treat POF in lupus mice has not been reported. Human umbilical cord mesenchymal stem cells (HUC-MSCs) are capable of several functions, such as self-renewal, multidirectional differentiation, and immune regulation. They are also easy to obtain and are not associated with ethical concerns. HUC-MSCs have been used in the clinical treatment of several diseases, including refractory lupus nephritis.5
The main aim of this study was to examine the ovarian function and the occurrence of POF in lupus mice. We also aimed to determine the effects of HUC-MSCs transplantation on ovarian function in lupus mice. Furthermore, we aimed to identify potential mechanisms underlying these effects, to provide a theoretical basis for the development of HUC-MSCs-based strategies for the treatment of POF in patients with SLE.
2 METHODS
2.1 Experimental animals and HUC-MSC transplantation protocol
We used 30 specific pathogen-free (SPF) female MRL/lpr mice (6 weeks old female; Shanghai Lingchang Biotechnology Co., Ltd., Shanghai, China, license number: SCXK, 2013-0018), with typical characteristics including hypersplenotrophy due to lymphocyte proliferation accompanying proteinuria and appearance of various autoantibodies, and 6 SPF female C57BL/6 mice (6 weeks old; Experimental Animal Center of Jiangsu University, license number: SCXK, 2013-0011). All experimental mice were raised in the SPF-class breeding area of the Animal Experiment Center of Jiangsu University until they were 20 weeks old. All animal experiments were approved by the Animal Experiment Ethics Committee of Jiangsu University. The MRL/lpr mice were randomly divided into the MSC1 (n = 6), MSC2 (n = 6), PBS1 (n = 6), PBS2 (n = 6), and model control (n = 6) groups. C57BL/6 mice (n = 6) were used as normal controls. The MSC1 group was injected with passage 3 (P3) HUC-MSCs (1 × 107cells/ml, 0.1 ml/10 g body weight) via the tail vein at 12 weeks. The MSC2 group was injected with P3 HUC-MSCs (1 × 107 cells/ml, 0.1 ml/10 g body weight) via the tail vein at 12 and 16 weeks. The PBS1 group, which was the control for the MSC1 group, was injected with the same volume of phosphate-buffered saline (PBS) as that for P3 HUC-MSCs at 12 weeks. The PBS2 group, which was the control for the MSC2 group, was injected with the same volume of PBS as that for P3 HUC-MSCs at 12 and 16 weeks. All mice were killed at 20 weeks, and serum and ovaries were collected. The ovaries were fixed in 10% formaldehyde.
2.2 Detection of serum hormone concentrations by enzyme-linked immunosorbent assay
Serum follicle-stimulating hormone (FSH), estradiol (E2), and anti-Müllerian hormone (AMH) concentrations were determined using an enzyme-linked immunosorbent assay kit (Nanjing Jiancheng Institute of Bioengineering Co., Ltd., Nanjing, China) according to the manufacturer's instructions.
2.3 Ovarian histomorphology and follicular count
After the mice were killed, the ovarian tissues were separated, fixed in formaldehyde fixative, embedded in paraffin, and stained with hematoxylin and eosin. The ovarian structure was observed under a microscope, and the number of follicles at various stages was noted. The follicular grading criteria were as follows: primordial follicles were defined as oocytes enclosed by a single layer of flat granular cells; primary follicles were defined as oocytes enclosed by a single layer of cubic granular cells; secondary follicles were defined as the appearance of follicular cavities, some forming cumulus, and two layers of follicular membranes; and atretic follicles were defined as a collapsed follicular wall, absent or unclear structure of the egg cell, and a shrunken zona pellucida.
2.4 Immunohistochemical staining of ovarian tissue
After the mice were killed, the ovarian tissues were separated. Fresh ovarian tissues were embedded in optimum cutting temperature compound (OCT) medium and cut into 5-μm-thick sections for immunohistochemical staining to detect hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1). Immunohistochemistry was performed by the streptavidin–peroxidase method according to the manufacturer's instructions.
2.5 Statistical analysis
SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA) was used for analysis. Data with a normal distribution are expressed as the mean ± standard deviation. The t-test was used for comparison between two groups, and one-way analysis of variance was used for comparison among groups. Dunnett's T3 test was used for paired comparisons. A p < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Changes in ovarian function in lupus mice
We evaluated ovarian function in the mice by measuring the serum concentrations of E2, FSH, and AMH in each experimental group. Serum E2 concentrations (296.75 ± 61.42 vs. 117.73 ± 25.87 ng/dl, p < 0.01) and AMH concentrations (308.41 ± 48.64 vs. 116.75 ± 31.10 ng/dl, p < 0.001) were significantly lower, and FSH concentrations (191.21 ± 76.80 vs. 742.80 ± 100.63 mIU/dl, p < 0.001) were significantly higher in the model control group than in the normal control group (Figure 1A).
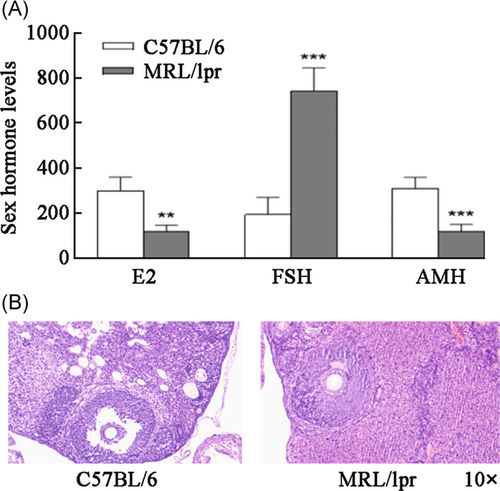
Hematoxylin and eosin staining was performed to observe ovarian structural changes, and we found that the follicles in the normal control group of mice grew actively and were clearly visible. The number of follicles in the model group was fewer than that in the normal control group and disorderly in arrangement with different shapes. The number of follicles in the treatment group was higher than that in the model group, and the level was clear, which was second to the shape of the control group. The numbers of primordial follicles (7.00 ± 1.83 vs. 2.50 ± 1.29, p < 0.01), primary follicles (10.25 ± 1.89 vs. 5.50 ± 1.29, p < 0.01), and secondary follicles (6.50 ± 2.08 vs. 2.00 ± 0.82, p < 0.01) were significantly lower, and the number of atretic follicles (1.00 ± 0.82 vs. 2.75 ± 0.96, p < 0.05) was significantly higher in the model control group than in normal control group (Figure 1B).
3.2 Effect of HUC-MSC transplantation on ovarian function in lupus mice
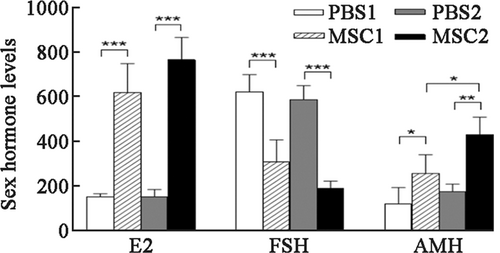
Serum E2, FSH, and AMH concentrations were significantly different between the PBS1, MSC1, PBS2, and MSC2 groups (Table 1 and Figure 2). There were no differences in serum concentrations of E2, AMH, or FSH between the PBS1 and PBS2 groups. Serum E2 (p < 0.001) and AMH (p < 0.05) concentrations were significantly higher, and serum FSH concentrations (p < 0.001) were significantly lower in the MSC1 group than those in the PBS1 group. Serum E2 (p < 0.001) and AMH (p < 0.01) concentrations were significantly higher, and serum FSH concentrations (p < 0.001) were significantly lower in the MSC2 group than those in the PBS2 group. There was no significant difference in serum E2 or FSH concentrations between the MSC1 and MSC2 groups. Serum AMH concentrations in the MSC2 group were significantly higher than those in the MSC1 group (p < 0.05).
| Items | PBS1 | PBS2 | MSC1 | MSC2 |
|---|---|---|---|---|
| E2 (ng/dl) | 153.38 ± 13.48 | 153.78 ± 31.97 | 622.74 ± 129.32 | 769.33 ± 100.99 |
| FSH (mIU/dl) | 626.00 ± 76.60 | 589.74 ± 63.31 | 310.15 ± 99.62 | 190.86 ± 32.50 |
| AMH (ng/dl) | 122.05 ± 71.70 | 177.12 ± 32.47 | 259.04 ± 83.10 | 433.73 ± 77.22 |
- Abbreviations: AMH, anti-Müllerian hormone; E2, estradiol; FSH, follicle-stimulating hormone; MSC, mesenchymal stem cell; PBS, phosphate buffer saline.
3.3 Effect of HUC-MSC transplantation on ovarian morphology of lupus mice
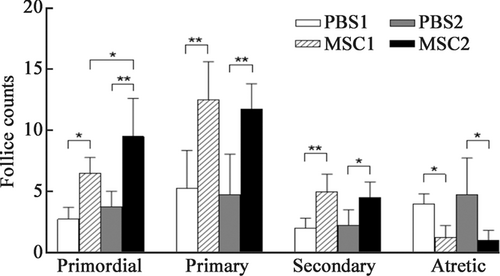
There were significant differences in follicular counts at all stages between the PBS1, MSC1, PBS2, and MSC2 groups (Table 2 and Figure 3). The numbers of primordial, primary, and secondary follicles were significantly higher (all p < 0.05), and the number of atretic follicles was significantly lower (p < 0.05) in the MSC1 group than that in the PBS1 group. The numbers of primordial, primary, and secondary follicles were significantly higher (all p < 0.05), and the number of atretic follicles was significantly lower (p < 0.05) in the MSC2 group than that in the PBS2 group. There was no significant difference in the number of primary or secondary follicles between the MSC1 and MSC2 groups. The number of primordial follicles in the MSC2 group was significantly higher than that in the MSC1 group (p < 0.05). Therefore, multiple infusions of HUC-MSCs significantly increased the number of primordial follicles. This finding suggested that multiple infusions of HUC-MSCs were more effective than just one infusion in improving ovarian function.
| Items | PBS1 | PBS2 | MSC1 | MSC2 |
|---|---|---|---|---|
| Primordial follicle | 2.75 ± 0.96 | 3.75 ± 1.26 | 6.50 ± 1.29 | 9.50 ± 3.11 |
| Primary follicle | 5.25 ± 3.10 | 4.75 ± 3.30 | 12.50 ± 3.11 | 11.75 ± 2.06 |
| Secondary follicle | 2.00 ± 0.82 | 2.25 ± 1.26 | 5.00 ± 1.41 | 4.50 ± 1.29 |
| Atretic follicle | 4.00 ± 0.82 | 4.75 ± 2.99 | 1.25 ± 0.96 | 1.00 ± 0.82 |
- Abbreviations: MSC, mesenchymal stem cell; PBS, phosphate buffer saline.
3.4 Effect of HUC-MSC transplantation on HGF and IGF-1 expression in ovarian tissue of lupus mice
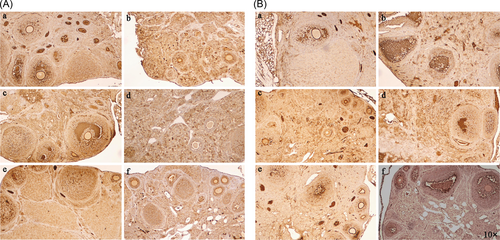
To determine the mechanisms by which HUC-MSCs could improve ovarian function, we used immunohistochemistry to detect HGF and IGF-1 expression in ovarian tissue of lupus mice. HGF and IGF-1 expression increased after HUC-MSCs transplantation (Figure 4), but this was not significant.
4 DISCUSSION
Most patients with SLE present with abnormal reproductive function.6 However, the specific cause of these abnormalities is unclear. Autoimmune oophoritis caused by SLE could lead to POF.7 Additionally, the use of drugs with gonadal toxicity, such as cyclophosphamide, can cause POF, and thus lead to premature amenorrhea and infertility.8 The chronic inflammatory state of SLE interferes with the function of the hypothalamic–pituitary–gonadal axis.9 Concomitant antiphospholipid antibody syndrome causes miscarriage, premature delivery, and stillbirth.10 However, the exact mechanism underlying POF in patients with SLE remains unclear, and there have been no reports of in vivo studies on this topic. In this study, the ovarian function of lupus mice was studied to determine whether SLE causes abnormal ovarian function. Our results suggested that patients with SLE had an abnormal ovarian function. However, the sample size was limited, and further studies with larger experimental groups are warranted.
The heterogeneity of POF is also reflected by a variety of possible causes, including autoimmunity, drugs, toxins, radiation, and infectious genetic defects.11 Our mouse experiments showed that SLE likely caused ovarian dysfunction, but whether SLE causes POF remains unclear. A cross-sectional study to assess the incidence of POF in 961 female patients with SLE showed that the incidence of POF in patients with SLE who did not receive cyclophosphamide treatment was not significantly lower than that in healthy individuals.12 This finding suggests that the autoimmune response in patients with SLE might not involve the ovary. However, some clinicians have observed that the ovarian reserve function of women with SLE is lower than that in healthy women.13 Patients with SLE of childbearing age who have never received any drug treatment have lower serum AMH concentrations, a higher incidence of abnormal menstruation, an imbalance of the lymphocyte ratio, a lower proportion of CD4+ T lymphocytes and NK cells, and a higher proportion of B cells and CD8+ T lymphocytes than healthy women.14 This finding suggests that abnormal autoimmune function in SLE leads to an impairment of ovarian reserve function. To determine the status of ovarian function in SLE, we studied ovarian function in lupus mice. We assessed ovarian reserve function by measuring serum E2, FSH, and AMH concentrations, and follicles were counted at all stages. E2 is a sex hormone that is synthesized and secreted by the ovaries. A decrease in E2 concentrations indicates a decline in the ovarian secretion of E2. FSH is a gonadotropin secreted by adenohypophysial FSH cells, which can promote the synthesis and secretion of estrogen, maturation of follicles, and ovulation. An increase in FSH concentrations indicates a decline in ovulation function. AMH is secreted by ovarian granulosa cells and promotes the development of gonads. AMH concentrations are not affected by gonadotropins and do not change with the menstrual cycle. Therefore, AMH is considered the most reliable indicator for evaluating ovarian reserve. In our study, E2 and AMH concentrations and the follicular count were significantly lower in lupus mice than in normal mice. FSH concentrations and the number of atretic follicles were significantly higher in lupus mice than in normal mice, which is consistent with clinical reports of ovarian reserve dysfunction in patients with SLE.13, 15 Therefore, SLE could cause POF, which could be exacerbated with the use of drugs with gonadal toxicity, such as cyclophosphamide. The ovarian function of patients with SLE should be protected during clinical treatment.
Several methods, including hormone replacement therapy, a gonadotropin-releasing hormone analog, melatonin supplementation, dehydroepiandrosterone supplementation, and cryopreservation of eggs, embryos, or ovarian tissue, are currently used to treat POF. However, these treatments have poor clinical efficacy and several limitations. Combined hormone replacement therapy is the most common. However, an optimal treatment plan and method of administration have not been established, and estrogen therapy is associated with risks of vaginal atrophy, venous thrombosis, and tumors.16 The use of a gonadotropin-releasing hormone analog during cyclophosphamide treatment could significantly reduce the incidence of POF in young women with SLE.17 Therefore, a gonadotropin-releasing hormone analog could be used to prevent POF in premenopausal women with SLE receiving cyclophosphamide treatment.17 European League Against Rheumatism (EULAR) recommendations on family planning, assisted reproduction, pregnancy, and menopause management in female patients with SLE state that a gonadotropin-releasing hormone analog should be considered to preserve fertility before using alkylating agents.18 Cryopreservation of eggs, embryos, or ovarian tissues is expensive, technically demanding, and relies on assisted reproductive technology. These fertility preservation methods are mainly suitable for young female patients with reproductive requirements and have limited clinical applications.19, 20 Therefore, there is an urgent need for safe and effective methods to treat POF in patients with SLE.
Stem cells are capable of self-replication and multidirectional differentiation. Stem cells can regenerate and repair body tissue, and also regulate the body's immune function via mechanisms such as cytokine secretion. Therefore, stem cell transplantation is considered a therapeutic method to repair damaged ovarian function.21 MSCs are pluripotent stem cells. Compared with embryonic stem cells and hematopoietic stem cells, MSCs have low immunogenicity and strong immunosuppressive activity. MSCs can restore ovarian function through a variety of mechanisms. These mechanisms include promoting the expression of various cytokines through paracrine pathways, improving the ovarian microenvironment, inhibiting granulosa cell apoptosis, and self-differentiation into estrogen-producing cells.22-25 The HUC-MSCs used in this study were derived from the umbilical cord tissue of healthy newborns. The umbilical cord is considered medical waste, and thus has the advantages of a noninvasive sampling process, availability, low cost, and no associated ethical issues. HUC-MSCs show strong immune regulation and damage repair functions, stable biological properties, considerable effects in the treatment of autoimmune diseases,26, 27 and great clinical application potential in the treatment of POF.28 In this study, HUC-MSC transplantation treatment significantly improved ovarian reserve in lupus mice, increased serum E2 and AMH concentrations, reduced serum FSH concentrations, increased the number of primordial, primary, and secondary follicles, and reduced the number of atretic follicles. The role of MSCs in vivo is not permanent, and previous data have suggested the necessity of repeating MSC infusions after 6 months in patients with refractory lupus.29 We found that the number of primordial follicles in the MSC2 group was significantly higher than that in the MSC1 group. This finding suggests that intermittent MSC therapy might benefit the recovery of ovarian function, and the treatment of POF with MSCs might be dose-dependent. Therefore, our results provide a theoretical basis for the development of novel strategies for the clinical treatment of patients with SLE and ovarian failure, particularly in women of childbearing age who have fertility requirements. In patients with SLE who choose cyclophosphamide therapy, preventive treatment could be considered; however, the preventive effects require extensive further study.
Experimental and clinical data suggest that MSCs are a promising strategy for severe and refractory systemic lupus erythematosus. Recently, a prospective, 4-year, follow-up study was published, aiming to evaluate the long-term safety and efficacy of allogeneic MSCT in 87 treatment-resistant patients with SLE.30 This previous study showed that disease activity declined as shown by significant changes in the systemic lupus erythematosus disease activity index score, and levels of serum autoantibodies, albumin, and complements. However, the role of stem cells in the treatment of POF is unclear. Human placental MSCs may promote ovarian function by secreting epidermal growth factors.31 HUC-MSCs may improve ovarian endocrine function by modulating ovarian cell apoptosis.32 Moreover, HUC-MSCs might ameliorate POF by regulating the autophagy of ovarian cells and the expression of CD8+CD28- T cells in peripheral blood.33 Additionally, HUC-MSC-derived exosome miR-17-5P may promote the functional recovery of POF induced by cyclophosphamide by regulating the expression of related genes.34 HUC-MSCs can repair ovarian function in a variety of ways, but the main mechanism is not completely clear.
HGF is an important factor in the environment of follicles and can accelerate the viability of growing follicles and promote the proliferation of ovarian surface epithelium to supplement the damaged areas formed during ovulation. IGF-1 is expressed in granulosa cells and healthy follicles, but cannot be detected in atretic follicles. IGF-1 is an important regulator of granulosa cell proliferation in the early stages of follicular development. The expression of ovarian IGF-1 stimulates the production of progesterone and estradiol and enhances the responsiveness of FSH in granular cells by improving the expression of FSH receptors. Therefore, IGF-1 is an important indicator of ovarian function. We studied the changes in ovarian protein expression before and after HUC-MSC transplantation to identify the mechanisms by which HUC-MSCs could improve ovarian function. IGF-1 and HGF expression in the lupus mouse with POF that received HUC-MSC transplantation slightly increased, but this was not significant. Our result is not consistent with previous studies, which suggested that HUC-MSCs can promote ovarian expression of HGF and IGF-1, resulting in improved ovarian reserve function and preventing ovarian senescence.28 However, how HUC-MSCs act on ovarian tissue to promote IGF-1 and HGF expression requires further study. Additionally, the reason why our result is different to that in previous studies needs to be further investigated.
5 CONCLUSIONS
This study suggests that SLE causes abnormal ovarian function, mainly manifesting as changes in ovarian structure and secretory function. This finding requires validation in clinical trials. HUC-MSC transplantation can repair ovarian tissue structure and enhance ovarian secretion function, and repeated HUC-MSC transplantation is required for effective treatment. Our results suggest novel strategies to treat patients with SLE with POF, but the specific mechanism remains unclear and merits further study.
AUTHOR CONTRIBUTIONS
Defang Meng contributed to the experimental operation, manuscript writing, and literature review. Yu Tang was responsible for animal experiments. Jing Xie and Hui Li were responsible for data gathering and analysis. Hua Wei contributed to funding support and the final editing of the manuscript content. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the Experimental Animal Center of Jiangsu University for their technical assistance. This work was supported by the Wu Jieping Medical Foundation (No. 320.6755.15035).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Our investigation using experimental animals was conducted on the basis of the Animal Experiment Ethics Committee of Jiangsu University.
Open Research
DATA AVAILABILITY STATEMENT
Some data from this study are available from the corresponding author by reasonable request.