Mechanism-driven strategies for prevention of rheumatoid arthritis
V. Michael Holers, Kristine A. Kuhn, and M. Kristen Demoruelle are co-first authors.
Abstract
In seropositive rheumatoid arthritis (RA), the onset of clinically apparent inflammatory arthritis (IA) is typically preceded by a prolonged period of autoimmunity manifest by the presence of circulating autoantibodies that can include antibodies to citrullinated protein antigens (ACPA) and rheumatoid factor. This period before clinical IA can be designated preclinical RA in those individuals who have progressed to a clinical diagnosis of RA, and an “at-risk” status in those who have not developed IA but exhibit predictive biomarkers of future clinical RA. With the goal of developing RA prevention strategies, studies have characterized immune phenotypes of preclinical RA/at-risk states. From these studies, a model has emerged wherein mucosal inflammation and dysbiosis may lead first to local autoantibody production, which should normally be transient, but instead is followed by a systemic spread of the autoimmunity as manifested by serum autoantibody elevations, ultimately driving the development of clinically identified joint inflammation. This model can be envisioned as the progression of disease development through serial “checkpoints” that in principle should constrain or resolve autoimmunity; however, instead, the checkpoints “fail” and clinical RA develops. Herein we review the immune processes that are likely to be present at each step and the potential therapeutic strategies that could be envisioned to delay, diminish, halt, or even reverse the progression to clinical RA. Notably, these prevention strategies could utilize existing therapies approved for clinical RA, therapies approved for other diseases that target relevant pathways in the preclinical/at-risk state, or approaches that target novel pathways.
Key points
Significant findings of the study:
-
Seropositive rheumatoid arthritis (RA) evolves over years before the development of clinically apparent inflammatory arthritis.
-
Emerging data support a model wherein mucosal inflammation precedes the development of systemic autoimmunity, followed by the development of synovitis.
-
The development of RA may entail multiple “endotypes” across individuals who ultimately develop clinical disease.
What this study adds:
-
Mechanisms that should normally constrain RA-related autoimmunity at specific checkpoints, including mucosal and systemic processes that limit autoimmunity, appear to fail during the preclinical development of RA.
-
By understanding and targeting these failed mechanisms, it may be possible to prevent, or minimally ameliorate, the development of classified RA.
1 INTRODUCTION TO THE PROBLEM
Rheumatoid arthritis (RA) is a chronic autoimmune disease, which clinically manifests as both a locally destructive and systemic illness.1 RA exhibits a prevalence of ~0.8%–1.0% in the general population, and a ~3–5-fold increase in first-degree relatives (FDRs).2 In addition to genes associated with the presence of clinically apparent RA, including HLA-DRB1 alleles containing the shared epitope (SE),3-5 PTPN22,6 and >100 additional non-HLA loci,7, 8 there are environmental factors that are both risk-elevating as well as protective for the development of RA.2, 9
Patients receive a clinical diagnosis of RA when inflammatory arthritis (IA) is present, which is characterized by objective swelling and tenderness, although there are also typical local joint-based signs and symptoms, including stiffness and arthralgias (defined for studies as joint/musculoskeletal discomfort with phenotypic characteristics concerning for RA).10, 11 Furthermore, while a diagnosis of RA is typically made through a clinical determination by a healthcare provider, individuals may also fulfill established classification criteria, for example, 1987 ACR or 2010 ACR/EULAR classification criteria.12, 13 The majority (~70%–80%) of clinical RA is termed “seropositive” because patients exhibit blood elevations of anticyclic citrullinated peptide/protein antibodies (ACPA) and/or rheumatoid factors (RFs)1 as well as other autoantibody systems, including antibodies to modified protein antigens (AMPA) (e.g., anticarbamylated antibodies).14 Systemic inflammation also occurs as evidenced by elevated serum cytokines and blood mononuclear cell abnormalities. In addition, there may be extra-articular features, including symptoms (e.g., fatigue) as well as tissue injuries, such as nodules, vasculitis, and lung fibrosis. Despite recent therapeutic advances, RA can still lead to disability, increased mortality, and substantial personal and societal costs.15
Although the initiation of clinically apparent arthritis was long considered the onset of disease, and indeed is still required for a diagnosis of clinical RA, it is now known that there is a prolonged period in seropositive RA before the onset of arthritis that is characterized by the presence of specific RA-related autoimmunity (Figure 1) detected by the serologic presence of ACPA, AMPA, and/or RF. The autoantibody elevations are on average 3–5 years before the onset of clinical IA/RA, although some studies have documented autoantibody elevation up to ~20 years prior.16-18 This period of autoimmunity and inflammation can be defined retrospectively as the “preclinical” period in subjects who eventually develop a clinical diagnosis of RA, and an “at-risk” status in a population without arthritis studied through cross-sectional or longitudinal means and identified because of familial or ethnic-/racial-based risk factors (e.g., FDRs or populations with high-risk of incident RA, such as indigenous populations in the Americas), or biomarker-based risk assessment.19
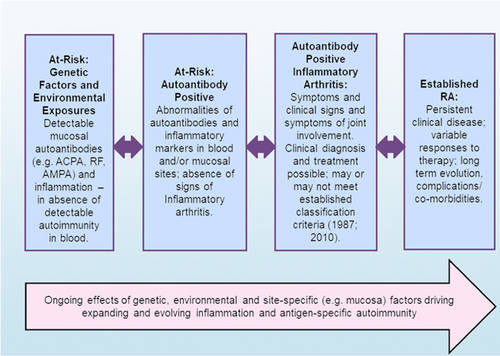
During this period there can be asymptomatic states, as well as periods of articular symptoms (e.g., arthralgia) in the absence of clinical IA. In addition, other changes to autoantibodies include avidity maturation,20 incorporations of amino acids encoding glycosylation sites,21 and an increase in epitope spreading resulting in recognition of a wider variety of targeted citrullinated peptide antigens.22, 23 Notably, there also appear to be “endotypes” in preclinical RA that can be identifiable by patterns of autoantibody changes over time. In particular, one study demonstrated distinct subsets of individuals in preclinical RA characterized by duration and type of autoantibody elevations, suggesting that there is no one set pattern of RA development.24 Furthermore, along with autoantibodies, there are changes in systemic levels of cytokines/chemokines,23, 25 alterations of innate immune responses in monocytes,26 and altered T-cell subsets.27
With regard to the etiology of RA and in particular, the processes that trigger and propagate autoimmunity and inflammation from preclinical RA to a transition to clinical RA, data from an increasing number of studies strongly suggest that the processes begin as subclinical inflammation and/or dysbiosis at mucosal sites. These early processes can be associated with local autoantibody generation, and evolve over a prolonged period of time in a subset of individuals to systemic autoantibodies and then clinically apparent RA28 (Figure 2). Evidence suggests that local mucosal ACPA and RF production may be present transiently in many individuals, is associated with neutrophil extracellular trap (NET) formation, and normally resolves as the inflammatory process subsides.29 As immunoglobulin A (IgA) itself is an isotype that enhances recognition and clearance of microbiota and suppresses inflammation by enhancing noninflammatory removal of cellular and bacterial debris,30 the development of transient locally produced ACPA can be viewed as protective, likely by decreasing citrullinated antigen exposure and potential loss of tolerance to those antigens, or alternately by impairing NET formation.31 However, chronic local mucosal expression of this IgA and IgG autoantibody, in association with continued inflammation and local RF, can be envisioned as failed resolution.
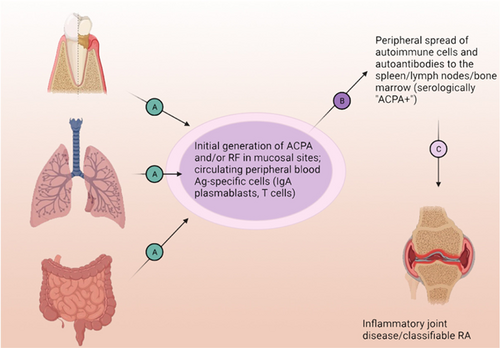
This mucosal hypothesis of preclinical RA initiation and evolution32 posits that a subset of individuals evolves from this normally localized process into the development of systemic ACPA as well as likely AMPA and RF production. Following the serologic ACPA+ at-risk period, individuals can transition to develop IA through a number of likely mechanisms, including traditional immune complex-mediated and synovial cell activation processes.33 The disease then becomes chronic and is perpetuated by cytokine production and other immune mechanisms characteristic of fixed inflammation and tissue remodeling.33
Conceptually, this evolving process can be envisioned as a series of steps wherein normal checkpoints that control and correct the aberrant immune responses are overcome (Figure 3). In particular, the failure of checkpoints could reduce the normal clearance of potential microbial pathogens or pathobionts and/or fail to maintain the mucosal barrier and resolution of local innate immune cell-driven inflammation. In addition, the checkpoints could fail to restrict or resolve the adaptive ACPA and RF immune response to the mucosal site, and particularly fail to suppress pathogenic T and IgG B cell autoimmune responses that are associated with epitope spreading and affinity maturation to systemic sites. Finally, there may be a failure of the resolution of transient joint inflammation that with perpetuation becomes a fixed synovial inflammatory process that is difficult to control and may be impossible to reverse once tissue damage develops.
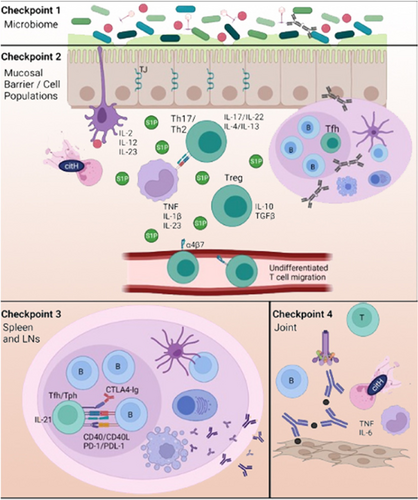
Within this conceptual approach, new opportunities exist to prevent or modify the evolution of disease processes at these checkpoints before the development of clinical IA/RA. This is an appropriate goal because, although major advances in treatment have been made, only a subset of patients with classified RA respond to each therapeutic, and none are consistently curative.34
Following is a discussion of various potential therapeutic strategies, using as a model the progression of the disease as outlined in Figure 3 and a subset of the data that underlie each process. The therapeutic examples presented are not meant to be comprehensive but rather illustrative of strategic approaches at each step. Notably, these stages are to some extent an artificial distinction, and various therapeutic strategies could work at multiple sites and multiple stages. Nevertheless, this approach provides a framework under which mechanism-based prevention strategies can be tailored to and utilized at different stages of disease evolution. This approach also emphasizes the importance in prevention of “stage-specific” research strategies that define the essential drivers of the processes at each checkpoint and approach their understanding as relevant to identifying therapeutic targets. Importantly, these strategies may go well beyond the “synovitis-modifying” drugs that are approved for clinical RA and that are also used in current prevention trials in RA, with the goal to more effectively target pathways that are relevant in preclinical RA.
2 STRATEGIES FOCUSED ON CHECKPOINT 1: COMPOSITION AND HOMEOSTASIS OF THE MUCOSAL MICROBIOME
2.1 Rationale
The human microbiome at mucosal sites has multiple components, including bacteria, bacteriophages, mycoplasma, viruses, and fungi, any of which individually or in combination, especially in the setting of other environmental (e.g., tobacco smoke) and host (e.g., genetic) factors, could be important in the development of autoimmune diseases.35-37 Following decades of speculation and the intermittent use of directed therapeutic approaches to address the potential role of focal infection in the development of RA,38 the majority of recent studies are focused on bacteria and viruses and have utilized serologic reactivity and/or DNA/RNA sequencing to characterize the composition of organisms from the periodontium, mouth, gut, and lung in subsets of individuals at different stages of disease development.
To that end, initial serologic cross-reactivity studies in patients with established RA suggested a molecular mimicry mechanism (Table 1) based on the cross-reactivity of antibodies that recognized both Proteus species antigens as well as the SE sequence of HLA-DR1/4 and collagen type XI.39 Subsequently, Porphyromonas gingivalis was proposed to play a major role by linking the clinical association of periodontitis and RA through pathogenic mechanisms mediated by this organism, including both molecular mimicry and the activity of an endogenous peptidyl-arginine deiminase (PAD) that could modify both P. gingivalis proteins, such as enolase as well as host proteins.40 Challenging that hypothesis to some extent were studies of the periodontal microbiome in new-onset RA, which identified expansions of Prevotella and Leptotrichia taxa and an association of P. gingivalis only with the severity of periodontal inflammation but not RA.41 However, further evaluation of the periodontium in individuals at-risk for future RA based on an ACPA+ status demonstrated a relative abundance of P. gingivalis,42 and thus the role of this organism remains undefined and requires additional study.
|
Studies of another strain associated with severe periodontitis, Aggregatibacter actinomycetemcomitans, suggest a different mechanism whereby LtxA, a bacterial protein that can induce cytolysis, could induce intracellular hypercitrullination through activation of endogenous PADs and the generation of a large number of citrullinated targets of ACPA that could be released into the local environment, either promoting the loss of tolerance or enhancing inflammation by recognition of these targets in tissues such as the synovium by ACPA.38 Although an intriguing candidate to expand inflammation in classified RA, neither discovery-based studies of at-risk42 nor new-onset RA41 identified this organism as expanded in the periodontium at these stages of disease development.
Studies of the oral microbiome have also identified Streptococcaceae as major contributors to an enhanced dysbiosis and periodontal inflammation score in at-risk FDRs, and that streptococcal cell walls from unique isolates induced joint inflammation in arthritis-susceptible ZAP-70-mutant SKG mice.43 Other studies of the oral microbiome of ACPA+ at-risk individuals have identified dysbiosis with different characteristics and taxa alterations.44
In addition to the periodontium and mouth, many studies have been performed on the gut microbiome in patients with RA. Perhaps the most prominent candidate to arise from this approach is Prevotella copri, which was initially described as expanded in a subset of seropositive new-onset RA patients.45 Subsequent studies revealed an increase in the relative proportion of this strain in a healthy population that are carriers of an RA risk SE HLA allele,46 and also individuals who are defined as at-risk based on the presence of ACPA+, RF+, or FDR status.47 Notably, T- and B-cell reactivity to specific P. copri antigens has been found in subsets of individuals with active RA,48 and recent collaborative studies in the Studies of the Etiology of Rheumatoid Arthritis (SERA) cohort have identified signals of enhanced antibody responses to P. copri in a subset of at-risk individuals (Seifert, Deane, Steere, and Holers, submitted).
Other studies of fecal samples from patients with RA have identified additional alterations of the gut microbiome, for instance, a lower alpha-diversity index associated with the expansion of the bacterial genera Bacteroides and Escherichia–Shigella and decreases in Lactobacillus, Alloprevotella, Enterobacter, and Odoribacter.49 In another study, expansion of rare Collinsella genera was reported to correlate with the production of interleukin-17A (IL-17A), and this strain altered gut permeability and increased disease severity in experimental arthritis.50 Finally, studies of the lung microbiome have identified in the bronchoalveolar lavage fluid of patients with new-onset RA a diminished diversity and abundance of the microbiota in a manner similar to sarcoidosis patients.51 Notably, the dysbiosis was characterized by the reduction of Actynomyces and Burkhordelia as well as the periodontal taxa Treponema, Prevotella, and Porphyromonas. In contrast, the genus Pseudonocardia was over-represented and associated with increased disease activity.
In addition to bacteria, multiple studies have explored potential relationships between viruses and RA pathogenesis, with a current resurgence of focus on Epstein–Barr virus (EBV) given a long background of EBV-specific immune dysregulation in patients.52, 53 In particular, data have emerged demonstrating that the EBV encoded transactivator EBNA-2 interacts with a large number of risk alleles associated with autoimmune diseases, including RA.54 This finding provides a different type of link between disease risk loci and the effects of EBV infection. With regard to individuals in the preclinical period, antibody responses against EBV, especially those which are indicative of increased numbers of viral reactivation cycles55 or are directed to citrullinated EBV antigens,56 are elevated in this period of RA.
Of note, bacteriophages, which are viruses that infect bacteria, have also been implicated to have a pathogenic role in RA development through recent studies in the SERA population, demonstrating that at-risk individuals exhibit intestinal phage compositions that are dominated by those associated with Streptococcaceae, Bacteroidaceae, and Lachnospiraceae.57 Additionally, these phages encode unique families of auxiliary metabolic genes in a manner, which suggests that they could influence the metabolic and immunomodulatory capability of bacteria and indirectly the human immune response.
Importantly, as there are likely many “endotypes” of RA development, it is possible that these studies are all relevant and that multiple pathways, mucosal sites, and organisms can lead ultimately to clinical RA in a manner depending on the different environment and genetics of the populations and individuals studied.
2.2 Potential therapeutic strategies
There are several mechanisms by which the microbiome could influence the development of RA, including cross-reactivity/molecular mimicry, alteration of host antigens, mediating or promoting metabolomics changes that affect host cells, chronic inflammation leading to a milieu promoting continued ACPA production and autoimmunity, carriage of pathogenic bacteriophages, and acting as a cofactor for other environmental factors such as tobacco smoke (Table 1). Because of this, therapeutic strategies to modify the microbiota could take multiple forms, in large part dependent upon the exact mechanism(s) by which RA development is promoted and whether a specific strain would be targeted.58 Depending on the clinical setting and desired outcomes, approaches include first- and second-generation probiotics, prebiotics, narrow-spectrum antibiotic treatment, and fecal microbiome transplantation. Newer approaches incorporate the potential use of bacteriophages to introduce strain-specific modifications,59 antibodies given either by the oral route,60 or as a secretory IgA biologic therapeutic delivered through traditional systemic means.30 In addition, the use of a commensal bacterial strain, Prevotella histicola, ameliorated arthritis in a murine model of RA, collagen-induced arthritis, through the generation of myeloid suppressors and expansion of gut-localized Treg cells.61 Finally, modulation of diet- and gut-derived and endogenous metabolites is a promising new approach that allows one to utilize pro- and anti-inflammatory metabolite and lipid-catalyzed pathways that have recently been identified and which work through specific receptors and signal transduction pathways.62 To that end, intriguing results have suggested that levels of anti- and proinflammatory lipids are associated with differential progression to IA in at-risk individuals.63, 64
3 STRATEGIES FOCUSED ON CHECKPOINT 2: MAINTENANCE OF THE MUCOSAL BARRIER AND THE ASSOCIATED IMMUNE CELL POPULATION HOMEOSTASIS
3.1 Rationale
A major means by which dysbiosis or mucosal inflammation could influence the development of RA is through disruption of mucosal barrier function. The mucosal barrier consists of both extracellular physical (mucus layer and intercellular tight junction proteins) and cellular components, primarily epithelial and goblet cells, whose functions are to limit and modulate the interactions between the microbiota and the underlying immune cell populations.65, 66 While disruption of the mucosal barrier is often found in human disease states and consists of pore, leak, or unrestricted denuded surface transport mechanisms, it remains relatively uncertain in each instance whether this effect is a primary driver of disease, or a secondary finding associated with inflammation. Nevertheless, certain cytokines, including IL-13, IL-9, tumor necrosis factor (TNF), and IL-4 can in excess play a major role in opening up tight junctions by modulating the amounts and relative ratios of claudin-2 and occludin, which functionally act as regulated extracellular pores.66 In addition to promoting a more permeable barrier, some bacteria can transit the mucous and epithelial cell layer into the underlying tissues using one or more mucolytic, adherence, toxin, or cell modifying mechanisms.67, 68
With regard to RA, a role for tight junction proteins has been suggested by findings that serum zonulin is elevated in patients and models of RA, and is associated with a more permeable barrier.69 In addition, therapeutic restoration of the barrier through modulation of zonulin levels in a mouse model reduces the development and severity of experimental arthritis.
Well-established contributors to intestinal barrier homeostasis and inflammation in human inflammatory bowel disease (IBD) include the cytokines TNF, IL-6, IL-12, IL-17, IL-23, IL-27, the chemokines and their receptors CC-chemokine receptor 6 (CCR6), CC-chemokine ligand 2 (CCL2), and CCL13, and the intracellular signaling pathways regulated by signal transducer and activator of transcription 1 (STAT1), STAT3, STAT4, and Janus kinase 2 (JAK2). In addition, the integrin α4β7 is involved in disease pathogenesis through its ability to mediate cell trafficking into the site. Similarly, sphingosine-1-phosphate (S1P) signaling through S1P receptor isoforms 1 and 5 mediates lymphocyte mobilization to inflamed intestinal tissue.70 The close relationship of these factors to Crohn's disease and ulcerative colitis pathogenesis is known through genetic association, biomarkers, and especially, therapeutic strategies.71 Targeting subsets of these molecules has resulted in a revolution in care for patients with the IBD spectrum of diseases.72
Mucosal cell populations that have received attention in inflammatory and autoimmune diseases include neutrophils, which through the generation of citrullinated-antigen replete NETs are activated and expanded in the complement and cytokine enriched at-risk pulmonary mucosa.29 Additional intestinal cell populations that appear to play major pro- and anti-inflammatory roles include macrophages, dendritic cells, innate lymphoid cells, mucosal-associated invariant T cells, Th1, Th2, and Th17 cells.72
With regard to other mucosal sites, especially the lung, an increasing number of mechanisms are understood through therapeutic, and biomarker- and mechanism-based approaches to modulate inflammation.73 Although many of the same pathways are implicated in lung disease pathogenesis through studies of disease models, for example, TNF, clinical trials with inhibitors that are effective in IBD are typically not beneficial in inflammatory lung diseases. Additional mechanisms that have been suggested to play important roles in the lung include IL-8 and thymic stromal lymphopoietin, and with regard to the latter, an inhibitory monoclonal antibody was recently shown to be effective in patients with severe asthma74 and may be an important modulator of intestinal homeostasis.75 These differences in clinical effectiveness between gut and lung may be caused by either endogenous differences in the protective mechanism or by distinct types of exposures, in that the lung is exposed to a large number of environmental pollutants and aerosolized factors, and the gut is bathed in a very large microbial mass and is exposed to digested food products. In addition, the microbiome that is adapted to the lung is distinct from that found at other sites.76
3.2 Potential therapeutic strategies
With regard to the mucosal barrier, there are no therapies focused on this target, although once available this would be an appropriate approach to investigate RA prevention at this stage of the disease. However, there are an increasing number of therapeutic strategies that modulate mucosal inflammation in the gut and are approved for the treatment of forms of IBD. These include TNF blockers, IL-12/IL-23 inhibitors, JAK inhibitors, and lymphocyte-trafficking inhibitors.77 In principle, one or more of these could be utilized to modulate preclinical RA-related dysbiosis and mucosal inflammation. In particular, IL-17A inhibition remains an intriguing approach, even though outcomes of this approach in established RA have been modest.78 Nevertheless, given that the outcome measures therein were based on synovial findings, the use of this approach, as well as clinically available IL-12/IL-23,79, 80 S1P,81 and α4β7 antagonists,82 in the preclinical phase are appropriate considerations for prevention trials. Each has the potential to modulate ongoing intestinal mucosal inflammation and potentially block the expansion of systemic citrullinated antigen recognition while allowing localized reconstitution of a homeostatic state.
In addition to systemic therapies, it will be important to consider mucosal-site targeted immunosuppression in RA prevention. These therapies could include nonspecific immune modulators, such as inhaled or oral rinse corticosteroids, as well as directed therapies that target specific factors that affect immune cell dysregulation, such as inhaled DNAse, which degrades NET remnants in the lung and is commonly used to treat cystic fibrosis.83
Additional approaches could be directed at promoting inflammation resolution at mucosal sites, following on the finding that elevated intake and levels of omega-3 fatty acids, the precursors of resolvins and maresins,84 appear to be protective against both systemic autoimmunity85 and progression to IA in at-risk populations.63 Finally, the successful use of JAK inhibitors in IBDs and other inflammatory/autoimmune diseases72, 86 as well as RA,87 suggests that this broadly-acting class of therapeutics might be an appropriate strategy for evaluation, taking into account risk-benefit considerations.
In sum, there are likely to be a number of processes involved in mucosal inflammation that are drivers of the development of systemic autoimmunity and arthritis development, and along with modulation of the microbiome are likely to be important therapeutic strategies going forward.
4 STRATEGIES FOCUSED ON CHECKPOINT 3: MODULATING SYSTEMIC EPITOPE SPREADING AND AFFINITY MATURATION
4.1 Rationale
It is well accepted that the adaptive immune cell response evolves in the preclinical period to recognize more citrullinated and synovial antigen targets with a higher affinity/avidity and is associated with posttranslational modifications of autoantibodies that promote the engagement of effector functions.88 As there is now the ability to study regional lymph nodes in patients and at-risk individuals, it is in principle possible to evaluate the effects of therapies targeting these processes,89 Indeed, CTLA4-Ig, which interferes with bidirectional antigen-specific B- and T-cell activation and immune evolution through interruption of costimulation,90 an essential process in the adaptive response,91 are an effective therapeutic even after the development of classified RA.92 Thus, the use of costimulation blockade that is designed to dampen epitope spreading and effector function generation is a very appropriate means by which disease prevention should be evaluated.93
4.2 Potential therapeutic strategies
With regard to the inhibition of costimulation, a large ongoing clinical trial of CTLA4-Ig is underway in at-risk individuals,94 and the use of this strategy in a small number of patients with symptoms of RA and imaging findings but without classifiable disease gave encouraging results.95 In addition, another promising therapeutic approach with a similar anticipated outcome would target the CD40-CD40L pathway interaction.96 With regard to T-cell-directed approaches, a particularly intriguing approach involved the use of teplizumab to induce T cell tolerance in individuals at-risk for the development of type 1 diabetes (T1D),97 resulting in a statistically significant delay in the development of insulin requirements. Specific T cell subpopulations that are garnering increasing attention in patients with clinical RA are T helper cells designated T follicular helper98 and T peripheral helper,99 which promote adaptive immunity through multiple mechanisms, including production of the cytokine IL-21, a pleiotropic molecule with major effects on B-cell responses.100 Targeting this cytokine pathway has substantial possibilities in the at-risk population. Newer approaches under development include the use of PD-1 agonists to induce a generalized state of unresponsiveness.101
In addition to targeting T cells and their products, restriction of antigen presentation or recognition of citrullinated targets by inhibitors of PADs may limit the ability of ACPA autoantibodies, as well as T cell responses, to recognize their target antigens, among other potential effects that may influence later stages of the disease.102
Beyond antigen-agnostic interventions, antigen-specific tolerance is an important potential approach.103, 104 This approach would follow upon the increasing understanding of the antigen-specific T cell repertoire in patients with classified RA105-108 as well as at-risk individuals (James, Buckner et al., unpublished). In addition, the approaches would involve means by which to modulate antigen-specific B cells, which are expanded and dysregulated in RA23, 109 as well as other autoimmune diseases.110 One early approach to the elimination of B cells, including autoreactive, was the use of rituximab in the PRAIRI study to deplete B cells in ACPA+ at-risk individuals, which resulted in a modest delay in the diagnosis of RA.111
Additional approaches could follow the lead of studies on disease prevention in T1D.112 Therein, dietary manipulation, B cell depletion, global and antigen-specific T cell tolerance induction, Class II antigen presentation blockade, and other approaches have been or will be explored. These trials have been greatly facilitated by the development of a global network of disease prevention centers.
5 STRATEGIES FOCUSED ON CHECKPOINT 4: PROTECTION OF THE SYNOVIAL TISSUE AND JOINT STRUCTURES
5.1 Rationale
One of the current mysteries in the development of clinically apparent and classified RA is the mechanism, or mechanisms, by which the state of nonarticular systemic autoimmunity that is associated with a substantial number of immune alterations transitions to target the synovium and cause inflammation and damage.33, 88, 113 The process likely involves both systemic and local factors, the latter of which can be vascular, synovial, cartilage, or bone marrow in origin. The initiation of clinical signs and symptoms is known to be highly variable from patient to patient,1 and may reflect the contribution of different preclinical endotypes that can exert variable long-term effects on the pathways that lead to joint disease development.24 In addition, it is also likely that the synovial process evolves over time, as the transcriptional signatures of fibroblast-like synoviocytes obtained from early and later disease stages are different.114
5.2 Potential therapeutic strategies
This phase of RA has been extensively explored through the initiation of treatment at earlier and earlier time points after the appearance of clinical IA/RA using conventional therapies, especially corticosteroids and methotrexate, alone115 and in combination with other conventional and biologic disease modifying anti-rheumatic drugs (DMARDs).116 Ongoing prevention trials in RA targeting individuals with abnormal autoantibodies in absence of clinical IA/RA have also used DMARDs, including hydroxychloroquine in the StopRA trial17 as well as abatacept in the APIPPRA trial94; in these studies, the hypothesis is that these agents may act to prevent the initiation of articular inflammation although they may also affect nonarticular sites and “checkpoints” in RA development. Aside from conventional small molecules and biologics, it is also possible that immune complex-mediated processes that utilize Fc receptors and activated complement fragments play important very early roles, as suggested in an intriguing synovial biopsy finding,117 or that restricting the generation of citrullinated antigens in the joint as targets of ACPA may be beneficial.
6 ADDITIONAL CONSIDERATIONS RELEVANT TO RA PREVENTION TRIALS
Beyond the choice of the therapeutic target and drug(s) used in the intervention, there are many additional considerations that are necessary to address to undertake and finish a successful RA prevention trial.17 These points are beyond the scope of this review but include the appropriate cost-efficient screening strategy, utilization of enrollment criteria with sufficient positive predictive values to answer the question in a reasonable period of time, establishment of relevant outcome measures and a determination as to whether surrogate measures (e.g., biomarker changes) are appropriate, ethical considerations around intervening in individuals without clinical IA/RA, consideration as to what are the intervention and control populations, and whether an adaptive trial design might be entertained. In addition, an RA prevention therapeutic would need an exceptionally favorable safety and tolerability profile to avoid toxicity in the individuals who are at lower risk to progress to clinical IA/RA.
7 SUMMARY AND NEXT STEPS
The authors hope that this review provides a “checkpoint” based experimental and therapeutic framework and considers the rapidly emerging opportunities in RA prevention. It is recognized, indeed anticipated, that the evolving science may well identify additional therapeutic strategies not yet envisioned and that the presence of a substantial number of preclinical disease endotypes may force a more personalized approach to prevention that may preclude the successful application of single approaches across the at-risk population. Nevertheless, it is an exciting period in prevention sciences both in RA as well as across autoimmune diseases. There are opportunities to change treatment paradigms and also to consider autoimmune diseases as a set of entities whose natural histories will share pathogenic features, such as a mucosal initiation process, which will then generate innovative prevention approaches that focus on this very early stage.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception of the hypotheses included in, as well as the writing of, this review manuscript.
ACKNOWLEDGMENTS
Relevant studies by the authors are supported by NIH U19 AI101981 (V. Michael Holers), P30 AR079369 (V. Michael Holers), R01 AR051749 (V. Michael Holers), R01 AR078268 (William H. Robinson), R01 AR075033 (Kristine A. Kuhn), R01 AR076450 (M. Kristen Demoruelle), UM1 AI110498 (Kevin D. Deane), Janssen Research and Development Sponsored Research Award: Targeting Immune Responses for Prevention of Rheumatoid Arthritis (TIP-RA) (Kevin D. Deane, V. Michael Holers, Gary S. Firestein, William H. Robinson, Jane H. Buckner), NIH U01 AI101981 (V. Michael Holers, William H. Robinson, Jane H. Buckner), NIH R01 AR071321 (Gary S. Firestein), and R01 AR065466 (Gary S. Firestein). Work performed at the Benaroya Research Institute at Virginia Mason was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program (PRMRP) Investigator-Initiated Research Award under Award No. W81XWH-15-1-0003 (Jane H. Buckner). All opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
CONFLICTS OF INTEREST
V. Michael Holers is an Advisory Editorial Board member of Rheumatology & Autoimmunity. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of these Editorial Board and Editorial Office members and their research groups; William H. Robinson is a founder, member of the Board of Directors, and consultant to Atreca, Inc.; Jane H. Buckner has consulting (Janssen, BMS, GentiBio, Hotspot Therapeutics, Neoleukin) and stock ownership (GentiBio); Kristine A. Kuhn has consulting (UCB, Solarea); Eddie A. James has consulting (Provention) and sponsored research funding (BMS, Novartis); Gary S. Firestein has consulting (Jubiliant Therapeutics, Evommune, Xinthera, Teijin) and sponsored research funding (Eli Lilly); M. Kristen Demoruelle has sponsored research funding (Pfizer, Boehringer Ingelheim); and Kevin D. Deane has consulting (Inova/Werfen, BMS, ThermoFisher, Microdrop/imaware, Exagen, Janssen) and sponsored research funding (Janssen Research and Development).
ETHICS STATEMENT
The authors have followed ethical guidelines as published by Wiley.
Open Research
DATA AVAILABILITY STATEMENT
There are no sharable data in this review manuscript; relevant data would be available through the cited publications.