Crystal structure of the endopolygalacturonase from the phytopathogenic fungus Colletotrichum lupini and its interaction with polygalacturonase-inhibiting proteins
INTRODUCTION
When infecting a plant tissue, pathogenic fungi secrete hydrolytic enzymes to degrade the plant cell wall. Among them, endo-polygalacturonases (PGs) cleave the α-1,4 linkages between D-galacturonic acid residues in homogalacturonan, the main component of pectin.1 PGs are important virulence factors: for instance disruption of a PG gene in Botrytis cinerea results in a strong reduction of virulence on tomato and broad bean.2
To recognize and inhibit fungal PGs, plants secrete polygalacturonase-inhibiting proteins (PGIPs).1 The PG-PGIP interaction limits the aggressive potential of PGs and favors the accumulation of oligogalacturonides, short fragments of the homogalacturonan capable of activating plant defense responses.3, 4 PGIPs belong to the superfamily of leucine-rich repeat (LRR) proteins5 and are coded by small gene families in both dicotyledonous and monocotyledonous plants.6, 7 PGIPs have been shown to be players of plant innate immunity: Arabidopsis, tobacco, tomato, and grapevine plants overexpressing endogenous or exogenous PGIP genes display increased resistance against Botrytis cinerea.8 Conversely, Arabidopsis plants with antisense expression of AtPGIP1 are more susceptible to B. cinerea.9
The structural bases of the PG-PGIP interaction are still elusive. PGIP isoforms differ in affinity and specificity for PGs secreted by different pathogens. Moreover, a single PGIP is often capable of inhibiting different fungal PGs with different mechanisms: for example PGIP2 from Phaseolus vulgaris (PvPGIP2) inhibits Fusarium moniliforme PG (FmPG), Aspergillus niger PGII (AnPGII), and Botrytis cinerea PG1 with a competitive, noncompetitive and mixed mode of action, respectively.10-12
We have determined the crystal structure of a PG from the phytopathogenic fungus Colletotrichum lupini (CluPG1), the causal agent of lupin anthracnose. Moreover we have characterized its enzymatic activity, analyzed the inhibition mode exerted by PvPGIP2 on the enzyme and compared our data with those on AnPGII and FmPG, whose 3D structures and inhibition kinetics are already known. We also analyzed the propensity of these three PGs to be involved in protein–protein interactions with computational methods. We show that different inhibition mechanisms are correlated to nonequivalent low surface desolvation energy profiles, supporting the idea that the PG-PGIP interaction involves different binding surfaces depending on the PG ligand.
MATERIALS AND METHODS
Fungal cultures and cloning of the CluPG1 gene
C. lupini var. setosum strain SHK78813 was grown at 20°C on 24 g/L Potato Dextrose Agar (Duchefa Biochemie BV, The Netherlands) until sporulation. 5 × 105 conidia per mL were inoculated in Czapex–Dox medium supplemented with 0.3% (w/v) glucose and incubated at 20°C. Pectin (1.5%) was added after 3 days of culture and mycelium was collected after 24 h. Total RNA was isolated using Tri Reagent (Sigma) and RNA samples were treated with RNase-free DNase I (Promega, France) for 30 min at 37°C. One microgram of total RNA was subjected to reverse trascription using ImProm-II Reverse Transcriptase (Promega, France). To clone the gene encoding CluPG1, two degenerate oligonucleotides [FwDeg: 5′-GGCAA(A/G)ACCACCTTCGGC-3′ (forward) and 5′-RGGCGCAGAGGATGTA(A/G)AC-3′ (reverse)] were designed on the basis of C. lindemuthianum sequence. The oligonucleotides were used as primers for PCR using GoTaq DNA Polymerase (Promega, France) and, as a template, one-tenth of the reverse trascription reaction. The amplified fragment (790 bp) was sequenced and two primers for inverse PCR [IFw: 5′-GGCAC CAACGTCTACATCCTCT-3′ (forward) and IRev: 5′-ACCGGAGAAGGAGATAAGGGGT-3′ (reverse)] were designed and used to complete the sequence of the gene. Inverse PCR was carried out using a modified version of the method described by Ochman et al.14 Fungal genomic DNA was extracted from mycelium using the Wizard Genomic DNA Purification Kit (Promega), digested with XbaI and SpeI at 37°C for 4 h, and self-ligated for 18 h at 16°C using T4 DNA ligase (Promega). Self-ligated DNA was used as a template for Inverse PCR and the obtained PCR product was sequenced.
Finally, to confirm the nucleotide sequence of the CluPG1, two additional primers [CluPgFw: 5′-ATGGTTTC TTCACTTCTCGCC-3′ (forward) and CluPgRev: 5′-TTAG CAAGCAGCACCGCTGCC-3′ (reverse)] were used to amplify the entire open reading frame using either genomic DNA or cDNA prepared from the pectin-induced mycelium, as templates. The nucleotide sequence of CluPG1 was deposited in the GenBank with accession number EF094978.
Expression and purification
The fungus was grown in Czapex–Dox medium containing 1.5% citrus pectin (Sigma), The culture was harvested after 5 days and mycelium was pelleted. The supernatant was loaded on a DEAE-cellulose column (Sigma) after adjusting pH to 4.0, the flow through was collected and loaded on a SP-sepharose column (Pharmacia). The protein was then eluted with 1M NaCl solution. Ammonium sulphate to a final concentration of 3M was added to the protein solution, which was shaken for 20 min. The supernatant was loaded onto a HiTrap Phenyl HP column (Amersham Bioscience) equilibrated with sodium acetate 20 mM pH 4.6, 3M ammonium sulphate. The protein was eluted with sodium acetate 20 mM pH 4.6, dialyzed against the same buffer and concentrated to 10 mg/mL. Endopolygalacturonase activity was monitored throughout the process by reducing sugar15 and agarose gel diffusion assays.12 PvPGIP2 was purified from PVX-infected tissues of Nicotiana benthamiana as previously described.16
Crystallography
Crystals were obtained by vapor diffusion at 21°C, using the hanging drop method; 2 μL drops were made mixing equal volumes of protein solution (10 mg/mL concentration) and of a reservoir solution made up of 24–30% PEG3000 and 0.1M CAPS pH 10.5 or 0.1M CHES pH 9.5. Crystals grew up in 2–3 days and reached their maximum dimensions in one week. They were cryoprotected with a reservoir solution supplemented with 20% PEG200 and flash-frozen in liquid nitrogen.
X-ray diffraction data were collected at the ELETTRA synchrotron (Trieste, Italy) using a wavelength of 1.2 Å, at a temperature of 100 K. Data were processed and scaled using the programs DENZO and SCALEPACK.17 The crystals belong to space group P212121 with unit cell dimensions of a = 85.640 Å, b = 127.266 Å, and c = 205.828 Å and diffract to a maximum resolution of 1.94 Å (Table I). Seven molecules, named A, B, C, D, E, F, and G are present in the asymmetric unit, corresponding to a calculated solvent content of 41%. The structure of CluPG1 was determined by molecular replacement with the program PHASER,18 using AnPGII structure (Protein Data Bank code 1CZF) as a search model. The first six solutions were correctly found by PHASER and the seventh molecule was manually positioned into fairly clear electron density of the initial 2Fo-Fc map. After refinement, performed with the program REFMAC5,19 the electron density was clear enough to build the missing parts of the model and to modify the aminoacid side chains. Several cycles of refinement, using REFMAC5, and manual rebuilding, using COOT,20 were performed to improve the model. The 2Fo-Fc map was then inspected to find eventual N-glycosylation sites and interpretable electron density was found in proximity of Asn209 in all monomers: two N-acetylglucosamine residues were added to monomers B, C, D, E, F, and G and two N-acetylglucosamine and four mannose residues were added to monomer A. Solvent molecules were then added into the 2Fo-Fc contoured at 4σ, using the “find waters” tool of COOT; only solvent molecules with a B factor less than 50 Å2 after refinement were kept in the model. Other refinement cycles were performed after solvent addition with the TLS and restrained refinement mode of REFMAC5,21 using seven TLS groups each containing a single CluPG1 molecule. The final model consists of 2372 aminoacids, 18 sugar residues, 9 PEG molecules, one acetate ion and 2449 water molecules and results in crystallographic R and Rfree values of 15.2% and 19.6%, respectively. Stereochemistry of the model is good, as results from the analysis performed with the program PROCHECK,22 with 85.4% of residues in the most favored region of Ramachandran plot, 14.1% in thegenerously allowed region and 0.5% in the additionally allowed region (see Table I). Coordinates and structure factors are deposited in the PDB with the entry 2IQ7. Structural superpositions were performed with the program CE.23 Optimal docking area (ODA) calculations were performed according to Fernandez-Recio et al.24 ODA values for PGs of known structure are available from authors upon request. Figures were created with Pymol (DeLano Scientific).
| Data collection | |
| Wavelength (Å) | 1.2 |
| Resolution limits (Å) | 50.00–1.94 (2.01–1.94)a |
| Space group | P212121 |
| Molecules in the asymmetric unit | 7 |
| Unit cell dimensions (Å) | a = 85.640, b = 127.266, c = 205.828 |
| Reflections | |
| Total (No.) | 1514222 |
| Unique (No.) | 165995 |
| Completeness % | 99.1 (97.8) |
| Rmerge % | 8.1 (39.6) |
| Average I/σ | 9.4 (3.4) |
| Average redundancy | 9.2 (8.2) |
| Structure refinement | |
| Resolution limits (Å) | 50.00–1.94 |
| Rcryst (%) | 15.2 |
| Rfree (%) | 19.6 |
| Root mean square deviation, bond length (Å) | 0.011 |
| Root mean square deviation, bond angles (°) | 1.257 |
| Ramachandran statistics | |
| Percentage of residues in most favored regions | 85.4 |
| Percentage of residues in generously allowed regions | 14.1 |
| Percentage of residues in additionally allowed regions | 0.5 |
| Percentage of residues in non allowed regions | 0.0 |
- a The values in parentheses refer to data in the highest resolution shell.
Enzymatic and inhibition assays
PG activity was determined measuring the increase of reducing end groups over time.15 Polygalacturonic acid (Sigma) was used as a substrate in concentrations ranging from 0.04 to 4.0 mg/mL and D-galacturonic acid (Sigma) was used as a standard. The experiments were performed at 30°C, in 50 mM sodium acetate buffered at pH 5.0. A final CluPG1 concentration of 2 nM was used in all experiments and PvPGIP2 concentrations of 4 and 8 nM were used in inhibition assays. One activity unit (RGU) was defined as the amount of PG producing one microequivalent of reducing groups per minute at 30°C, using 1.0% polygalacturonic acid as a substrate. Km and Vmax values were calculated by nonlinear regression analysis using the Michaelis–Menten equation.
RESULTS AND DISCUSSION
CluPG1 folds in a right-handed parallel β-helix of approximately 65 × 35 × 35 Å3, made up of 10 complete coils. Each coil is formed by three or four β-strands forming four parallel β sheets named PB1, PB2a, PB2b, and PB3, and connected by turns named T1, T2a, T2b, and T3 [Fig. 1(A)]. The interior of the β-helix is highly hydrophobic and is capped on the N-terminal side by an α-helix and a short β strand belonging to PB2b, constrained by a disulfide bridge between Cys26 and Cys41. The C-terminal side of the β-helix is shielded from the solvent by an additional coil with no secondary structure forced in a defined geometry by a disulfide bridge involving the C-terminal cysteine and Cys351. The extensions formed by the T1-turns on the 7th, 8th, and 9th coil and the T3-turns on the 3rd, 4th, and 5th coil shape a deep, long substrate-binding cleft, lined by PB1 sheet, which is obliquely directed with respect to the β-helix axis. The cleft spans from the 3rd to the 9th coil and is approximately 8 Å wide and 30 Å long; it is compatible with the binding of an unbranched polygalacturonic acid stretch and is open at both ends, as expected for an enzyme with an endo mode of action.
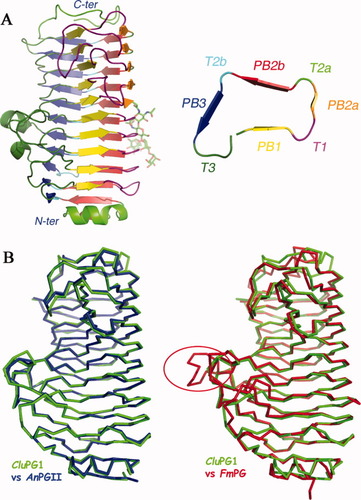
(A) Ribbon representation of CluPG1. A cross section of the β-helix is shown on the right panel; the color code indicates the secondary structure elements in a representative coil. (B) Structural superposition of CluPG1 with AnPGII and FmPG. The red circle indicates an eight residues insertion in the structure of FmPG that likely plays a role in the interaction of this enzyme with PGIPs.
A comparison of CluPG1 with PGs of known structure shows that the scaffold of these enzymes is very well conserved, with the largest rms deviation being ∼2.3 Å for the superposition of CluPG1 with the bacterial PG from Erwinia carotovora.25 However, despite their structural similarity, PGs differ in their capability to interact with PGIPs. While all bacterial and plant PGs tested so far are not recognized by PGIPs, many fungal PGs are selectively recognized and inhibited by certain PGIP isoforms and not by others.6 CluPG1 shares with AnPGII and FmPG the property of being inhibited by PvPGIP2. Interestingly, AnPGII and FmPG are inhibited with different mechanisms, that is, noncompetitive and competitive, respectively.10, 11
CluPG1 and AnPGII26 are 65% identical in sequence and their superposition results in a rms deviation of 0.6 Å, for 334 equivalent Cαs [Fig. 1(B)]. Three insertions are present in CluPG1 with respect to AnPGII: the first is an Asn residue in position 117, in the middle of the 3rd T3-turn, the second is a Ser residue in position 153, on the 3rd T1-turn and the last one is a two residue (Gly356 and Ser357) insertion in a loop of the C-terminal tail. Asn117 insertion narrows the active site cleft with respect to AnPGII, reducing the distance between the 3rd T3-turn and the 9th T1-turn from 11 to 8 Å.
CluPG1 and FmPG10 share a sequence identity of 48% and can be superimposed with a rms deviation of 1.0 Å for 321 equivalent Cαs [Fig. 1(B)]. Although the T1-turns delimiting the active site cleft are conserved, the T3-turns on the opposite side of the funnel are different between the two enzymes, being longer in FmPG. Three single aminoacid insertions are present in FmPG in the T3-turns, Asp84 on the 2nd coil and Asp127 and Asn121 (FmPG numbering) on the 4th coil. Moreover an insertion of eight residues (Asn178-Pro185) on the 5th T3-turn of FmPG is the most notable difference between the enzymes [red circle in Fig. 1(B)]. This insertion, together with that of Asn121, contributes to make the substrate binding cleft of FmPG deeper and narrower than in the other fungal enzymes. Other minor differences are present in the C-terminus of CluPG1, where an Asn residue insertion is located on the 3rd T1-turn of FmPG. N-glycosylation occurs in all three enzymes on the opposite side of the β-helix with respect to the active site cleft [Fig. 1(A)]. The Asn residues are located on solvent exposed turns between PB2a and PB2b in both CluPG1 and AnPGII, whereas they are located between PB1 and PB2b in FmPG.
To assess how structural similarities and differences among CluPG1, AnPGII and FmPG correlate with their activity and their mode of interaction with PGIPs, we have characterized CluPG1 kinetic parameters for the hydrolysis of polygalacturonic acid in the absence and in the presence of PvPGIP2 [Fig. 2(A)]. CluPG1 exhibited a Km of 0.15 mg/mL and a specific activity of 1187 RGU/mg. The Km value was similar to that of AnPGII (<0.15 mg/mL)27 and significantly lower than that of FmPG (0.56mg/mL),10 whereas specific activity was intermediate between that of AnPGII (2000 RGU/mg) and FmPG (500 RGU/mg). Since the active site dimensions decrease from AnPGII to CluPG1 to FmPG, a correlation with the decreasing specific activities may occur. The Km values did not vary significantly in the presence of increasing concentrations of PvPGIP2 (4 nM, 8 nM), while Vmax decreased from a value of 1187 RGU/mg for the uninhibited enzyme to a value of 530 RGU/mg in the presence of 8 nM PvPGIP2 [Fig. 2(A)]. The observed variation indicates a noncompetitive mode of inhibition, such as that already observed for AnPGII with both tomato PGIP28 and PvPGIP2.11
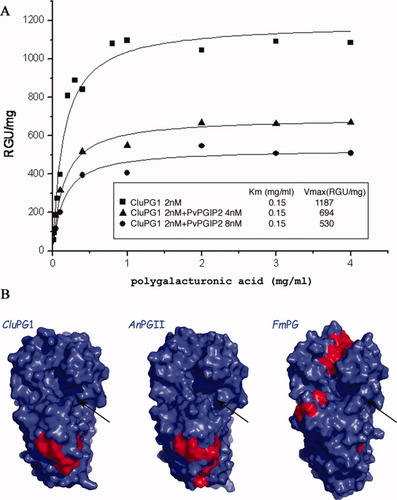
(A) CluPG1 enzyme kinetics and inhibition played by PvPGIP2. The uninhibited enzyme is represented in squares; triangles and circles represent the data obtained in the presence of 4 and 8 nM PvPGIP2, respectively. Curves represent the fits of experimental data with non-linear regression analysis using the Michaelis–Menten equation. Km and Vmax values are reported in the inset. A decrease in Vmax while Km remains constant is indicative of noncompetitive inhibition. (B) Optimal docking areas of CluPG1, AnPGII, and FmPG. Surface residues with an ODA value lower than −2 kcal/mol are colored in red, otherwise in blue. Black arrows mark the position of the active sites. The putative interaction areas of CluPG1 and AnPGII, located below the binding cleft, are almost superimposable. Differently, FmPG shows two distinct patches on opposite sides of the active site. A different PG-PGIP interaction area for the latter enzyme with respect to CluPG1 and AnPGII is suggested, which accounts for the different modes of inhibition observed. The view is ∼90° anticlockwise rotated over the z-axis with respect to Figure 1.
An analysis of the potential interaction areas of the various PG enzymes with PvPGIP2 was performed using the Optimal Docking Area algorithm (ODA) [Fig. 2(B)].24 The ODA algorithm has been successfully used to identify portions of a protein surface that are prone to interact with a protein–ligand, by means of surface desolvation energy calculation. The analysis for CluPG1 identifies a patch of residues with favorable desolvation energy upon ligand binding, that is, a putative protein–protein interaction site, located at the N-terminal portion of the enzyme, just below the lower side of the active site cleft [Fig. 2(B)]. A second identified patch is located in the rear side of the β-helix with respect to the cleft. Both potential interaction sites are compatible with the noncompetitive inhibition observed since PGIP, in engaging those patches, would not hamper the interaction with substrate, allowing the formation of the enzyme–substrate-inhibitor complex. The interaction with PvPGIP2 might then cause or prevent a conformational change in the loops delimiting the active site cleft, resulting in a reduced Vmax.
Interestingly, AnPGII has two patches of residues with low desolvation energies that are almost completely superimposable with those of CluPG1 and located below the binding cleft [Fig. 2(B)] and in the rear side of the β-helix. In contrast, FmPG shows a completely different desolvation energy profile, lacking both the potential interaction sites conserved between CluPG1 and AnPGII, and being instead characterized by two distinct low desolvation energy patches: one in the C-terminus, above the binding cleft, and one in the loop delimiting the lower part of the binding cleft [Fig. 2(B)]. Such ODA distribution suggests that the inhibitor contacts FmPG on both sides of the binding cleft, preventing substrate access, in accordance with the competitive mode of inhibition observed for this enzyme.10 Notably the FmPG loop that is predicted to be involved in the interaction with PvPGIP2 contains an insertion of eight amino acids with respect to CluPG1 and AnPGII [Fig. 1(B)]. This analysis is also corroborated by the observation that the bacterial PG from Erwinia carotovora,25 which is not recognized by PGIPs, does not show any defined cluster of ODA residues in this region (data not shown).
In conclusion, the structure of CluPG1 improves our current understanding of the structural requirements of fungal PGs for the interaction with PGIPs. There is now compelling evidence that the different modes of inhibition observed correlate with peculiar structural features of the enzymes and that PGs from different fungi may engage the same plant PGIP with different areas of their surface. This is the consequence at the protein level of the counter-adaptation of PG and PGIP genes in the host-pathogen arms race. Since the perception of PGs by PGIPs plays an important role in activating plant immunity, our combination of functional, structural and computational approaches to study the details of this protein–protein interaction may be useful to rationally design or select PGIP mutants with improved characteristics for crop protection.
Acknowledgements
We thank the staff scientists at the ELETTRA synchrotron (Trieste, Italy) for setting-up the experimental beamline for data collection.




