Old and new damage-associated molecular patterns (DAMPs) in autoimmune diseases
Abstract
All organisms living in complex environments have evolved effective mechanisms of dynamic responses to extracellular stimuli. The immune system activates when damaged or injured cells release damage-associated molecular patterns (DAMPs). In addition to well-characterized DAMPs such as high-mobility group box 1 and adenosine triphosphate, studies on new classes of DAMPs have emerged. Here, we review recent reports of a new class of isoprenoid-derived DAMPs, including farnesyl pyrophosphate and geranylgeranyl pyrophosphate, both of which are pivotal metabolic intermediates of the mevalonate pathway. We also explore the roles of old and new DAMPs in autoimmune diseases that result from dysregulated inflammation. The findings highlight that understanding the functional mechanisms of DAMPs is important to enrich the DAMP family and decipher their immunoregulatory mechanisms to provide new therapeutics for the prevention and treatment of autoimmune diseases.
Key points
-
The mevalonate pathway plays a major role in autoimmune diseases resulting from dysregulated inflammation.
-
Isoprenoid metabolites from the mevalonate pathway show that farnesyl pyrophosphate and geranylgeranyl pyrophosphate are new damage-associated molecular patterns (DAMPs).
-
Comprehensive analysis of isoprenoid metabolites derived DAMPs may lead to new treatments for autoimmune diseases.
-
Immune dysfunction contributes to the pathology of cross-sectional DAMPs in autoimmune diseases.
1 INTRODUCTION
Multicellular organisms have sophisticated signaling cascades and networks that carry out specific and dynamic responses following diverse stimuli and stresses, among which exist potentially life-threatening events disguised as a danger signal. The molecules associated with danger typically arise from endogenous elements within injured cells and tissues, which are known as damage-associated molecular patterns (DAMPs). DAMPs released from injured or dying cells activate the immune system and invoke inflammatory responses in autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).1-3 DAMPs broadly exist in both mammals and plants.4, 5 Several novel molecules, including alarmins, endokines, and DAMPs, have been recently identified as potent proinflammatory factors of innate immunity because of the release of these molecules by activated or damaged cells under stress conditions. Recognition of DAMPs by their cognate receptors activates signaling cascade events that lead to, for example, activation of nuclear factor-κB (NF-κB), which is an essential regulator of immune responses during inflammation.6 Some reports have elucidated that DAMPs play a crucial role in aggravating the pathogenesis of autoimmune diseases, including RA, SLE, and systemic sclerosis because they are concurrently involved in the maturation of various immune pathways through a series of proinflammatory cytokine mediators.7 For example, high mobility group protein 1 (HMGB1), S100, and heat shock proteins belong to a protein category that gives rise to amelioration of inflammatory diseases. Well-characterized compounds, such as ATP and uric acid, participate in immune responses and wound healing. Mechanistically, HMGB1 protein diversely functions as a regulator of toll-like receptors (TLRs), advanced glycation endproducts (RAGE), and upstream elements of matrix metalloproteinases (MMPs), generating proinflammatory mediators to robustly elicit chronic inflammatory responses.8, 9 ATP released via a corresponding ATP receptor contributes to NLRP3 inflammasome activation in myeloid-derived suppressor cell dysfunction, brain damage, and pre-eclampsia.10-12 Furthermore, ATP released from damaged cells induces the formation of pannexin-1-mediated pores, which are permeable to large molecules and subsequent lysis by contributing to plasma membrane depolarization and calcium influx after activation of the P2X7 receptor.13, 14 Typically, in RA, the influx of several extraneous DAMPs in the synovial cavity causes inflammatory mediators (e.g., cytokines, chemokines, lipid mediators, and DAMPs) to be released into the synovial fluid by synovial cells, macrophages, and fibroblasts, which activate chondrocytes that produce metalloproteinases, resulting in aggravation of autoimmune conditions.15 However, to date, the functions of lipids as intermediates involved in isoprenoid metabolism, which undertake the danger signal role, remain largely unexplored.
Recently, a novel concept has been proposed, suggesting how isoprenoid metabolites, specifically farnesyl pyrophosphate (FPP) and geranylgeranyl diphosphate (GGPP), play a pivotal role in cholesterol metabolism and cell signaling, which are used as a novel signal to induce acute cell death.16 DAMPs and mevalonate metabolites are the focus of this review in which we describe their roles in immune responses and immunological pathology. Furthermore, through modulation of the abundance of these molecules, it is vital to understand the mechanisms of inflammation induced by old and new DAMPs to develop novel therapeutic strategies for various autoimmune diseases.
2 DAMPs AND AUTOIMMUNE DISEASES
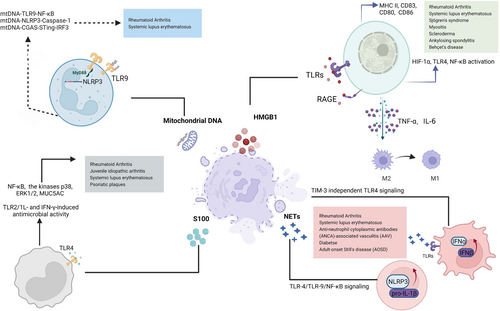
DAMPs are endogenous molecules released by cell death, namely endogenous danger signals, derived from immune cells activated by damaged or necrotic tissue. In addition to activating innate immune cells and responses, DAMPs may directly or indirectly influence adaptive immune responses. Recent studies suggest that certain DAMPs, such as HMGB1, S100 protein, neutrophil extracellular traps (NETs), and mitochondrial DNA (mtDNA), mediate inflammation and are considered to play pathological roles in aggravating inflammatory diseases such as RA and SLE (Figure 1).
2.1 HMGB1
HMGB1 is an enzyme mainly found in the nucleus, which binds to DNA and plays a role in DNA replication, recombinant transcription, and repair. However, after its active secretion or passive release, extracellular HMGB1 usually acts as a DAMP. Because of the lack of a signal peptide, HMGB1 is not folded and modified by the endoplasmic reticulum or Golgi apparatus and is transported to the cell membrane by forming vesicles at the edge of the Golgi apparatus through lysophospholipids, triggering the extracellular release of nuclear HMGB1 in an atypical vesicular manner. Macrophages, monocytes, pituitary cells, and epithelial cells are stimulated by lipopolysaccharides (LPS), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin (IL)-1, HMGB1 can be actively secreted from cells through the abovementioned mechanism, resulting in local or systemic inflammation. HMGB1 can also be released after cell damage or necrosis, but not apoptosis. Additionally, self-tissue cells automatically secrete HMGB1 through nonclassical pathways under external environmental changes, acting on themselves or surrounding tissues to induce cell migration, differentiation, and regeneration. Subsequently, HMGB1 interacts with several immune receptors and sensors, including RAGE, TLR2, TLR4, and TLR9. Consequently, it devastates the pathophysiological process of various autoimmune diseases, including RA, SLE, Sjögren's syndrome, myositis, scleroderma, ankylosing spondylitis, Behçet's disease, and crystal-induced arthritis. Additionally, extracellular HMGB1 is an inflammatory cytokine in endotoxemia, sepsis, and other conditions requiring lipopolysaccharide or TNF to activate macrophages/monocytes and pituitary cells.
In SLE patients, serum HMGB1 and an anti-HMGB1 antibody are abnormally elevated, especially in patients at the active stage and with concomitant renal damage, and are positively correlated with the SLEDAI score, proteinuria, and anti-dsDNA expression. However, a monoclonal anti-HMGB1 antibody does not reduce renal damage in a mouse model of lupus nephritis, suggesting that HMGB1 is a valuable biomarker for SLE rather than a treatment target.17 Similarly, in the case of cutaneous lupus erythematosus, higher levels of HMGB1 have been detected in addition to increased expression of TNF-α and interleukin-1β (IL-1β). Further investigations have shown that cytoplasmic and extracellular HMGB1 exhibit peak clinical activity in experimentally induced cutaneous lupus lesions. HMGB1 robustly activates macrophages after binding to lymphocyte-derived DNA activated by TLR2/TLR4 or by skewed differentiation of M2-like macrophages into M1-like macrophages as evidenced by TNF-α and IL-6 secretion, depending on the origin of the extracellular HMGB1.18-20 Furthermore, HMGB1 and C1q typically organize as a tetramolecular complex architecture on lipid rafts with intricate binding through RAGE and LAIR-1 at monocyte surfaces, driving the polarization of M2-like macrophages under proinflammatory conditions.21 Additionally, HMGB1 promotes CpG-ODN-induced pDC activation and interferon production mediated by RAGE, which further activates B cells to produce autoantibodies mediated by RAGE and TLR9, thereby promoting autoimmune responses.22, 23 Similarly, patients with Sjörgen syndrome show significantly enhanced expression of cytoplasmic and extracellular HMGB1 in mononuclear infiltrates compared with healthy individuals, suggesting that elevated HMGB1 expression robustly aggravates autoimmune disease through a TNF-α and IL-1β proinflammatory loop.24
In the case of RA, HMGB1 is highly expressed in peripheral blood, synovium, and joint tissue. This increases the extension of the inflamed synovium through rapid activation of HIF-1α, TLR4, and NF-κB, resulting in the transcription of multiple proinflammatory genes, and initiating and maintaining an inflammation cascade.25, 26 Collagen-induced arthritis in rodents is significantly alleviated by antagonistic HMGB1-specific antibodies, indicating a crucial role of HMGB1 in arthritis pathogenesis. Furthermore, HMGB1-elevated secretions have been speculated to trigger dendritic cell and macrophage antigen-presenting abilities by upregulating major histocompatibility complex II (MHC II), CD83, CD80, and CD86 expression on macrophage surfaces. Consequently, this leads to induction of dendritic cell maturation, activation of T-helper cells and B lymphocytes, and differentiation of proliferating CD4 + T cells to Th2 and Th17 cells.27-32 Although BD patients have reportedly elevated the expression levels of HMGB1 compared with healthy controls, controversy about the correlation between HMGB-1 expression and the Behçet's disease current activity form and Behçet's syndrome activity score.33, 34 Despite substantial progress in understanding HMGB1, the precise mechanism underlying HMGB1, especially in diseases such as Behçet's disease, remains to be explored.
2.2 S100 protein
Among many chronic inflammatory diseases including RA and psoriatic plaques, S100 proteins function as a danger signal by binding to pattern recognition receptors, such as TLR and RAGE, which activate the immune system.35, 36 S100 proteins are typically released from cell surfaces to regulate target cell proliferation and the activities of macrophages, white blood cells, and inflammatory mediators, which produce cytokines and MMPs. S100 proteins, including calgranulins, S100A8/A9, S100A15, and S100A7, are highly enriched in RA and other associated inflammation.37-42 Typically, the secretion levels of TNF-α, IL-1β, and IL-6 depend on the type of S100 protein released by neutrophils and monocytes, thereby creating a hostile inflammatory environment to aggravate the pathological condition.43 Additionally, target-specific secretion of S100 proteins at the local site of inflammation makes these molecules valuable markers to monitor disease activity. For example, elevated S100 protein expression is a potential biomarker of several autoimmune disorders, including systemic juvenile idiopathic arthritis. Similar speculations have shown that elevated concentrations of S100 proteins typically lead to the occurrence of premultiple sclerosis, suggesting that S100 proteins are an indicator of neuronal damage.44
Recently, neutrophils from RA patients have been demonstrated to synthesize and secrete S100A11 that is externalized via the NETosis pathway, resulting in an inflammatory response by increasing IL-6 and TNF production by neutrophils.45 S100A4 protein triggers the onset of an inflammatory cascade in RA patients through the activation of TLR4, Nuclear factor kappa B (NF-κB), and ERK1/2.46 S100A8 and S100A9 induce TLR4-dependent MUC5AC expression, whereas S100A12 induces TLR4 and RAGE-dependent MUC5AC expression mediated through ERK and NF-κB-dependent pathways, suggesting that they all act as crucial mediators linking airway inflammation caused by neutrophils to mucin hyperproduction.47 These studies revealed the involvement of S100 protein in regulating the innate immune responses in RA. Plasma levels of S100 proteins, including S100A4, S100A8/9, and S100A12, were significantly higher in SLE patients compared with HCs, which revealed the potential utility of S100 proteins as diagnostic or activity-specific biomarkers of SLE. S100a9 has an immunosuppressive function to effectively moderate lupus-like disease development by inhibiting type I interferon production, lymphocyte activation, autoantibody production, and the development of renal disease.48-51
S10012 induces TLR2/1L- and IFN-γ-based antibacterial activity against mycobacterium tuberculosis.52 Furthermore, S100A7—an antimicrobial peptide—is released in a biphasic manner dependent on TLR5 signaling in the early acute phase, whereas it relies on caspase-8 downregulation in chronic and sustained release, both of which are mediated by p38 MAPK/NF-κB/caspase-1/IL-1α.53
2.3 NETs
NETs are fibrous structures of DNA histones, granular proteins, and cytoplasmic proteins produced by bacteria, fungi, viruses, and parasites and released by neutrophils. Anti-neutrophil cytoplasmic antibody-associated vasculitis and RA are diseases in which NETs play a major role.54-56 NETs, which serve as a DAMP complex, are a major source of autoantigens in autoimmune diseases, activating the NLRP3 and complement system, breaking auto-tolerance, and accelerating the innate inflammatory response. Consequently, these NET actions led to activation and stimulation of other immune cells, including B and T cells, which modulate their immune functions. SLE patients and a mouse model with lupus-prone skin and kidneys have elevated levels of NET deposition. Additionally, NETs typically released by LDGs of SLE patients harbor augmented levels of autoantigens, including LL-37 and IL-17, which are considered essential hallmarks in aggravating the disease pathogenesis.57
NET formation requires activation of neutrophils and ROS production by NADPH oxidase. NET-induced activation of neutrophils typically depends on phosphorylation of Akt, ERK1/2, and p38 in autoimmune disorders.58 In SLE and vasculitis, NET stimulation further activates plasmacytoid dendritic cells via TLR9, TLR7, and TIM-3-independent TLR4 signaling, which promotes type I IFN expression and drives autoimmune pathology in animal models.59-61 Several studies have explored the possibility of modulating NETs to treat RA. For example, blocking FcαRI by antibodies decreases NET formation in isolated neutrophils after exposure to immunoglobulin A (IgA) immune complexes, suggesting that such an approach reduces neutrophil activation and prevents NETs from developing in RA patients. In adult-onset Still's disease, overproduced NETs trigger NLRP3-mediated inflammasome activation and IL-1β release in macrophages. Additionally, NETs induce NLRP3 and pro-IL-β through the TLR-4/TLR-9/NF-κB signaling pathway, generating reactive oxygen species, which concomitantly enable interaction between NLRP3 and thioredoxin-interacting protein, leading to inflammasome activation.62, 63 Furthermore, complement proteins stimulate NET formation, which releases C3a and C5a that have proinflammatory properties including leukocyte-endothelial cell and platelet recruitment and activation.64 NETs significantly enhance autoantibody production in naïve B cells, which is a hallmark of SLE devastation. Similar studies have shown that NETs lead to immunoglobulin G (IgG) upregulation in memory B cells, suggesting that NETs cause lupus nephritis and SLE through the persistence of IgG2c.65 Furthermore, NETs activate autoreactive B cells by secreting proinflammatory chemokine IL-8 and B cell-activating cytokines such as B cell activating factor, inducing CD4 + T cells to differentiate into Th1 and Th17 cells, and releasing IL-12 and TNF-α.60, 66
2.4 Mitochondrial DNA
Mitochondria contain genetic material called mtDNA. Alterations in the mtDNA copy number (CN) have been reported to be a hallmark of autoimmune and inflammatory diseases.15 There is strong evidence that a decreased leucocyte mtDNA CN contributes to the progression and development of SLE and secretion of proinflammatory cytokines, and may contribute to the persistence of low-grade inflammation in SLE. mtDNA significantly increases after femoral fracture in the synovial fluid of RA patients, but not in healthy individuals.67-71 Furthermore, mtDNA activates polymorphonuclear neutrophils under the interaction of CpG/TLR-9 to cause aseptic systemic inflammatory response syndrome.72 Numerous studies have demonstrated that oxidized mtDNA significantly increases TNF production in mouse immune cells, which significantly aggravates arthritis symptoms when administered to mice. Similar studies have confirmed the inflammatory nature of mtDNA by demonstrating that mtDNA directly activates various pattern recognition receptors to trigger an inflammatory response. By injecting mice with mtDNA, neutrophils are activated via p38 MAPK to produce excess proinflammatory cytokines, including IL-1β, IL-6, IL-8, MMP-8, and TNF-α, and induce inflammation.73, 74
To date, mtDNA functions in adaptive immunity have been rarely reported. T-cell receptor (TCR) activation triggers mtDNA production. However, abnormal mtDNA production significantly contributes to impaired adaptive immune responses such as B-cell lymphoma development.75-79 Comparatively, the effect of mtDNA on innate immunity has been intensively studied, showing that mtDNA also activates certain intrinsic signaling cascade events, such as NLRP3 inflammasome, TLR-9, and cyclic GMP–AMP synthase-stimulator of interferon genes (cGAS-STING) signaling pathways, to induce innate immune responses against pathogenic microorganisms. MtDNA interactions with TLR-9 mediate the myeloid differentiation factor 88 (MyD88)-dependent signaling pathway, which activates the NF-κB pathway and promotes the expression of inflammatory factors.80
MyD88 and TRIF adaptors, which are triggered by TLR signals, enhance transcription of CMPK2, a crucial enzyme that further provides deoxyribonucleotides to induce mtDNA synthesis. CMPK2-dependent synthesis of mtDNA is perpetually required to produce cytosolic oxidized mtDNA fragments that are associated with and necessary for the activation of the NLRP3 inflammasome.81 Caspase-1, as an important effector protein of the NLRP3 inflammasome, activates to cleave precursors of proinflammatory factors IL-1β and IL-18 to initiate an immune response. Conversely, activated caspase-1 also induces pyroptosis of innate immune cells.82, 83 Furthermore, mtDNA, which escapes from damaged mitochondria to the cytosol, is recognized by cGAMP and STING, which initiates activation of TBK1 and the transcription of several inflammatory genes, including Interferon regulatory factor (IRF3), interferon, and IRF7.84-86 In summary, mtDNA is involved in various innate immune pathways, including the mtDNA-TLR9-NF-κB axis, mtDNA-NLRP3-Caspase-1 pathway, and mtDNA-CGAS-STING-IRF3 signal transduction pathways, which lead to various innate immune responses. However, its role in adaptive immune cells remains to be explored.
3 MEVALONATE PATHWAY AND AUTOIMMUNE DISEASES
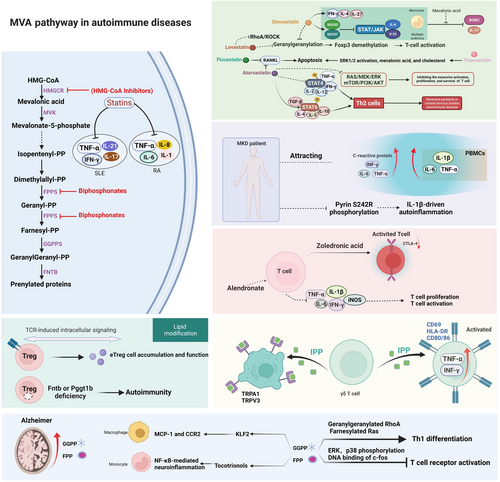
Isoprenoids in the mevalonate pathway, including cholesterol, dolichol, heme-A, isopentenyl tRNA, and ubiquinone, are vital to maintaining organic metabolic homeostasis. In recent years, the mevalonate pathway has become a hot topic with many experimental and clinical studies, indicating additional effects of the mevalonate pathway beyond cholesterol regulation, particularly anti-inflammatory and immunomodulatory effects.87 Several immune disorders are caused by abnormal mevalonate pathway functions, such as SLE and RA (Figure 2).88-90
3.1 Statins
Statins are typically recognized to reduce mevalonate (MVA) pathway expression by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A, the potential enzyme of mevalonate pathways, which has proven clinical significance in certain autoimmune pathologies such as antiphospholipid syndrome, SLE, and RA.91, 92 It is still controversial whether statins are capable of reducing SLE activity. However, statins are widely accepted to induce substantial downregulation of certain proinflammatory cytokines, especially IL-17, IL-21, IFN-γ, and TNF-α, in SLE patients, perhaps through in vitro blockade of the Rho-associated signaling cascade and downstream HMG-CoA metabolites. Numerous studies have documented the immunomodulatory potential of these compounds in experimental autoimmune encephalitis (EAE) models independently of their lipid-lowering properties. These properties include inhibiting various processes, including myelin–antigen presentation, T-cell activation and differentiation into proinflammatory T-cell lineages, and leukocyte agglomeration into the central nervous system. Moreover, several studies have shown that statins typically inhibit MHC-II expression and the expression of several costimulatory molecules (e.g., CD40, CD80, and CD86) in various cell types, which prevents antigen presentation to CD4 + T cells by inhibiting their expression.93 Lipid parameters deteriorate in RA patients, specifically in those with a high activation index. Therefore, statins are routinely prescribed for RA patients as part of their treatment regimen. Moreover, statins inhibit disease progression by suppressing several inflammatory factors, including TNF-α, IL-1, IL-6, and IL-8.94, 95 Additionally, fluvastatin and atorvastatin induce apoptosis and suppress osteoclastogenesis in RA synoviocytes by decreasing RANKL expression.96, 97 Lovastatin protects against experimental autoimmune encephalomyelitis through the blockade of mevalonate pathways, particularly inhibition of protein geranylgeranylation and RhoA/ROCK pathways.98-101 Atorvastatin promotes the growth of myeloid-derived suppressor cells, suppressing T-cell responses by producing nitric oxide, which is reversed with mevalonate acid.102 In central nervous system-interlinked autoimmune disease, atorvastatin induces STAT6 phosphorylation, secretion of Th2 cytokines, and transforming growth factor-beta (TGF-β), and inhibits STAT4 phosphorylation and secretion of Th1 cytokines to promote differentiation of Th0 cells into Th2 cells.103, 104 Statins induce the expression of several inflammatory markers in cultured human endothelial cells, such as leukocyte adhesion molecules, tissue factor, and monocyte chemoattractant protein-1, and increase the degree of inflammatory activation.105
Statins were recently shown to possess anti-inflammatory activities. The mechanisms of statins in regulating immune diseases are well studied and they are potential drug-repositioning candidates for the treatment of T-cell-mediated autoimmune diseases.106 Simvastatin blocks protein geranylgeranylation, which enhances arrested endosomal maturation. This results in long-lasting retention of antigens, enhanced antigen presentation, and T-cell activation, which are also involved in the demethylation of the Foxp3 promoter.107, 108 In the case of immune thrombocytopenia, atorvastatin potentially restores CD4 + T cell functions by inhibiting abnormal activation, proliferation, and survival of CD4 + T cells through coordination of rat sarcoma/mitogen-activated protein kinase kinase/extracellular regulated protein kinases (RAS/MEK/ERK) and mammalian target of rapamycin/phosphatidylinositol-3-kinase/protein kinase B (mTOR/PI3K/AKT) pathways.109 Furthermore, atorvastatin inhibits antigen-specific responses and attenuates T-cell proliferation independent of MHC class II and lymphocyte function-associated antigen-1 upon direct TCR engagement.110 Regulation of the STAT/JAK signal transduction pathway in CD4 + T cells and expression of IL-6 and IL-23 genes in monocytes induced by simvastatin is negatively regulated by mevalonic acid, which is reversed by simvastatin.111 Pitavastatin inhibits T- cell proliferation by activating the ERK1/2 pathway and inducing apoptosis through mevalonic acid and cholesterol, which causes apoptosis.112 In addition to inhibiting mevalonate metabolism, pitavastatin and atorvastatin suppress autoimmune neuroinflammation by controlling the balances of Treg/Th17 cells and Th1/Th2 cells.113, 114
Apart from their effect on T cells, statins, including fluvastatin, simvastatin, rosuvastatin, and atorvastatin, modulate the monocyte phenotype and activation by lowering the levels of proinflammatory cytokines and oxidized low-density lipoproteins.115-120 By activating IGF1-R and mTOR in inflammatory pathways, statins prevent mevalonate from inducing trained immunity in human monocytes.121 In summary, statins function as immunomodulators in a mevalonate-dependent manner by regulating T-cell activation, monocyte activation, and coenzyme Q.
3.2 Mevalonate kinase
Mevalonate kinase dysfunction results in autoinflammatory symptoms. Mevalonate kinase deficiency (MKD) results from impaired isoprenoid biosynthesis, causing autoinflammation. To date, there is an incomplete understanding of MKD pathogenesis. However, some studies have reported that reducing the amount of the downstream mevalonate-derived intermediate GGP specifically triggers caspase-1 activation and IL-1 secretion.122 MKD patients typically experience periodic episodes of systemic inflammation marked by enhanced secretion of inflammatory mediators. As a result of attacks, MKD patients display high levels of acute-phase proteins (C-reactive protein) as well as IL-6, TNF-α, and INF-γ. Additionally, compared with the resting state, peripheral blood mononuclear cells from MKD patients produce IL-1β, IL-6, and TNF-α after stimulation with activating ligands. Mevalonate kinase is an indispensable enzyme for the mevalonate pathway, and its deficiency influences the production of downstream isoprene products. Reversion of mevalonate acid accumulation via the HMG-CoA reductase inhibitor lovastatin induces distinct upregulation of IL-1β by healthy peripheral blood mononuclear cells, whereas isoprenoid farnesyl reverses the cytokine oversecretion in MKD patients. These findings demonstrate that isoprenoid product deficiency appears to be the dominant reason for inflammatory hyper-responsiveness in MKD patients.
In RNA-seq analyses, monocytes from mevalonate kinase-deficient patients exhibited a well-guided immune phenotype, suggesting that statins reduce symptoms in these patients.123 A metabolic defect in the mevalonate pathway is also correlated to hereditary periodic fever syndromes, which indicates the importance of these proteins in regulating inflammation.124 Furthermore, MKD causes the loss of pyrin phosphorylation in S242R, leading to IL-1β-guided auto-inflammatory responses as exemplified by PAAND, a pyrin-associated auto-inflammatory condition.125 Despite the lack of clarity surrounding the exact pathogenesis of MKD, increasing evidence suggests that protein prenylation dysfunction contributes to innate immunity activation and systemic inflammation.
3.3 Biphosphonates
N-BP molecules downregulate the expression of farnesyl pyrophosphate synthase (FPPS), a potent enzymatic target. These activities lead to the accumulation of several phosphonate substrates. For example, dimethylallyl diphosphonate and isopentenyl diphosphonate (IPP) dramatically enhances T-cell proliferation and activation, resulting in enhanced production of proinflammatory cytokines such as TNF-α and interleukin-6.126 Some studies have shown that non-amino bisphosphonates typically inhibit the secretion of several proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, by activated macrophages. Amino-BPP typically regulates histidine decarboxylase, which triggers the aggregation of granulocytes and macrophages, thereby exacerbating experimental autoimmune arthritis.127 Moreover, nitrogen-containing BPs (N-BPs) such as zoledronic acid have been studied for their immunomodulatory functions, including the induction of proinflammatory cytokines, activation of T cells, and downregulating cytotoxic T-lymphocyte antigen-4.128-130 Alendronate and nitrogen-containing bisphosphonates substantially reduce the inflammatory cascade in the spinal cord after induction of experimental autoimmune encephalomyelitis, thereby suppressing the proximal assembly of CD68-positive inflammatory cells. In turn, this leads to the suppression of various inflammatory mediators such as IFN-γ, TNF-α, and inducible nitric oxide synthase.131
3.4 IPP
In juvenile idiopathic arthritis, IPP functions as a specific potent antigen for Vγ9Jγ1.2(+) T cells and increases secretion of IFN-γ and TNF-α in synovial fluid and peripheral blood Vγ9(+) T cells, which produces CD69(+) activated Vγ9(+) T cells.132 Additionally, recovery and magnitude of the disease severity index are interlinked to the proportion of SF Vγ9 + T cells and the extent to which Vγ9 + T cells respond to IPP stimulation. Furthermore, inhibiting FPPS in vitro by zoledronate or short hairpin RNA targeting FPPS accumulates IPP, converting memory T cells to the phenotypic characteristics of antigen-presenting cells, such as high HLA-DR expression and CD80/86 expression.133-136 Furthermore, FPPS knockdown cells activate Vγ9Vδ2 T cells, thereby upregulating CD69 and CD107a expression and triggering IFN-γ secretion.135 Similar to FPP, IPP as a blocker of TRPV3 and TRPA1 inhibits the elevation of H+ gated [Ca2+], which is a potential therapeutic target of white matter ischemia.137, 138 A substantial increase in γδ T cells in synovial fluid from arthritis patients is amplified by ethanol and isopentenyl pyrophosphate, indicating that ethanol concentrations, which further enhance IPP-stimulated expansion of γδ T cells. Moreover, these expanded γδ T cells might target fibroblast-like synoviocytes, explaining the substantial protective effects of ethanol against RA and other autoimmune disorders.139
3.5 FPP and GGPP
Inhibition of protein geranylgeranylation interferes specifically with CD40-dependent B-cell activation and consequently reduces T-cell-guided immune responses.140 In response to TCR activation, GGPP promotes cell proliferation, but its precursor, specifically farnesyl-PP, may reduce phosphorylation of p38 and ERK to regulate c-Fos-oriented DNA binding.113 Rheumatoid fibroblast-like synoviocytes secreting pentraxin 3 and monocyte chemoattractant protein-1 respond to geranylgeranyl-pyrophosphate via a Rho-dependent mechanism.141 Statins modulate the immune system because they deplete isoprenoids (e.g., FPP and GGPP) instead of cholesterol in an EAE model of multiple sclerosis.142 Furthermore, LKB1 modulates STAT5-positioned signaling events mediated through IL-2 activities, which supports the functional stability of Treg cells by activation of the mevalonate pathway via GGPP.143 GGPP, the intermediate metabolite of the mevalonate pathway, and Rho GTPase loading prevent HMGCR-deficient lymphocyte death and promote T-cell survival and migration, indicating that prenylation is fundamentally required to sustain T-cell migration and survival.144, 145 In autoimmune SLE, geranylgeranyl pyrophosphate reverses IFN-α production in plasmacytoid dendritic cells, the main molecular and cellular components of the disease.146
FPP and GGPP accumulate in AD patients and are involved in NF-κB-mediated neuroinflammation via regulation by tocotrienols, which affects chemokine expression in mononuclear cells.147, 148 FPP and GGPP control the expression of inflammation genes, such as C-C motif chemokine receptor 2 and membrane cofactor protein-1, by inducing kruppel like factor 2-guided activities of THP1 macrophages.149 Furthermore, exogenous GGPP significantly mediates IL-6 and IL-8 associated inflammation through GTPase translocation when applied to LPS-challenged HUVECs, but not FPP.150 From these perspectives, FPP/GGPP-mediated immune responses are largely indirect.
Recently, isoprenoids, such as FPP and GPP, were reported to act as pivotal intermediates for cholesterol homeostasis and to react as a danger signal by interacting with receptor transient receptor potential (TRP) cation channels, resulting in an influx of calcium into the cell. Subsequently, acute cell death occurs independently of downstream mevalonate metabolites and conventional cell death pathways. Additionally, after FPP accumulation in the brain, the FPP and TRP danger signal axis play a predominant role in ischemic injury.16 While none of the previous studies described the relationship between FPP/GGPP and TRP directly, FPP has been reported to be a specific activator of the TRPV3 channel.151 FPP not only increases intracellular calcium levels in DRG neurons but also serves as the endogenous ligand for GPR92, which colocalizes with TRPV1 in abundance, indicating a possible relationship between FPP and TRPV1.152 An upstream metabolite of mevalonate metabolism, IPP, has also been reported to regulate TRPA1 and TRPV3 activation.138, 153 Furthermore, dimethylallyl diphosphonate produced in this pathway acts as a TRPV4 activator.154 Thus, it is clear that multiple components of the mevalonate pathway interact with TRPs in various manners. These data raise the issue of which roles the mevalonate pathway and TRP family play in the immune system. Although novel DAMPs, such as FPP and GGPP, increase our understanding, there are still many issues to be resolved.
4 PERSPECTIVES
Numerous lipid products, such as oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-glutaryl phosphatidylcholine, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine, and peroxidation lipid products, including oxidized phosphatidylcholine, cardiolipin, and phosphatidylserine, have been identified as DAMPs.155-157 Lipid-derived metabolites FPP and GGPP are unprecedented novel discoveries. The major roles of DAMPs in autoimmune diseases are obvious, but only a fraction of DAMPs have been studied extensively to reveal clear signaling mechanisms. It is essential to identify other DAMPs and clarify their functions. Although DAMPs appear to play an influential role in the progression of inflammatory diseases, there remain some issues. There is limited information available about the pattern recognition receptors (PRRs) of DAMPs. To develop specific inhibitory molecules that suppress the interaction of DAMPs and PRRs and regulate inflammation, it will be crucial to define how these molecules interact. Deciphering the complex interactions among immune sensors, cytokines, DAMPs, and cellular death may be important to find new therapeutics for autoimmune diseases. In addition to assessing the underlying signaling cascade, DAMPs would be useful biomarkers to determine the prognosis of autoimmune diseases and several inflammatory disorders. Furthermore, it is crucial to uncover the roles of FPP, GGPP, and other DAMPs in the context of assessing their relative contributions to targeting and treating autoimmune and hypersensitivity disorders. This review is the first to link DAMPs and MVA pathways to uncover their crucial roles in immune response and autoimmune diseases. In particular, novel DAMPs FPP and GGPP are synthetic products of cholesterol metabolism, which are also drug discovery targets. As we uncover new insights about the roles of FPP and GGPP in various diseases, drug targeting of FPP and GGPP may become an increasingly important area of investigation, which may lead to novel and effective treatments for autoimmune diseases.
AUTHOR CONTRIBUTIONS
All the authors have contributed to this review. Na Kang drafted the manuscript. Na Kang and Xiaohang Liu drew the figures. Kabeer Haneef revised the manuscript. Wanli Liu designed, revised, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors acknowledge support from the Tsinghua University Spring Breeze Fund, Center for Life Sciences, and Institute for Immunology, Tsinghua University.
CONFLICT OF INTEREST
Wanli Liu is Editorial Board member of Rheumatology & Autoimmunity. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journals standard procedures, with peer-review handled independently of these Editorial Board and Editorial Office members and their research groups.
ETHICS STATEMENT
Ethical approval is not applicable to this article.
Open Research
DATA AVAILABILITY STATEMENT
Data availability statement is not applicable to this article as no new data were created or analyzed in this study.