MSCs relieve SLE by modulation of Th17 cells through MMPs–CCL2–CCR2–IL-17 pathway
Bo Jiang and Genhong Yao contributed equally to this study.
Abstract
Objective
To explore the modulation of mesenchymal stem cells (MSCs) on T helper 17 (Th17) cells in systemic lupus erythematosus (SLE) and underlying mechanism.
Methods
The concentration of matrix metalloproteinases (MMPs), CC chemokine ligand-2 (CCL2), and interleukin-17 (IL-17) in the serum of SLE patients and mice were detected by enzyme-linked immunosorbent assay. The expression of CCR2 and IL-17 of T lymphocytes were determined by flow cytometry. The effects of MSCs on Th17 cells were analyzed in lupus mice and coculture system in vitro.
Results
The levels of MMPs, CCL2, IL-17, CCR2, and percentages of Th17 cells were significantly increased in SLE patients. These molecules and numbers of Th17 cells were downregulated by umbilical cord-derived MSCs (UC-MSCs) which relieve SLE disease. CCL2 neutralizing antibody blocked the effects of MSCs on Th17 cells. MMPs reversed the function of CCL2.
Conclusion
The beneficial effects of MSCs on SLE patients rely on secreting MMPs, which reverse the activity of CCL2 to inhibit Th17 cells, suggesting the crucial MSCs–MMP–CCL2–CCR2–Th17–IL-17 pathway in SLE.
Highlights
-
CCL2 activates Th17 to participate in SLE.
-
UC-MSCs secret MMPs to hydrolyze CCL2.
-
Hydrolyzed CCL2 inhibit Th17 to relieve SLE.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a common refractory multiorgan involvement autoimmune disease. In recent years, mesenchymal stem cells (MSCs) transplantation is proved effective for SLE especially for lupus nephritis (LN).1, 2 Researchers found that T helper 17 (Th17) cells and interleukin-17 (IL-17) participated in the occurrence and development of SLE.3 The number of Th17 cells and the concentration of IL-17 in SLE patients and mouse model are much higher than normal controls,4 and could be evidently decreased by MSC transplantation.5 Thus, MSCs may relieve SLE by inhibiting differentiation of Th17 cells and its IL-17 secretion.
CC chemokine ligand-2 (CCL2), also named as monocyte chemoattractant protein-1 (MCP-1), involves in the recruitment, metabolism and function of lymphocytes. The concentration of CCL2 is obviously increased in SLE patients and positively correlates with proteinuria, autoantibody and SLE disease activity.6 CCL2 receptor-CCR2 is abundant on the surface of Th17 cells.7 MSCs could produce CCL2.8 MSCs could secrete matrix metalloproteinases (MMPs)-a kind of calcium-dependent zinc-containing endopeptidases which have been reported changing the activity of CCL2 by hydrolyzation.9, 10 Therefore, MSCs may alleviate SLE by suppressing Th17 cells through MMPs–CCL2–CCR2 pathway.
Here, we show that the production of MMPs, CCL2 and IL-17 in serum and supernatant of MSCs were evidently higher in SLE patients. MSCs decrease the level of MMPs–CCL2–CCR2–IL-17. CCL2 neutralizing antibody blocked the effect of MSCs on Th17 cells. And MMPs reverse the function of CCL2 on Th17 cells. In summary, the effects of MSCs on SLE is through MMPs–CCL2–CCR2–Th17 pathway.
2 MATERIALS AND METHODS
2.1 Patients and mice
All patients fit for the classification criteria of SLE established by American College of Rheumatology (ACR) in 1982. Their clinical features were shown in Table S1. After approved by medical ethics committee of Drum Tower Hospital (No. 2008017) and got informed consent from SLE patients and healthy control, the bone marrow and anticoagulant venous blood were collected.
Sixteen-week-old female C57BL/6 mice and female MRL/lpr mice (lupus mouse model) were purchased from Beijing Experimental Animal Center of Military Medical Sciences. All mice were raised in SPF grade animal housing. MRL/lpr mice were randomly divided into three groups: umbilical cord-derived MSCs (UC-MSCs) transplantation combined with IgG2b isotype control group (n = 6), UC-MSCs transplantation combined with CCL2 antibody group (n = 6) and untreated group (n = 6). C57BL/6 mice were used as negative control. 1 × 106 UC-MSCs were injected into 16-week-old MRL/lpr mice through tail vein. In the same time, 25 µg CCL2 neutralizing antibody or IgG2b was injected intraperitoneally every week. All mice were sacrificed at 29-week-old.
2.2 Reagents
Culture medium including DMEM-F12, RPMI 1640, fetal bovine serum (FBS) were purchased from Gibco. Lymphocyte separation liquid was bought from TBD Company. To purify mouse naïve T lymphocytes, immune magnetic bead was gained from Miltenyi. Antimouse-CD3, antimouse-CD28, recombinant mouse IL-1β, IL-6, IL-21, IL-23, and TGF-β1 were from Santa Cruz Biotechnology. Recombinant human MMPs and CCL2 were purchased from Peprotech. Fluorescent antibody of CD4-FITC, IL-17-APC, CCR2-PE, and isotype control were from eBioscience. Enzyme-linked immunosorbent assay (ELISA) kits for IL-17, MMP1, MMP3 and CCL2 were from R&D System. Luminex kits of MMPs were bought from Merch & Millipore Company.
2.3 Isolation and culture of UC-MSCs
Fresh umbilical cord originated from informed healthy mothers in maternity department after normal deliveries was placed in balanced salt solution. After blood eliminating and vessel were removed, Wharton's jelly was separated, washed and cut into 1–4 mm3 tissue. Cells (1 × 106/cm2) were incubated in in low sugar DMEM medium with 5% FBS at 37°C and 5% CO2. Culture medium was removed 1 week later and replaced by complete medium every 3 days. When adherent cells reached 60%–80% fusion, they were digested with 0.25 g/L trypsin and passaged (1 × 104/cm2). Flow cytometry (FCM) was used to identify the surface markers of stem cell including CD106, CD105, CD44, and CD29. Their positive percentage was ≥95%. And the percent of CD45, CD34, CD14, CD79 and HLA-DR is ≤2%. Second to fifth generation of UC-MSCs were used for transplantation.
2.4 Isolation and culture of human bone marrow-derived MSCs (BM-MSCs)
Ten milliliter heparin-anticoagulated bone morrow from SLE patients and healthy controls was diluted with 0.9% saline and added onto same volume of lymphocyte separation liquid, then centrifuged 400g for 20 min at 4°C. Mononuclear cells were collected, washed and cultured in bottle (5 × 106cells/ml) in complete DMEM medium with 10% FBS at 37°C and 5% CO2. One week later, the culture medium was changed and non-adherent cells were removed. After that, culture medium was replaced by low sugar complete DMEM every 3 days. When adherent cells were more than 90%, they were harvested by digestion using 0.25% trypsin and passaged according to the proportion 1:2. Amplified BM-MSCs were counted and identified.
2.5 Isolation of naïve T lymphocytes from mouse spleen
Naïve T lymphocytes were isolated by magnetic-activated cell sorting (MACS). Mononuclear cells of mouse spleen were suspended in 40 μl magnetic buffer and 10 μl CD4+ T cell biotin-antibody cocktail II MicroBeads, then incubated at 4°C for 15 min. After incubation with anti-Biotin MicroBeads for 15 min at 4°C, the cells were washed, centrifuged 400g for 10 min and resuspended. Cells suspending liquid was added onto LS column. Cells were collected from LS column after sorting and centrifuged 400g for 10 min, incubated with CD62L microbead at 4°C for 15 min. Then the cells were washed and added into MS column. Positive cells were collected.
2.6 Coculture of UC-MSCs with Th
The three to five generations of UC-MSCs were incubated overnight in lower chamber of 24-hole-transwell (104cells/well) in DMEM-F12 medium. Naïve T cells were cultivated in RPMI 1640 with Th17 stimulation agents: anti-mCD3 (2 μg/ml), anti-mCD28 (2 μg/ml), IL-1β (10 ng/ml), IL-6 (30 ng/ml), IL-21 (50 ng/ml), IL-23 (20 ng/ml), and TGF-β1 (3 ng/ml) in the upper chamber (104 to 106cells/well) for 72 h. According to different groups, various reagents were added: anti-human-CCL2-Ab, IgG2b isotype control, recombinant human CCL2, MMP-processed CCL2 (mpCCL2), and recombinant human MMPs, respectively.
2.7 Detection of IL-17 and CCR2 expression on T lymphocytes
The expression of CD4, IL-17 and CCR2 on T lymphocyte were measured by flow cytometry. Antibody staining was performed according to manufacturer's instruction. Peripheral blood mononuclear cells (PBMC) separated by Ficoll were washed and added into 80 µl RPMI 1640 culture medium with 10 µl PMA, 10 µl Ionomyocin, 5 µl BFA in 37°C, 5% CO2 for 4 h. After washing, the cells were stained by FITC-CD4 and CCR2-PE darkly at room temperature (RT) for 15 min, then were membrane-broken by agent A/B for 15 min, stained by IL-17-APC for 30 min. The washed stained cells were resuspended and tested.
2.8 Detection of the concentration of cytokines
The concentration of CCL2, MMPs, IL-17, IgG, anti-nuclear antibody (ANA) and anti-double-stranded DNA (dsDNA) in serum and supernatant of BM-MSCs were detected by ELISA and Luminex according to manufacturer's instructions. The procedure of Luminex: assay buffer was added to pre-wet and closing, then microtiter plate was placed on a shaker for 10 min. Assay buffer was removed. Standard set and suitable substrate solution was added. 25 µl diluted sample and 25 µl pre-mix microbead were added into every well. The plate was covered with special plastic film board and shook at 4°C for 18–20 h. After washed twice, the sample was incubated with secondary antibody (25 µl/well) at RT for 2 h. Then 25 µl Streptavidin-Phycoerythrin was joined and incubated at RT for 30 min. The content of inner panel was removed. Sheath fluid was added to each well and shook for 5 min. The board was placed in Luminex 100™ IS, 200™ to analyze.
2.9 Generation of MMP-processed CCL2
To generate MMP-processed CCL2 (mpCCL2), we added 10 ng pure recombinant human MMP (rhMMP) directly to 50 μg pure recombinant human CCL2 (rhCCL2) at 37°C for 4 h. Mp-CCL2 was directly used in assay.
2.10 Detection of the kidney of lupus mice
Twenty-four-hour urine was collected using metabolism cages. Urine protein was measured by Coomassie Blue staining method. The kidneys of MRL/lpr mice and C57BL/6 mice were cut into small pieces and fixed in 10% formalin at 4°C for 24 h. Paraffin sections (4 µm) of renal tissues were stained with hematoxylineosin (HE). The presence of immune complex in kidney was detected by immune fluorescent assay.
2.11 Statistics analysis
Data are expressed as mean ± standard deviation. Data analysis was done using two-tailed Mann–Whitney and Wilcoxon t test by SPSS 13.0 software. p Value < 0.05 was considered as statistically significant.
3 RESULTS
3.1 The level of CCL2-CCR2-Th17-IL-17 increased in SLE
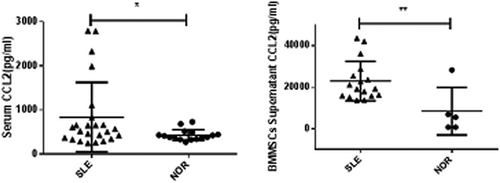
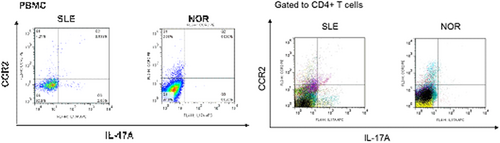
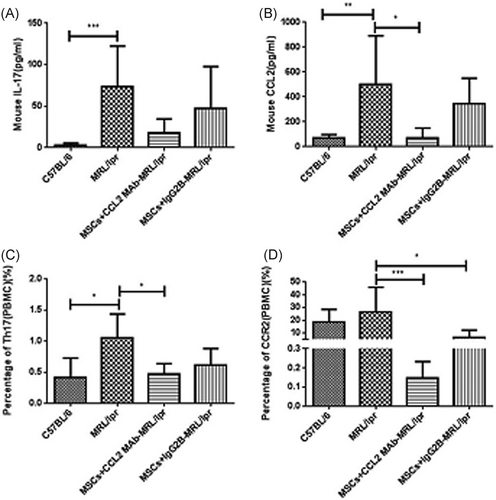
The percentage of IL-17+CD4+ T lymphocytes (Th17) (3.077 ± 0.515% vs. 0.223 ± 0.084%, p < 0.0001) and the concentration of IL-17 in peripheral blood of SLE patients (7.56 ± 1.69 pg/ml vs. 3.62 ± 0.42 pg/ml, p < 0.05) was significantly higher than health control (shown in Figure S1). The concentration of chemokine-CCL2 in serum and supernatant of BM-MSCs (Figure 1) and the expression of CCR2 on Th17 cells in blood (Figure 2) were obviously increased in SLE patients. It is also found that the concentration of IL-17 (73.13 ± 24.67 pg/ml vs. l3.14 ± 1.14 pg/ml, p < 0.001) and CCL2 (498.0 ± 175.6 pg/ml vs. 69.29 ± 15.53 pg/ml, p < 0.01) in the serum, and the percentage of Th17 cells (1.06 ± 0.17% vs. 0.42 ± 0.14%, p < 0.05) and CCR2+ Th17 cells (26.59 ± 8.62% vs. 18.94 ± 5.12%, p > 0.05) in PBMCs of MRL/lpr mice were higher than those of C57BL/6 mice. These results displayed that CCL2-CCR2-Th17-IL-17 was involved in SLE.
3.2 MSCs inhibit IL-17 production through CCL2–CCR2 pathway to relieve SLE
We further investigated the effects of MSCs and CCL2 on SLE disease. Compared with normal control mice, the proteinuria, serum ANA concentration and immune complex in kidney were all significantly higher in MRL/lpr mice with disease activity of lupus (Figure 3). MSCs transplantation (MSCs + IgG2b isotype control group) evidently decreased these indexes especially for urine protein (p < 0.05), ANA concentration (p < 0.01), and immune complex in kidney of MRL/lpr mice (Figure 3). It is interesting that antimouse-CCL2-Ab strengthened effects of MSCs on lupus mice (Figure 3), suggesting that CCL2 participate in the immune-regulation function of MSCs in SLE.
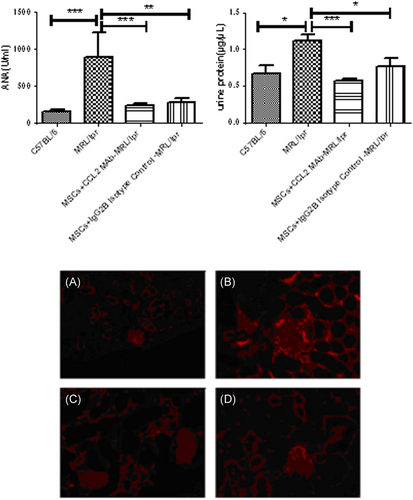
Next, to explore if MSCs alleviate SLE through CCL2–CCR2–Th17 pathway, the levels of IL-17, CCL2 and CCR2 after UC-MSC transplantation in lupus mice were detected. The serum IL-17 and CCL2, Th17 cell percentage and CCR2 expression on PBMCs in lupus mice were all decreased by UC-MSCs (Figure 4). On the other hand, the effects of UC-MSCs combined with CCL2-Ab were much stronger than UC-MSCs along, though without statistic difference (Figure 4). These results confirmed the modulation of MSCs on CCL2-CCR2–Th17–IL-17 pathway.
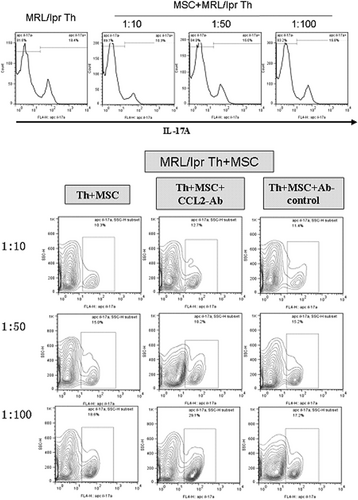
Then coculture experiment was carried out to further identify the molecule pathway of MSCs on Th17 cells. UC-MSCs were cocultured with spleen CD4+ Th cells from lupus mice in transwell system for 72 h with different ratio (UC-MSC: Th cells = 1:10, 1:50, 1:100). Then IL-17A expression on CD4+ Th cells was detected. It is found that UC-MSCs could significantly inhibit the production of IL-17A especially in the group of ratio 1:10 (Figure 5). However, IL-17A expression was increased by anti-human-CCL2-Ab in coculture system of MSCs and Th17 cells (Figure 5). CCL2-Ab reversed the inhibition of UC-MSCs on Th17 cells, suggesting that CCL2 was crucially involved in the effect of MSCs on Th17 cells.
3.3 MSCs secrete MMPs to reverse CCL2 function and modulate Th17 cells
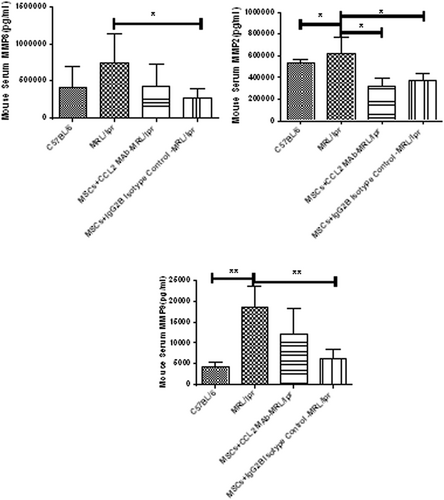
We next explore how CCL2 modulates the control of MSCs on Th17 cells. MSCs could produce MMPs. It is found that the level of MMP2/3/8 in the serum and supernatant of BM-MSCs of SLE patients were evidently elevated than normal controls (Figure S2). Similarly, in MRL/lpr mice, the concentration of MMP2/8/9 in the serum were also much higher than those of C57BL/6 mice, and transplantation of UC-MSCs obviously decreased them (Figure 6). Therefore, MMPs may participate in SLE and normal MSCs could relieve SLE through secreting MMPs.
To explore the modulation of MMPs on CCL2–CCR2–Th17–IL-17 axis, recombinant human CCL2 (rhCCL2, 100 ng/ml), rhMMPs (10 ng/ml), MMP-hydrolyzed CCL2 (mpCCL2, 100 ng/ml) and UC-MSCs were added into the culture system of naïve T cells of lupus mouse respectively. RhCCL2 and rhMMP improved, but mpCCL2 and UC-MSCs decreased the percentage of CCR2+ IL-17+ T lymphocytes (see Figure S3), suggesting that the hydrolysis of CCL2 by MMP reverse its function on Th17 cells.
4 DISCUSSION
In the past few years, the involvement of Th17 cells (IL-17+ CD4+ T lymphocytes) and IL-17 in SLE is getting more and more attention. Researchers found that the number of Th17 cells and the concentration of IL-17 in peripheral blood, spleen and kidney of SLE patients were significantly increased and positively correlated with disease activity. In our study, similar result was found in lupus patients and model mice, which further confirmed their participation in SLE pathogenesis. Th17 cells express CCR2. CCL2 has been proved to promote the function of immune cells and involving in some autoimmune diseases. Our study discovered that serum concentration of CCL2 and the expression of CCR2 on Th17 cells in SLE patients and lupus mice were evidently increased. And recombinant CCL2 upregulated the expression of IL-17 and CCR2 on naive T lymphocytes, suggesting CCL2 promotes the differentiation of Th17 through CCR2 resulting the occurrence and development of SLE.
MSCs could secret CCL2. BM-MSCs from SLE patients produce higher level of CCL2 in vitro than health control, consistent with higher CCL2 and IL-17 concentration in the serum of SLE patients. Our coculture experiment displayed that UC-MSCs suppressed the differentiation of Th17 cells and the production of IL-17. And in vivo normal UC-MSCs transplantation, IL-17 concentration and percentage of Th17 in lupus mice was decreased, consistent with the meta-analysis study by Zhou et al.11 Luz-Crawford also proved that normal BM-MSCs inhibit the differentiation and activation of spleen Th17 cells from normal mice in vitro,12 but the detailed pathway was not explored. Chen et al made efforts to discover that the inhibition of Th17 by MSCs was inhibited by anti-HGF-antibody, suggesting the modulation on Th17 is partly attributed to HGF secreted by the MSCs.13 In other diseases, the inhibition of MSCs on Th17 was also proved. Song et al.14 found that the extracts of MSCs relieve dermatitis in atopic dermatitis (AD) mice and decrease levels of IL-17 (Th17) through NF-κB pathway possibly. The study by Milosavljevic suggested that MSCs suppress liver Th17 cells, then attenuate liver fibrosis in IDO-dependent manner.15 Different with above, we try to find out if chemokines take part in the effect of MSCs on Th17 cells. Our results showed that the inhibition of MSCs on Th17 cells could be blocked by CCL2 antibody. Therefore, CCL2 should be an important molecule in the inhibition of MSCs on Th17. Thus, MSCs transplantation may treat SLE partly through CCL2–CCR2–Th17–IL-17 pathway.
Next, we aim to discover why CCL2 secreted by MSCs participates in the inhibition of Th17, different with recombinant CCL2 activating Th17. MSCs could secrete MMPs which maybe the upstream molecule of CCL2 and regulate its activity.9, 10 In autoimmune experimental autoimmune encephalomyelitis (EAE), MSCs were found to secrete MMPs to transfer CCL2 from activator to inhibitor by hydrolyzation.16 Our results showed that increased the concentration of MMPs in SLE patients and mouse were all reduced by UC-MSCs transplantation. Thus, MMPs may take part in SLE disease and lupus remission by MSCs. In vitro, rhCCL2 improves the expression of CCR2 and IL-17 in Th17 cells, but MMP-treated CCL2 (mpCCL2) decreases them, which is consistent with the effects of UC-MSCs alone. Our previous study also shows that MSC-mediated inhibition of B cell is dependent on MMPs proteolytic processing of CCL2.17 This suggests MSCs–MMPs–CCL2–CCR2–Th17–IL-17 pathway in SLE.
In conclusion, our study proved that MSCs transplantation has significantly therapeutic effect for SLE by inhibiting the differentiation of Th17 cells and the production of IL-17 through secreting MMPs which reverse the activity of CCL2. In the future, we will study the AQ8detailed mechanism how MMPs reverse the activity of CCL2 and how CCL2 modulates function of Th17 cells in SLE.
ACKNOWLEDGMENTS
This study is financially supported by National Nature Science Foundation of China (81102257), Fundamental Research Funds for the Central Universities (1012016), and Nanjing Science and Technology Development Plan (201715021).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Xuebing Feng and Bo Jiang contributed to the study conception and design. The experiments was mainly done by Yang Xi and directed by Genhong Yao. Data analysis was performed by Xiaojun Tang. The draft of the manuscript was written by Bo Jiang and edited by Genhong Yao. All authors have read, approved and agreed to take full responsibility for the final manuscript.
ETHICS STATEMENT
This study has been approved by medical ethics committee of Nanjing Drum Tower Hospital (No. 2008017).
DATA AVAILABILITY STATEMENT
Some or all data that support the findings of this study is available from the corresponding author by reasonable request.