Stereotactic radiotherapy: An educational narrative review
Abstract
Stereotactic radiotherapy is a term collectively used to describe the radiation treatment techniques that allow for the delivery of highly precise ionizing radiation. It is usually a high dose per session in single or few fractions. This treatment approach has been in medical use for over six decades and has primarily evolved in the last two decades. Many patients benefit from this unique non-conventional radiotherapy approach. Its indications include various malignant, benign and functional problems in cranial and body sites. This technique is not widespread in developing countries compared to developed countries. This work is an educational narrative review for learners in radiation oncology. We aim to share the knowledge of this practice to improve precision radiation oncology globally. This review summarizes the basics of stereotactic radiotherapy, the technical prerequisites, the clinical considerations, the practical recommendations and the learning points from each site-specific region.
1 INTRODUCTION
Radiation Oncology techniques have evolved recently. This helps achieve conformal treatment volumes, reduce margins around targets, and reduce radiation doses to critical structures, thereby reducing radiation toxicities. It also helps escalate radiation doses to the targets to improve tumor control, and, in some settings, it even creates the opportunity to increase focal doses to ablative levels. The concept of stereotactic was coined from brain surgery, which aimed to minimize invasive procedures by utilizing planned coordinate systems, immobilizing devices, and diagnostic radiology.1 Lars Leksell was a visionary neurosurgeon who integrated stereotactic surgical devices to offer RT and introduced Stereotactic Radiosurgery (SRS).2
Stereotactic radiation treatment has emerged as a non-invasive alternative to surgery at multiple sites, avoiding invasive procedures.3 The current evidence shows that the stereotactic technique is feasible for relatively smaller tumors, and data is emerging for larger lesions.3, 4 Stereotactic Ablative Radiotherapy (SABR), also referred to by many scholars as Stereotactic Body Radiation Therapy (SBRT), is an advanced technique that necessitates specialized training for all members of the RT team to acquire expertise in procedural documentation, simulation and quality assurance protocols, the use of specialized equipment, audits, and network development to improve educational and training programs, ultimately improving patient selection and optimizing patient care.5
During the last two decades, many developed countries have adopted precision radiation oncology and leapt toward their goal.6 Its utilization in developing, lower and lower-middle-income counties (LMIC) is still in the early phases. A survey from the LMIC region reported only 10% utilization of the SABR modality from their potential case and identified lack of recourse, infrastructure and training as the main limiting factors for adaptation of this modality.7 This makes it an essential core of learning at the postgraduate residency level in any well-structured radiation oncology certification program.8, 9 The Accreditation Council for Graduate Medical Education (ACGME) recommends logging 30 cases (10 with SRS and 20 with SABR) to fulfill residency training requirements in radiation oncology.10 More recently, the role of SABR in inoperable oligometastases has led to encouraging reports showing a new paradigm in radiation oncology, as reported by the SABR COMET group.11
We intend to summarize the basics of stereotactic radiation. We focused on indications, contraindications, prerequisites, motion management, target volume delineation, planning objectives regarding targets and site-specific learning points, planning objectives for organs at risk (OARs), and image-guided radiotherapy (IGRT) with treatment delivery tips for learners to achieve better global precision radiation oncology.
2 BASICS
2.1 General indications and benefits
Stereotactic techniques have emerged as a non-invasive modality compared to surgery. It is particularly useful in treating patients with oligometastatic disease or certain early-stage cancers (including non-small cell lung, prostate, and hepatocellular carcinomas) and in settings of reirradiation. It has the advantage of shorter treatment schedules for patient convenience and better resource utilization compared to the conventional schedule and cost-effectiveness.12
2.2 Definition, terminologies and acronyms – SRS, SRT, FSRT, SABR & SBRT
Techniques that allow delivering highly precise (stereotactic) ionizing radiation (Gamma-ray or X-ray) and usually of high dose (of 6 Gray and more) in single or few fraction(s) (fx). The terms can be classified based on intracranial and extracranial treatments. If treatment is limited to the cranium region with a single fx, a single-day procedure mimicking surgery is termed Stereotactic Radio-Surgery (SRS). If intracranial treatment is given over 2 to 5 fx and the dose per fx is high, it is termed Stereotactic Radio-Therapy (SRT). If the number of fx and dose per fx are conventional, it is referred to as fractionated SRT (FSRT). Stereotactic Ablative Radiotherapy (SABR) is used for any site outside the cranial region for single or multiple fx, and it is also known as Stereotactic Body Radiotherapy (SBRT).13 In this work, we will use SABR as a synonym for SBRT and refer collectively to all of these techniques as “stereotactic radiotherapy”.
2.3 Radiobiology
Historically, the 5 “R's” of tumor biology includes repair of sublethal cellular damage, repopulation of the cell after radiation, redistribution of cells within the cell cycle, reoxygenation of the surviving cells and radiosensitivity. With new high-dose-per-fraction, ablative doses to the tumor introduce potent anti-tumor immune response and enhanced vascular injury, which can produce advantageous oncologic outcomes. Modern technology precision allows the safe delivery of these doses while keeping normal tissue injury to a minimum. Moreover, it overcomes the reoxygenation, redistribution and repopulation phenomena predominating alpha killing in the LQ model,14, 15 as treatments may be effective in as little as a single fraction, and are completed in a short time frame that negates the effects of repopulation.
2.4 General contraindications and toxicities
The general contraindications are the larger lesions, numerous small lesions or the sensitive location of the targets, as these concerns will make stereotactic radiotherapy prohibitive - either due to concern for unacceptable normal tissue toxicity with large dose per fraction hitting a large volume of surrounding normal tissue, or unacceptable treatment toxicity to a sensitive area. The general toxicities can be more or less similar those of any RT, with the chance that late radiation-related toxicities might be higher compared to conventionally fractionated RT.
3 TECHNICAL PREREQUISITES
3.1 Safety and quality in stereotactic radiotherapy
The importance of quality checks is mandatory. Still, stereotactic radiotherapy is not an immune technology, and incidents may occur during contouring, planning, quality assurance or treatment positioning. So, it is pivotal that all disciplines work as a team to prevent any incident that may result in a missed tumor target or damage to normal tissue with a high radiation dose. Quality and safety culture can be achieved by proper training of “Men”, knowing the options of “Machine”, and being adherent to guidelines and protocols, i.e., “Methodology” with the patient-centered approach being kept on the highest level.16
3.2 Imaging and registration for precise contouring
Delineating a precise target with the sparing of OARs is the goal for contouring. This requires carefully selecting imaging modalities, including a particular MRI sequence or PET/CT scan fusion with the simulation CT. While registering these imaging modalities, one may use rigid or deformable fusions depending on the case-to-case variation. All fusions have challenges, including random fusion errors, image distortion from MRI, intensity variation from PET and artefacts from organ motion are few examples. Image fusion always helps define the correct target volume while keeping all the above potential errors.17 A list of complementary imaging modalities for fusion according to each tumor is suggested in Table 1.
3.3 Motion management systems
The main aim is to minimize radiation around the target region. The use of 4D-CT can reduce the uncertainty in target delineation due to respiratory motion. Managing the movement of the targets and OARs by reducing internal motion (e.g. respiration) and monitoring the setup errors results in accurate stereotactic radiotherapy, which can translate into reduced toxicity.18 Motion management systems are divided into two main regions: cranial and extra-cranial.
3.3.1 Cranial
- Framed: The history of stereotactic radiotherapy started using a frame-based invasive system with a bolt over the skull to fix the frame (by screws). This frame helps to achieve the following three factors, including (i) immobilization by fixing the skull anatomy, (ii) target localization by identifying coordinates and (iii) treatment setup for delivering stereotactic radiotherapy.19
- Frameless: These may include base plates, CT localizers, treatment localizers, head holders and clamps with a mask system. Usually, a specially designed thermoplastic mask has a fixed shell with a cushion and is now commonly used. A small field of view (FOV) with high resolution covers the anatomy and all immobilization devices in the field. A non-invasive method of immobilization systems has made it a popular and convenient choice.20
3.3.2 Extracranial
The greatest challenge in extracranial stereotactic radiotherapy is motion management. The main focus is to manage and limit the internal motion of organs, especially the lung, liver and pancreas. Restricting movement to less than 5 mm is the primary objective. Table 1 illustrates five techniques are commonly used in this regard:
| Types | Details | |
|---|---|---|
| 1 | Motion-encompassing methods | 4D, the fourth dimension, is time. Therefore, 10 phases with normal breathing are suggested to acquire the target's movement in all directions. The 50% phase is the target region where contours should be made. |
| 2 | Respiratory Gating | This should be assessed on the first clinical visit. Coaching plays a vital role in acquiring the desired results. A patient who cannot breathe regularly will not be a candidate for the gating technique, as out-of-phase treatment will result in misadministration of the offered stereotactic radiotherapy. |
| 3 | Breath Holds | Either as deep inspiration breath hold (DIBH) or deep expiration breath hold (DEBH). During DIBH, the arching of the back muscles is observed. The aim for breath hold is 20 seconds. |
| 4 | Forced Shallow breathing methods | The diaphragm mainly contributes to the internal organ movement. Very useful for Liver treatment. |
| 5 | Respiration synchronized technique | If movement is more than 5 mm, it is advised to control the motion via gating, breathing hold, chest compression or target tracking |
During Simulation and treatment, different gadgets are used. The most used systems are whole-body Vac-Lok™ or Pro-Lok™ bags. Other options include using an abdominal compression plate to reduce diaphragm movement and implementing breath hold in a specific phase of treatment delivery with three times the scan to assess reproducibility.21, 22
3.4 Target contouring
In RT, GTV is the gross tumor volume clinically examined or seen by different imaging modalities. The CTV is the clinical target volume for microscopic disease, depending on the pattern of failure of the disease. The ITV is the internal target volume margin added to compensate for the internal physiologic movement and variations in tumor position for particular body sites affected by respiratory motion, such as lung or liver tumors. The PTV is a safety margin that accounts for systematic and random uncertainties in dose delivery and treatment planning.23 In stereotactic radiotherapy, GTV is usually equal to CTV, and both are sometimes equal to the PTV; however, the latter depends upon the immobilization and tracking system used.
3.5 Planning objectives
Historically, dose inhomogeneity was considered a disadvantage. Still, with stereotactic radiotherapy, this “Double Trouble” effect has been turned into an advantage by ablating the tumor tissue with doses up to 160% of the prescription inside the GTV. This approach minimizes the dose outside the target region, sparing normal tissue with a rapid fall-off. Therefore, optimum coverage for a stereotactic radiotherapy plan is generally between 60–95% of the prescribed isodose line to the target region.24
The TV is the target volume, TV (PIV) is the target volume covered by the prescription dose, and V(RI) is the body covered by the prescription dose measured in cubic centimeters (cc). For plans with equivalent CI, the gradient index (GI) can be calculated as the ratio of the 50% isodose volume to the 100% isodose volume. It is typically > 2, with lower values representing a steeper dose fall-off outside the PTV.25
Another critical consideration usually in practice is evaluating stereotactic radiotherapy dose spillage by two different parameters. First, “high dose spillage”, which is any dose > 105% of the prescription dose, should occur primarily within the PTV itself and not within the normal tissues outside the PTV. The other is “low dose spillage”, a fall-off gradient evaluated by the maximum total dose to any point 2 cm from the PTV in any direction.26
3.6 Image Guided Radiation therapy (IGRT) with treatment delivery tips
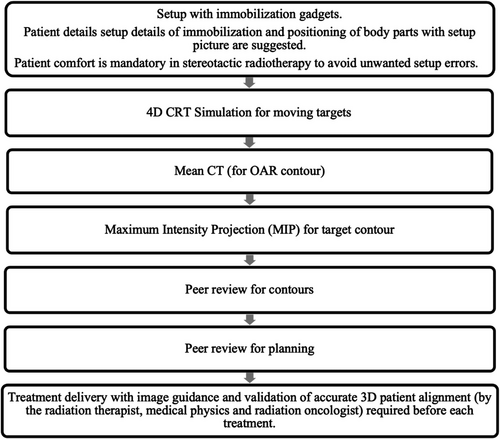
IGRT is a mandatory component to visualize and reduce inter & intra-fraction variabilities, which include setup (patient positioning, out-of-plane rotations, weight changes, registration errors, shift of skin marking, patient discomfort or involuntary motion) or organ motion variabilities (volume changes, diaphragm movement, bladder and rectal filling, bowel gases & peristalsis & cardiac motion). Figure 1 illustrates IGRT workflow for optimizing stereotactic radiotherapy.
3.7 Machines' options
There are some machines that can be used for stereotactic radiotherapy, and Table 2 summarizes this issue.
| Linac Based | Gamma Knife (GK) | CyberKnife (CK) | |
|---|---|---|---|
| Source | Photons | Gamma rays | Photons |
| Site Treatment | Whole body | Cranial | Whole body |
| Mechanism | Micro MLC small field. | Pencil Beam | Pencil Beam |
| Imaging during Treatment | CBCT +/- KV imaging system | CBCT, live imaging with real-time infrared motion detection | KV tracking system (6D Skull, Spine, LOT, Fiducial) |
| Design | C shape arm | Helmet shape | Robotic Arm |
| Duration | Short | Long | Long |
| Conventional fractionation | Yes | No | Yes |
| Setup Margin | More as compared to CK & GK | Lesser as compared to Linac bases | Lesser as compared to Linac bases |
3.8 Treatment delivery monitors
During stereotactic radiotherapy, there are several techniques to monitor treatment delivery with tighter margins; this includes various gating techniques, including amplitude or phase-based gating. A newer approach is surface-guided radiation therapy (SGRT).27 KV, MV imaging via bony real-time matching, monitors delivery over CyberKnife (CK) and Linac-based treatment monitoring system.28, 29 Another option for certain moving targets involves target tracking via the insertion of fiducial markers in the target region with real-time tracking.30
3.9 On board imaging tolerance
The general principle for SRS / SRT is kept at 1 mm for all translational shifts and 1 degree for rotational shifts. For SABR, it is kept at between 3–5 mm for all translational shifts and 1 degree for rotational shifts.
4 CLINICAL CONSIDERATIONS
Here, we highlight a few of the most important and practical considerations when treating each disease site. While this is not a universal or comprehensive list, it should help the learner with essential treatment considerations, such as a primary or secondary malignant lesion, a benign lesion, or a functional disorder.
4.1 Primary malignant lesions
4.1.1 Head &Neck (H/N)
There is considerable heterogeneity in patient selection and techniques in H/NSABR practice among experienced centers. The main indication for SABR in H/N includes (a) Unresectable H/N sarcomas. (b) Reirradiation of H/N carcinoma; ideally > two years from treatment but certainly > six months from previous treatment. Careful selection of patients is advised. The best outcomes are seen in patients with smaller tumors and no skin involvement. For example, the locally recurrent nasopharyngeal carcinoma, due to its anatomic location & proximity to the optic structures, brain, brainstem, and spinal cord, where stereotactic radiotherapy can improve local control and survival with sparing of OAR.31, 32 Currently investigated schedules range from 35–44 Gy in 5 fx. The target for SABR is typically GTV with a narrow PTV margin of 2–3mm without CTV expansion. Sujith et al. proposed that a target volume of < 25 cc is the candidate for stereotactic radiation treatment, while larger target regions should be treated with a moderate hypo-fractionated schedule.33 Caution should be considered in cases of circumferential carotid artery involvement. Carotid reirradiation dose max is 33 Gy. The risk of carotid blowout quoted in the reirradiation setting ranges from 3–20%. When considering protecting the larynx in the setting of H/N reirradiation, a safe rule of thumb is to keep the mean dose to the larynx below 15 Gy.34
4.1.2 Primary Brain
Limited data is available even in reirradiation for gliomas, and no evidence of clear benefit for upfront SRS boost is available. Most of the literature is driven by the meningioma, which is continuously evolving, like for small (<3.5cm in diameter), recurrent, partially resected, surgically inaccessible (base-of-skull) meningioma and in patients who are poor surgical candidates. Target volume includes GTV with areas of nodular dural enhancement only, not linear enhancement. SRS doses typically range from 12.5 to 15 Gy, while SRT ranges from 21 to 30 Gy in 3 to 5fx, depending on the target's location. Due to brain parenchymal invasion, long-fractionated SRT is recommended for Grade II/III. OAR includes all brain and H/N structures near the target region.35
4.1.3 Pancreas
SABR is indicated in unresectable/borderline tumors. Fasting > 2 hours before planning CT is recommended. Triple-phased protocols (arterial, pancreatic, portal-venous phase) are performed. SABR with a dose of 40 Gy / 5 fx or 33Gy / 5 fx is attempted, depending on proximity to bowel, ensuring that the duodenum and large bowel critical structures receive a dose of 30 Gy <0.5cm3 and 32 Gy < 0.5cm3, respectively. Delineate GTVp in the pancreatic phase of the planning CT. Tumors smaller than 3 cm, especially in the pancreatic head, should consider using a CTV due to the size. The pancreas can have an average of 1 cm of motion in the cranio-caudal (CC) direction, and the PTV is generated after incorporating the ITV margin. Expiratory breath hold (DEBH) is preferred over inspiratory breath hold (DIBH) as expiration increases the distance between the bowel and the target. Limiting the motion with abdominal compression is an alternative and widely used option. OAR includes stomach & duodenum and small bowel around PTV with V15 < 9cc, V20 < 3 cc.36
4.1.4 Primary Liver
Primary liver SABR scenarios generally include (a) Unresectable primary hepatocellular carcinoma (HCC) with Child-Pugh A & limited patients of Child-Pugh B. (b) Tumor thrombus, as it is superior to TACE. (c) Bridging to liver transplant. Motion management and ITV margins are essential, similar to those described for pancreatic cases. The usual dose range is 35 – 50 Gy in 5 fx with the lower doses used for patients with worse liver function (e.g. Child-Pugh B or C). CT simulation with IV contrast is required to visualize the target on the arterial (bright) and portal venous phase (dull/washout). The red flags are bile duct stenosis and hepatitis. OAR includes the liver due to the Radiation-Induced Liver Disease (RILD).37, 38
4.1.5 Thorax
SABR is the standard of care for medically inoperable NSCLC (stage T1 – 2 N0 M0) with baseline Forced Expiratory Volume in 1 second (FEV1)<40%. A dose of 48 Gy / 4 fx to 54 Gy /3 fx is favored for peripherally located lesions with heterogeneity correction (up to 60 Gy/3 fx without heterogeneity correction), given with an inter-fraction interval of 40 hours to 7 days, with an overall treatment time of 8 to 14 days. A dose of 60 Gy/8fx, daily or every other day, is standard for central tumors, and moderately hypofractionated doses of 60 Gy / 15fx can be considered for ultra-central lesions. Respiratory motion management and ITV margins are critical, with greater motion present for lesions in the bases of the lungs (near the diaphragm). The OARs include lungs with V20 < 10%, chest wall (CW) with V30 <30 cc [V35 <1cc and Dmax<45 Gy for CW 2cm. Contour CW as a 2 cm tissue ring on the lung but should not compromise tumor coverage. Consider increased fractionation of 4–5 fx with high volume CW overlap with PTV] and brachial plexus of 20 Gy.39, 40
4.1.6 Chordoma & chondrosarcoma
The preferred treatment is maximal safe resection; nonetheless, radical removal is feasible in less than 50% of the cases due to the considerable associated post-operative morbidity and mortality. Adjuvant radiation is incorporated in residual disease and has favorable control with stereotactic radiotherapy compared to conventionally fractionated RT. Stereotactic radiotherapy offers good outcomes in the skull base and spine with modest toxicity. The median dose is 37.5 Gy (range 30 -40 Gy) / 5 fx.41
4.1.7 Pediatric tumors
- - Pediatric rhabdomyosarcoma: Protocol ARST1431 includes consolidation SABR to a metastatic lesion in patients with intermediate-risk stage IV for sites ≤5 cm with a dose of 35 Gy in 5 fx.42
- - Ewing's sarcoma: In metastatic lesions as in AEWS 1221, with a dose of SABR 40 Gy / 5 fx.43
- - Craniopharyngioma: Stereotactic radiotherapy is recommended for residual or recurrent tumors to improve local control and reduce in-field recurrences. A dose of 12 - 18 Gy SRS & 21 – 30 Gy/3 – 5 fx SRT is used.44, 45
4.1.8 Prostate
Due to the low α/β ratio (1.4-3 Gy), many trials suggested a hypo-fractionation regimen in prostate cancer providing the same or better tumor control than conventional RT. The most robust evidence for prostate SABR is for low and intermediate-risk patients with a Gleason score of 7 or less. The role of SABR alone for high-risk patients is still evolving.46 Some critical issues are (a) Fiducial placement for on-treatment tracking and matching. (b) CT sim could be done with and without Foley's catheter (to delineate the urethra carefully). (c) Rectal gel spacer, if possible, as it has proven to provide better sparing of the anterior rectal wall. (d) Contour CTV prostate on T2-MRI fused with CT sim scan, a superior most slice of CTV is where seminal vesicle separates from prostate & inferior slice of CTV is marked at least 8 – 10 mm cranial to the penile bulb. PTV is 5 mm in all directions except for 3 mm posteriorly. (e) Ensure the urethra is delineated with MRI fusion, urethrogram or Foley catheter at simulation. (e) The total prescription dose ranges from 36.25 – 42.7 Gy in 5 – 7 fx. (g) Especially when using higher dose prescriptions, avoid hot spots at the urethra; generally, urethra Dmax< 120%.
4.1.9 Renal cell carcinoma (RCC)
SABR may defer systemic therapy in oligometastatic RCC, but long-term follow-up data are unavailable. The most commonly used dose under clinical trials is 40 Gy / 5 fx.47
4.2 Secondary malignant lesions
4.2.1 Brain metastasis
The main aim is local control. SRS is preferred for patients with 1 – 4 GTVs and a median survival over four months (e.g., KPS > 70). The preferred dose is 20 to 24 Gy. SRS doses below 18 Gy are not recommended as they may result in lower local control. However, if the target lies on the brainstem or an eloquent structure, reducing the dose (e.g. to 16 or 17Gy) may be necessary to avoid causing a neurologic deficit. OARs include brain, brainstem, optic pathways, pituitary, and cochlea. GTVs ≥3 cm may have poor local control with SRS and may be better treated with SRT, like 27 Gy/3 fx or 30–35 Gy/5 fx.48 Post-cranial metastatectomy stereotactic radiotherapy can reduce local recurrence compared to observation, and it is recommended.49
Re-treatment in brain metastases often refers to stereotactic radiotherapy for newly developed brain lesions after a prior brain RT or stereotactic radiotherapy, which is safe and routinely performed whenever indicated. With re-treatment of previously treated GTVs or close by new GTVs with overlapping isodoses, there is an increased risk of symptomatic radio-necrosis. Para-sagittal GTVs are at greatest risk of radio-necrosis due to poor blood supply.50 Discussing these cases with a neuro-radiologist and ruling out pseudo-progression, radio-necrosis, and leptomeningeal involvement is essential. If unsure about a potential brain lesion, consider following up with the patient with short-term interval imaging (depending on the pathology, shorter for cancer and longer intervals for benign lesions) to allow time for the lesion to “declare itself”. This is better than prematurely committing to treatment if you are unsure.
4.2.2 Bone metastasis
RT is a cornerstone in bone metastasis treatment for local and pain control. The median time to pain response is ∼3 weeks with either single or multi-fraction SABR. The single fraction SABR dose range is 18–24 Gy, leading to>90% LC. Caution with 24 Gy in vertebral metastases as there is a 40% chance for vertebral compression fractures, which can be reduced to 10% if a single fx SABR dose< 20 Gy.51 OAR are the spinal cord and cauda equina, which can be marked with MRI or Myelogram.
4.2.3 Liver metastasis
Optimal candidates for SABR in the liver are patients with preserved performance status, adequate liver function, solitary liver metastasis, and uninvolved liver volume >700 cc. In some cases, treating new liver metastases can help extend a patient's break from chemotherapy or be selected for treatment if they threaten liver function (and would eventually impair the patient's ability to take chemotherapy). Generally, a preferred SABR dose range is 30 – 50 Gy / 3 - 5 fx.52, 53
4.2.4 Lung metastasis
The role of SABR in oligometastatic lesions is maturing. There are better outcomes in overall survival, progression-free survival with no grade III toxicity, and less chance of being dependent on cytotoxic chemotherapy.11 Typically, the biologically effective dose (BED) ≥ 100 Gy10 is targeted for SABR. Most trials report five or fewer as a safe number to target lung metastasis following the same principles of primary lung SABR.54
4.3 Benign lesions
4.3.1 Arterio-Venous Malformation (AVM)
As per Spetzler–Martin grade, high-risk lesions can be treated with SRS. The dose range is 16–24 Gy to the margin of the nidus. Nidus identification must be assessed with an angiogram, CT Angiography and Magnetic Resonance Angiography (MRA). Embolization before radiosurgery critically affects the accurate delineation of the nidus and may decrease obliteration rates if done with SRS. Complete obliteration of AVM is reported in two-thirds of the patients with SRS in 3–5 years (Latent period). During this latent period, patients still carry the risk of hemorrhage (5-8%) and mortality (2%).55
4.3.2 Glomus Tumor
Glomus tumors are in the base of the skull region at the jugular foramen. Symptoms include tinnitus, hearing loss, & cranial nerve deficits. Stereotactic radiotherapy provides excellent local control >90% at ten years and symptomatic improvement in half of the patients. SRS of 14 – 16 Gy or SABR of 21–30 Gy in 3–5 fx for larger tumors. OAR are facial nerve, brainstem and cochlea.56
4.3.3 Schwannoma (neuroma)
Targets up to 3 cm without significant mass effect are considered appropriate for management with SRS, with a dose of around 12.5 Gy. Surgical debulking followed by SRT is suggested for larger tumors with a risk of cranial nerve dysfunction. Alternatively, 18 – 25 Gy / 3 – 5 fx SRT can be given in selected patients without debulking surgery in these large tumors. A ventriculoperitoneal shunt sometimes needs to be placed before SRT.57
4.3.4 Pituitary adenoma
SRS doses range from 14 –16 Gy for non-secretory and 16 – 20 Gy for secretory tumors. Especially in secretory adenoma, the effects of radiation to normalize the hormonal levels may take years. Surgical debulking to decompress the optic chiasm is necessary to relieve the pressure effects and avoid permanent damage, effectively reducing hormonal levels. SRT dose ranges from 21 – 25 Gy/ 3 – 5 fx for non-secretory and 24– 30 Gy/ 3 – 5 fx for secretory tumors. Post-stereotactic radio therapy hypopituitarism is not uncommon. OAR is optic apparatus.58
4.4 Functional disorders
4.4.1 Trigeminal neuralgia
It is recommended for patients who are refractory to medical options such as carbamazepine, phenytoin and gabapentin; and are elderly or not good surgical candidates for microvascular decompression (MVD). The trigeminal nerve root in the prepontine cistern is the primary target area. Selecting proximal vs distal trigeminal root, an isocentric vs volume (6×3×3 mm target) varies per practice in different institutions and equipment. CT cisternography or 1mm MRI thickness (FIESTA/CISTERNO / 3D T1 Gadolinium or heavily weighted T2W sequence) fused with CT simulation are used for treatment planning. SRS dose range is 60– 90 Gy, prescribed to the 80 – 100% isodose line.59
4.4.2 Epilepsies
Mesial temporal lobe epilepsy (MTLE) refers to a chronic condition of recurrent seizure activity focally originating in the temporal lobe, namely the amygdala and hippocampus. The preferred treatment of MTLE is anti-epileptic medication. Medical refractory MTLE cases are treated with surgery. Alternatively, SRS can be given with a dose of 16–20 Gy.60
5 PRACTICAL RECOMMENDATIONS
5.1 Outcomes, dosimetric objectives and endpoints
The HyTEC group has published peer-reviewed data on doses, volumes and clinical outcomes for stereotactic radiotherapy and the normal tissue complication probabilities for stereotactic radiotherapy. This information is aimed to assist the treating physician in maintaining a balance and maximize the therapeutic ratio for patients undergoing stereotactic radiotherapy.61 The details can be viewed in the Tables 3 and 4.
| Tumor site/type | Targets and PTV margins used in studies | Image fusion (New tips by authors) | Number of fx | Dose (Gy)* or dose volume parameters | Endpoint | Rate (%) |
|---|---|---|---|---|---|---|
| Brain metastases | GTV + 0–2 mm | MRI T1W Contrast Enhanced (CE) | 1 | 18-24 Gy (≤ 2 cm) | 2-year local control by size | 80-95% |
| 18 Gy (2-3 cm) | 66% | |||||
| 15 Gy (> 3cm) | 47% | |||||
| 24–30 Gy (2-3 cm) | 65–85% | |||||
| 3 | 21-27 Gy (> 3cm) | 53–69% | ||||
| 30–35 Gy (2-3 cm) | 75–85% | |||||
| 5 | 25-30 Gy (> 3cm) | 59–69% | ||||
| Vestibular schwannoma | GTV + 0–2 mm | MRI T1W CE | 1 | >12 Gy | 3-5 year local control | >91% |
| 3 | 18 Gy | >91% | ||||
| 5 | 25 Gy | >91% | ||||
| Head & neck retreatment | GTV + 0–6 mm | PET-CT, MRI T1W CE | 5 | 45 Gy | 2-year local control | 50% |
| Lung T1-2 Lesions | ITV or IGTV + 3–8 mm | PET-CT with a lung window setting | 3 | >60 Gy | 1-5 year local control | >80-85% |
| 45–55 Gy | >75% | |||||
| 33 Gy | <50% | |||||
| 4 | >52 Gy | >80–85% | ||||
| 42–48 Gy | >70% | |||||
| 5 | >50 Gy | >80% | ||||
| Liver primary tumor | Variable | CT Triphasic, MRI Multiphase dynamic | 3-5 |
BED10 = 60–72 Gy Examples: 33–54 Gy in 3fx 48 Gy in 4fx 40–50Gy in 5fx |
2-year local control | 90% |
| Liver metastases | Variable | CT CE or MRI CE, PET-CT | 1-5 | BED10>100 Gy | 2-year local control | >90% |
| 1-5 | BED10<100 Gy | 65–76% | ||||
| Adrenal | ITV or IGTV + 3–10 mm |
PET-CT MRI T1W CE |
Median 5 |
BED10 >116 Gy Examples: 45Gy in 3fx 55Gy in 5fx |
1-year local control | >95% |
| Pancreas | GTV + 2–5 mm | CT Dual Phase, PET Scan | 1 | 20-25 Gy | 1-year local control without surgery | 79-88% |
| 3 | 30-36 Gy | 79–86% | ||||
| 5 | 33 Gy | 77% | ||||
| Prostate | CTV+ 0–5 mm | MRI T2W | 5 | 36.1 Gy (low-intermediate risk) | 5-year freedom from biochemical relapse | 95% |
| 5 | 38.7 Gy (high risk) | 95% | ||||
| Spine | CTV + 0–2 mm | MRI T1W CE and T2W | 1 | 18 Gy | 2-year local control | 82% |
| 20Gy | 90% | |||||
| 22Gy | 94% | |||||
| 24Gy | 96% | |||||
| 24 Gy | 82% | |||||
| 2 | 27 Gy | 78% | ||||
| 3 | 30 Gy | 85% |
- * Recall that the prescription isodose line used in stereotactic radiotherapy is typically 50–95%, depending on the technology used, disease site, and tumor size (smaller tumors can safely be prescribed to lower isodose lines than larger tumors).
| Organ | Volume segmented | Number of fx | Dose or DVH | Endpoint | Rate (%) |
|---|---|---|---|---|---|
| Brain: For the metastases | Total brain including target | 1 | V12Gy <5 cm3 | Symptomatic necrosis or edema | 10% |
| V12Gy <10 cm3 | 15% | ||||
| 3 | V12Gy <15 cm3 | 20% | |||
| V20Gy <20 cm3 | <10% | ||||
| 5 | V20 Gy <30 cm3 | <20% | |||
| V24 Gy <20 cm3 | <10% | ||||
| V24 Gy <30 cm3 | <20% | ||||
| Brain: SRS for AVM | Total brain including target | 1 | V12Gy <5 cm3 | Symptomatic necrosis | 10% |
| Optic pathway | Optic nerves and chiasm | 1 | Dmax<10-12 Gy | Neuropathy | <1% |
| 3 | Dmax<20 Gy | Neuropathy | <1% | ||
| 5 | Dmax<25 Gy | Neuropathy | <1% | ||
| Carotid artery (retreatment) | Each artery | 5 | Dmax<20- 30 Gy | Grade 3–5 bleeding | <2–12% |
| D0.5cc <20 Gy | <2–12% | ||||
| Lungs | Combine lungs minus Target | 2-5 |
Mean Dose <8 Gy V20Gy <20-15% |
Grade 2 or more | 10-15% |
| Liver, SABR for primary lesions | Liver minus GTV | 3 | Mean dose <13 Gy | Grade 3 or more enzyme changes | <20% |
| 6 | Mean dose <18 Gy | <20% | |||
| Liver, SABR for metastases | Liver minus GTV |
3 6 3–6 |
Mean dose <15 Gy Mean dose <20 Gy V(<15Gy) >700 cm3 |
Grade 3 or more enzyme changes Liver dysfunction and grade 3–5 GI toxicity |
<20% <20% <13% |
| Bladder | Bladder(as a solid organ) | 4-5 | V(Prescription Dose)<5-10 cm3 | Late Grade 2 or more urinary toxicity | <20% |
| Rectum | Rectum (as a solid organ) | 4-5 | Dmax<35-38 Gy | Late Grade 3 bowel toxicity | <3% |
| Urethra | Prostatic urethra | 4-5 | Dmax<38-42 Gy | Late Grade 2 or more urinary toxicity | <20% |
| Spinal cord | The spinal cord, canal or thecal sac | 1 | Dmax<12.4-14 Gy | Myelopathy | 1-5% |
| 2 | Dmax<17-19.3 Gy | 1-5% | |||
| 3 | Dmax<20.3-23.1 Gy | 1-5% | |||
| 4 | Dmax<23-26.2 Gy | 1-5% | |||
| 5 | Dmax<25.3-28.8 Gy | 1-5% |
5.2 Prophylactic treatment considerations
One potential side effect of stereotactic radiotherapy is the development of radiation-induced edema. Short-course corticosteroids, such as dexamethasone and prednisolone, have anti-inflammatory properties and can help to reduce swelling and pain.62
5.3 Follow-up imaging with outcomes
In stereotactic radiotherapy, follow-up imaging is crucial for assessing treatment response, monitoring for potential complications and guiding further management. The specific imaging plan may vary depending on the individual case, treatment type, and the treated pathology. For example, after stereotactic radiotherapy for malignant brain lesions, a contrast-enhanced MRI is performed after three months to assess the initial response and serve as a baseline for future follow-up (as chances of developing other brain metastasis are 60% in the first year, which reduces to 15% in the second year). In the case of benign lesions, the first post-treatment imaging is usually performed after six months. Long-term surveillance is essential for potential late toxicity for benign lesions and tumor recurrence at the treatment site or out-of-field recurrence or progression of the disease. Usually, a 3-monthly scan is scheduled for patients in the first two years.63
6 CONCLUSIONS
Stereotactic radiotherapy has proven to be an effective complex RT technique for managing various malignant, benign, and functional medical problems. This review provides a summary of the basics of stereotactic radiotherapy, the technical prerequisites, the clinical considerations and some practical recommendations. These focus on indications, contraindications, target contouring, organs at risk parameters, tissues’ constraints, immobilization devices, and the learning points from each site-specific region. This information is catered for early learners in radiation oncology to achieve better precision in global radiation oncology.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.