Rational redesign of neutral endopeptidase binding to merlin and moesin proteins
Abstract
Neutral endopeptidase (NEP) is a 90- to 110-kDa cell-surface peptidase that is normally expressed by numerous tissues but whose expression is lost or reduced in a variety of malignancies. The anti-tumorigenic function of NEP is mediated not only by its catalytic activity but also through direct protein–protein interactions of its cytosolic region with several binding partners, including Lyn kinase, PTEN, and ezrin/radixin/moesin (ERM) proteins. We have previously shown that mutation of the K19K20K21 basic cluster in NEPs' cytosolic region to residues QNI disrupts binding to the ERM proteins. Here we show that the ERM-related protein merlin (NF2) does not bind NEP or its cytosolic region. Using experimental data, threading, and sequence analysis, we predicted the involvement of moesin residues E159Q160 in binding to the NEP cytosolic domain. Mutation of these residues to NL (to mimic the corresponding N159L160 residues in the nonbinder merlin) disrupted moesin binding to NEP. Mutation of residues N159L160Y161K162M163 in merlin to the corresponding moesin residues resulted in NEP binding to merlin. This engineered NEP peptide–merlin interaction was diminished by the QNI mutation in NEP, supporting the role of the NEP basic cluster in binding. We thus identified the region of interaction between NEP and moesin, and engineered merlin into a NEP-binding protein. These data form the basis for further exploration of the details of NEP-ERM binding and function.
Introduction
Neutral endopeptidase (NEP; neprilysin, enkephalinase, CD10) is a 90- to 110-kDa zinc-dependent metallopeptidase that cleaves peptide bonds at the amino end of hydrophobic amino acids. NEP inactivates a variety of bioactive peptides, including atrial natriuretic factor, substance P, bradykinin, oxytocin, Leu- and Met-enkephalins, neurotensin, bombesin, endothelin-1, bombesin-like peptides, and amyloid-β.1-3 NEP is normally expressed by numerous tissues, including prostate, kidney, intestine, endometrium, adrenal gland, and lung. Loss or decrease of NEP expression has been reported in a variety of malignancies, including ∼ 50% of prostate cancers.4
The anti-oncogenic function of NEP has been found to be due not only to its catalytic activity but also due to direct protein–protein interactions with other proteins.5 In particular, the ezrin/radixin/moesin (ERM) proteins6 are important binding partners of NEP. ERM proteins consist of an N-terminal 4.1 protein, ezrin, radixin, moesin (FERM) domain followed by a coiled-coil segment and a C-terminal domain containing an actin-binding motif.7 ERM proteins are thought to switch between a closed conformation, which is inactive, and an open conformation. The open confirmation of ERM proteins mediates the association of certain plasma-membrane receptors with the actin cytoskeleton and regulates signaling by Rho family GTPases. Phosphorylation of a conserved C-terminal serine residue and phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] binding to the domain disrupts the head-to-tail interaction which maintains ERM proteins in a closed conformation, resulting in their activation.8 ERM proteins supply a functional linkage between integral membrane proteins and the cytoskeleton in mammalian cells to regulate membrane-protein dynamics and cytoskeleton rearrangement. Ezrin has already been shown to promote tumorigenesis. It is necessary for Net- and Dbl-mediated transformation of fibroblastic cells and it enhances metastasis in mouse models of osteosarcoma and rhabdomyosarcoma. However, the mechanisms governing ERM protein promotion of tumorigenesis need to be further elucidated.8 We have previously shown that NEP coimmunoprecipitates with ezrin, radixin, and moesin in NEP-expressing LNCaP prostate cancer cells and MeWo melanoma cells.9 Coimmunoprecipitation showed that ERM proteins associate with wild-type NEP protein, but not with NEP protein containing a truncated cytoplasmic domain or a NEP mutant in which the positively charged amino acids cluster in the cytoplasmic domain, K19K20K21, was replaced by residues QNI. In-vitro binding assays showed that the positively charged cluster is required for NEP binding to recombinant N-terminal fragments of ERM proteins. Binding of ERM proteins to NEP resulted in decreased binding between ERM proteins and one of their main binding partners, the hyaluronan receptor CD44.9 Cells expressing wild-type (but not mutated) NEP demonstrated decreased adhesion to hyaluronic acid and cell migration. These data suggest that NEP can affect cell adhesion and migration through direct binding of ERM proteins.5, 9
A protein that displays significant sequence similarity to the ERM proteins is merlin, encoded by the NF2 (neurofibromatosis type 2) gene. Merlin shares its domain organization with ERM proteins but does not contain a canonical actin-binding motif at its C terminus. The phosphorylated, presumably open form of merlin can form heterodimers with ezrin and other ERM proteins and localize at the cell cortex. The dephosphorylated form of merlin opposes cell proliferation and transformation and is, therefore, considered active. This anti-mitogenic and tumor-suppressive function of merlin is unique and contrasts with the ERM proteins' functions.8, 10
Here we investigate the binding between NEP and moesin, an ERM protein, and between NEP and merlin. We show that NEP does not bind merlin. We then use experimental data, threading, and sequence analysis to identify residues in ERM that are likely to be the binding determinants of NEP. We show that swapping these binding determinants in moesin to amino acids that occupy the same positions in merlin disrupts the binding of moesin to wild-type NEP. Swapping this region in merlin for the corresponding region in moesin results in gain of binding to wild-type NEP, but not to NEPs' QNI mutant. We thus identify the interacting region between NEP and moesin, and engineer merlin to get a NEP-binding protein. These data form the basis for further exploration of the structural details of NEP-ERM binding.
Results
Merlin and NEP do not coassociate
Our previous studies have shown that ERM proteins coimmunoprecipitate with NEP in prostate cancer cell lines9 and NEP coassociates with tumor-suppressor proteins such as PTEN.11 We considered whether NEP might also interact with the tumor-suppressor ERM-like protein merlin. For these experiments, we used (a) NEP-expressing LNCaP prostate cancer cells; (b) WT-5 cells, a derivative of Tsu-Pr1 bladder cancer cells, in which NEP expression is regulated by a tetracycline-repressible system (Tet-off)12; and (c) DU-145 prostate cancer cells that lack NEP expression. As shown in Figure 1, merlin protein was expressed in all three cell types. Western blotting of NEP following immunoprecipitation using anti-merlin antibody of cell lysates derived from WT-5 cells cultured without tetracycline (NEP expressed) or LNCaP cells indicated that NEP and merlin proteins do not associate with each other [Fig. 2(A), upper panel]. Similar results were obtained with Western blotting of merlin following immunoprecipitation using anti-NEP antibody [Fig. 2(A), lower panel]. Studies in mammalian cells have shown that phosphorylation of S518 in merlin is essential for intramolecular interactions between its C-terminal tail and the FERM domain. Hypophosphorylated merlin is localized to the membrane–cytoskeleton interface and interacts with many membrane-associated ERM partners via its FERM domain. It also interacts either directly or indirectly with the actin cytoskeleton.13-15 We therefore assessed whether phosphorylation of S518 influences merlin's interaction with NEP. We cotransfected NIH3T3 cells with vectors expressing NEP, and either the nonphosphorylatable mutant of merlin (S518A) or its phosphomimicking mutant (S518D). Western blotting of NEP immunoprecipitates showed that NEP and merlin proteins do not associate, regardless of the phosphorylation state of S518 [Fig. 2(B), lower panel]. Similar results showing no association between merlin and NEP were detected in merlin- and NEP-expressing NIH3T3 cells immunoprecipitated with an antibody to Flag and Western blotted with an antibody to NEP [Fig. 2(B), upper panel]. Finally, as we had previously shown that NEP associates with ERM proteins using an in-vitro binding assay,11 we performed a similar experiment using the N-terminal domain of merlin. Again, we could not demonstrate any association between merlin and NEP [Fig. 2(C)]. Together, these data show that in contrast to ERM proteins, merlin does not associate with NEP.
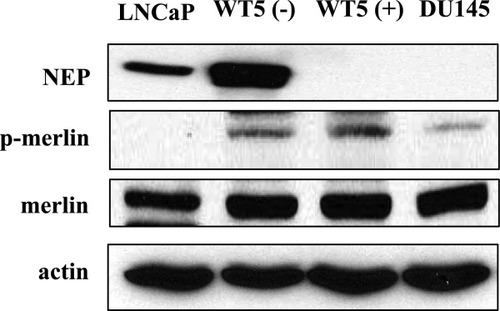
Merlin and NEP expression. Lysates from LNCaP, DU145, and WT-5 cells cultured with (+) or without (−) 1 μg/mL tetracycline were subjected to SDS-PAGE followed by immunoblotting using anti-NEP monoclonal antibody (Blot: NEP), anti-merlin polyclonal antibody (Blot: merlin), anti-phospho-merlin polyclonal antibody (Blot: p-merlin), and anti-actin antibody (Blot: actin) as a control. NEP expression was nearly completely repressed with 1 μg/mL tetracycline.
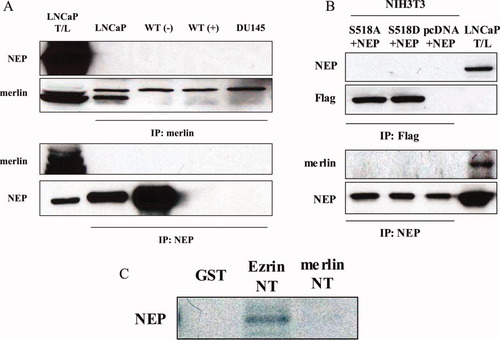
Lack of merlin-NEP coimmunoprecipitation. A: Cell lysates immunoprecipitated with anti-merlin antibody (IP: merlin) or anti-NEP antibody (IP: NEP) were separated by SDS-PAGE and immunoblotted with anti-NEP mouse monoclonal antibody (Blot: NEP) and anti-merlin antibody (Blot: merlin). LNCaP total lysate (T/L) was used as a control. Note that merlin did not coimmunoprecipitate with NEP in any cell type. B: Lysates from NIH3T3 cells cotransfected with the expression vector of NEP and the Flag-active mutant of merlin (S518A; lane 1) or Flag-inactive mutant of merlin (S518D; lane 2) were immunoprecipitated with anti-Flag antibody (IP: Flag) or anti-NEP antibody (IP: NEP). The immunoprecipitates were separated by SDS-PAGE followed by immunoblotting with anti-NEP mouse monoclonal antibody (Blot: NEP) and anti-merlin antibody (Blot: merlin). The pcDNA vector lacking the merlin sequence (lane 3) and LNCaP total lysate (T/L; lane 4) were used as controls. C: Lysates from LNCaP cells (500 μg) were incubated with 10 μg of glutathione-S-transferase GST-merlin N terminus (NT) or GST-ezrin NT fusion protein or GST control and immobilized on glutathione-Sepharose 4B beads for 3 h at 4°C. After washing, GST or GST fusion proteins were eluted with their binding proteins, separated by SDS-PAGE and transferred to a membrane. NEP was detected by immunoblot using anti-NEP antibody (Blot: NEP). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Prediction of moesin region involved in NEP binding
We previously demonstrated that the interaction between NEP and ERM proteins occurs via the positively charged amino acids within the cytoplasmic domain of NEP9: NEP and ERM proteins associated in cell lysates expressing wild-type NEP, but not in lysates from cells expressing cytoplasmic-domain-truncated or cytoplasmic-domain-mutated NEP containing amino-acid substitutions K19K20K21→Q19N20I21. To determine why ERM proteins bound NEP whereas merlin did not, we used computational analysis to predict which region in moesin is responsible for binding NEP. We used computational threading against another complex of moesin with a long peptide (its own C-terminal tail)16 and against a complex with ICAM-2 peptide17 to find the most feasible conformation for the NEP cytoplasmic domain interaction with this protein. The resulting predicted model of the protein–peptide complex suggested an association between NEP K21 and moesin E159Q160 (Supporting Information Fig. 2). The E159Q160H161K162L163 region is conserved in ERM proteins, but has a different sequence in merlin (see Fig. 3). Therefore, this region might be responsible for determining binding (as in ERM) or lack of binding (as in merlin) to NEP. We tested this hypothesis by mutagenesis of this region in both moesin and merlin proteins.
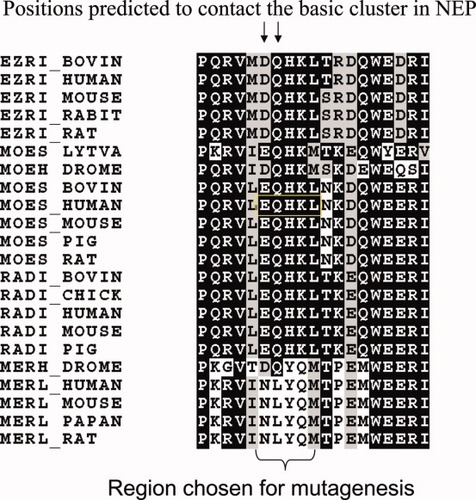
Sequence alignment of ERM and merlin proteins (obtained with ClustalW) in the region of predicted interaction with NEP. Conserved residues are shaded using BoxShade. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
E159Q160 residues in moesin are required for binding of NEP in vitro
To test our hypothesis that moesin residues E159Q160 constitute the determinants for NEP binding, we carried out a binding assay using in-vitro-translated wild-type moesin protein or the identical sequence mutated to the residues found in merlin (N159L160), and the GST-fusion cytoplasmic domain of NEP. We had previously demonstrated that the GST-fusion cytoplasmic domain of NEP binds in-vitro-translated wild-type ERM proteins.11 Glutathione-S-transferase GST alone, used as a control (Supporting Information Fig. 1, lanes 1 and 2), possessed negligible binding affinity for the recombinant N-terminal wild-type or mutant moesin. The N-terminal wild-type moesin bound to the GST-fusion protein of NEP cytoplasmic domain (GST-NEP1-19), whereas the moesin double-mutant (E159NQ160L) demonstrated weak binding, similar to the GST control (Supporting Information Fig. 1). These results suggested that residues E159Q160 in moesin are essential for the binding of NEP and ERM proteins in vitro. To confirm the importance of these residues for the interaction, we constructed an isopropyl-β-D-thiogalactopyranoside (IPTG)-inducible GST fusion with a merlin mutant in which residues N175L176 were replaced with EQ (merlin 2a.a mut) and residues N175L176Y177Q178M179 were replaced with EQHKL, the corresponding sequence in the ERM proteins [merlin 5a.a mut; Figs. 3 and 4(A)]. We then performed a binding assay using wild-type and mutant merlin GST fusion proteins and lysates from WT-5 cells grown in the presence (NEP not expressed) or absence (NEP expressed) of tetracycline. Wild-type merlin and the merlin 2a.a mut (not shown) exhibited no binding affinity for NEP protein. However, as shown in Figure 4(B,C), immunoprecipitation using NEP antibody and western blotting for merlin showed that NEP and the merlin 5a.a mut had coassociated. Importantly, the NEP protein containing the QNI mutation in the cytoplasmic domain did not bind to either the wild-type or mutant merlin proteins [Fig. 4(C)]. Our results suggest that the EQHKL motif in moesin and merlin is necessary and sufficient for in-vitro interaction with NEP and that the binding involves the basic cluster of NEP in the cytoplasmic domain.
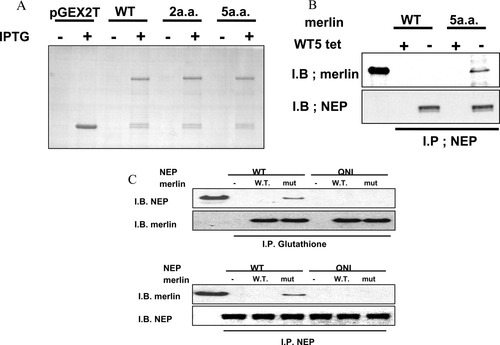
In-vitro binding assay of NEP with wild-type and mutant merlin. A: GST (pGEX2T) and GST fusion proteins (GST-merlin wild-type [WT], GST-merlin 2a.a mut, GST-merlin 5a.a mut) were produced in enhanced BL21 derivative competent E. coli cells with (+) or without (−) 0.1 mM IPTG. E. coli cells were disrupted by sonicator and incubated with glutathione-Sepharose 4B. The expression of GST fusion proteins was confirmed by SDS-PAGE followed by Coomassie Brilliant Blue staining. These purified GST fusion proteins were subjected to in-vitro binding assay. B: The lysates were immunoprecipitated with anti-NEP mouse monoclonal antibody and protein G-Sepharose beads (IP: NEP). The immunoprecipitates were separated by SDS-PAGE followed by immunoblotting with anti-merlin (NF2) antibody (IB: merlin) and anti-NEP antibody (IB: NEP). C (upper panel): GST fusion proteins of merlin were incubated with glutathione-Sepharose 4B and WT-5/QN-12 cell lysate expressing wild-type (WT) or mutant (QNI) NEP. The coimmunoprecipitates were separated by SDS-PAGE followed by immunoblotting with anti-NEP antibody (IB: NEP) and anti-merlin (NF2) antibody (IB: merlin). C (lower panel): Lysates from WT-5 or QN-12 cells were immunoprecipitated with anti-NEP mouse monoclonal antibody and protein G-Sepharose beads. The mutant NEP (QNI) did not show binding affinity with either wild-type (WT) or mutant (mut) merlin, whereas WT NEP showed binding affinity with mutant merlin.
Discussion
Members of the protein 4.1 superfamily have highly conserved FERM domains at their N-termini.18, 19 There are more than 40 identified members, generally divided into five subfamilies on the basis of sequence similarity, two of which are protein 4.1 molecules and ERM proteins. We used the NEP cytoplasmic domain to first identify ERM proteins as a NEP binding partner.9 The NF2 tumor-suppressor protein product merlin contains a highly conserved N-terminus FERM domain homologous to ERM proteins,18 which interacts with many of the same membrane proteins, such as CD44 and N-WASP,20, 21 whereas some proteins interact with one or the other ERM proteins.22 ERM proteins and merlin are similarly regulated by an intramolecular head-to-tail association between the FERM domain and the C-terminus that prevents binding of membrane partners.20 However, in contrast to ERM proteins in which the open phosphorylated form binds to CD44, it is the closed, dephosphorylated form of merlin that interacts with CD44 and mediates inhibition of growth through signals from the extracellular matrix.14 We investigated whether NEP interacts with merlin to determine the relationship between potential binding partners, to assess how these interactions may regulate cell motility. We found that no direct interaction occurs between NEP and merlin proteins, independent of the phosphorylation state of position S518 in merlin. Immunofluorescence experiments have shown that NEP and merlin do colocalize when NEP is expressed, although not to the same degree as NEP and ezrin (Nanus, unpublished data). Thus, although NEP does not directly bind to the FERM domain of merlin, they could potentially affect one another indirectly by affecting interactions with binding partners.
We set out to explore the structural basis of the interactions between ERM proteins and NEP and the lack of direct interaction between merlin and NEP. Our starting point was the information that mutation of the KKK cluster in NEP disrupts binding with ERM proteins and that merlin does not bind either the wild-type or the mutated NEP. Several structures were solved for the ERM protein FERM domain complexes with cognate peptides (summarized in Supporting Information Table 1). Figure 5 shows the different peptides for which structures in complex with ERM proteins were obtained. The actin C-terminal tail is a long peptide, part of which coincides with the binding mode of NHERF1 and NHERF2 peptides to radixin. Concurrent with our studies, a structure of NEP in complex with radixin, PDB: 2YVC,24 and a structure of CD44 in complex with radixin, PDB: 2ZPY,25 were published. These peptides bind in the same groove of radixin as ICAM-2 (Ref. 17) and PSGL-1 (Ref. 26; see Fig. 5). In the CD44-radixin structure, the KKK cluster in CD44 is located near regions in the FERM domain which are identical between ERM proteins and merlin,25 in accordance with the fact that both merlin and ERM proteins bind CD44. Notably, the basic cluster of NEP in the NEP-radixin complex does not overlap with the basic cluster of CD44 and is not in contact with radixin (see insert in Fig. 5).
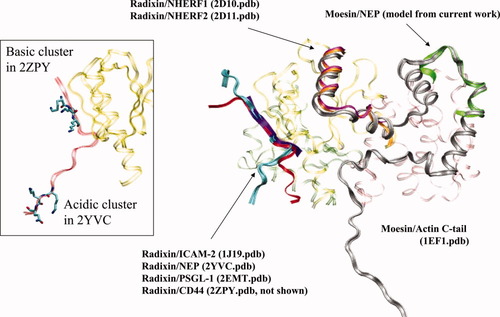
Interactions of different peptides with the ERM proteins. Structures of ERM proteins (obtained from the Protein Data Bank and listed in Supporting Information Table 1) were overlapped using Iterative Magic Fit in Swiss-PdbViewer. PDB: 1EF1 chain A is shown as a representative FERM domain in transparent ribbon representation (colored light green, yellow, and pink). The FERM domain partners from the different structures are color-coded as follows: the actin C-terminal domain (chain C in PDB: 1EF1) is shown in gray; the proposed moesin-NEP interaction validated in the current work resembles a region of the actin C-tail-moesin interaction and is shown in green; NHERF1 and NHERF2 peptides are shown in magenta and orange; the rest of the peptidic partners are shown in cyan, red and purple. Insert: The KKK clusters in NEP peptide (PDB: 2YVC) and CD44 peptide (PDB: 2ZPY) bound to radixin do not overlap and are not involved in interaction with the E159Q160 residues of radixin. The figure was prepared in VMD23 and rendered by POV-Ray (www.povray.org).
To elucidate the differences between the ERM protein moesin and merlin with respect to NEP binding and to further investigate the role of the basic cluster of NEP in binding, we constructed a computational model of the interaction between the NEP peptide and the FERM domain of moesin. Structural modeling of protein interactions by analogy has been useful in recent applications,27, 28 but because threading against the solved peptide provides multiple possible models, additional information is needed to prioritize these solutions. Constraining computational solutions using biochemical and biophysical data are an important tool in data-driven docking,29 which enables docking in cases of a large number of degrees of freedom30 as well as in conformational dynamics.31 We used 1EF1 and 1J19 structures as peptide-ERM protein structural templates for possible NEP peptide–moesin complexes, because these templates cover all the known ERM-peptide interaction regions known to date. Importantly, we used experimental data on the requirement for the cluster in NEP for binding to ERM proteins9 and chose a model that supports these data, namely, a model in which the basic cluster is involved in binding to the FERM domain (Supporting Information Fig. 2). The chosen putative model was analyzed for possible clues that would explain the lack of binding between NEP and merlin. Using multiple-sequence alignment, we identified a stretch of residues in merlin that differ from the residues in the ERM protein's putative NEP-contacting region (see Fig. 3). Indeed, introduction of moesin residues into merlin caused binding of NEP by the mutant, whereas swapping the corresponding residues in moesin for the residues found in merlin eliminated moesin binding to NEP. None of the constructs bound the mutated NEP, confirming the notion that the basic cluster is essential for binding. These biochemical results support the putative model of the NEP–moesin interaction and suggest that there are at least two different binding modes between ERM proteins and NEP: one exemplified by the radixin interactions with NEP, CD44, and ICAM-2 (see Fig. 5) and the other by the moesin-NEP interaction found in the current work. It should be also noted that there are slight differences in the sequences of the mouse NEP in the NEP-radixin complex24 and the human NEP used in this study. Furthermore, investigation and comparison of radixin, moesin, and ezrin interactions with NEP are currently underway.
Abbreviations:
ERM, ezrin/radixin/moesin; FERM domain, band 4.1 and ezrin/radixin/moesin proteins domain; NEP, neutral endopeptidase; NF2, neurofibromatosis type 2; PTEN, phosphatase and tensin homolog; Tet-off, tetracycline-repressible system.
Materials and Methods
Cells and Antibodies
LNCaP, DU145, and NIH3T3 were maintained in RPMI 1640 medium supplemented with 2 mM glutamine, 1% nonessential amino acids, 100 units/mL streptomycin, and penicillin containing 10% fetal calf serum. WT-5 cells or QN-12 cells, containing full-length (starting from the 8th amino acid in the sequence NP_009220) NEP cDNA or mutated NEP cDNA in which the positively charged amino acids in the cytoplasmic domain, K19K20K21, were replaced with Q19N20I21, subcloned into the tetracycline-repressible, transactivator-protein-responsive plasmid tel-regulated expression (pTRE/NEP), which were derived and maintained as described previously.13 The cells were cultured in medium with 1 μg/mL tetracycline to repress expression of NEP. Antibodies used included anti-actin, anti-merlin, anti-phospho-NF2 and anti-Flag antibodies (Santa Cruz Biotechnology, Inc.), mouse anti-NEP mAb NCL (Novocastra Laboratories, Ltd.), and mouse anti-NEP mAb J5 (Beckman Coulter, Inc.).
Immunoprecipitation, SDS-PAGE, and immunoblotting
Confluent monolayer cultures of cells on 10-cm dishes were washed twice with cold phosphate buffered saline (PBS) and then lysed in 1 mL of radioimmune precipitation assay buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% sodium deoxycholate, 0.1% sodium dodecyl (lauryl) sulfate (SDS), 1.2% aprotinin, 5 μM leupeptin, 4 μM antipain, 1 mM phenylmethylsulfonyl fluoride, and 0.1 mM Na3VO4) for 30 min. After centrifugation at 12,000g for 30 min, the supernatant was collected, and protein concentration was determined using BCA protein assay reagent (Pierce). Lysate (500 μg) was incubated 1 h to overnight with 2 μg of primary antibody and 30 μL of protein G-Sepharose beads (Amersham Biosciences) at 4°C. Immune complexes were washed three times with radioimmune precipitation assay buffer and then diluted in 2× sample buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 10% glycerol, 0.2% bromphenol blue, 4% 2-mercaptoethanol). The samples were separated by 10% SDS-PAGE, transferred to a nitrocellulose membrane (OPTITRAN; Schleicher and Schuell), and immunoblotted as described previously.32
Site-directed mutagenesis and vector constructs
Expression vectors for the Flag mutants of merlin (S518A and S518D) were kindly provided by Dr V. Ramesh (Massachusetts General Hospital, Boston, MA). Expression vectors for N-terminal and full-length moesin were kindly provided by Dr. Heinz Furthmayr (Stanford University, Stanford, CA).33 Moesin and merlin cDNA with mutations were constructed by site-directed mutagenesis (QuikChange; Stratagene). To substitute amino acids E159Q160 in the N terminus of moesin with N159L160, PCR-based site-directed mutagenesis was performed using a set of primers including point mutations (underlined) as follows: 5′-CG CAG AGA GTC CTG AAT CTG CAC AAA CTC AAC AA-3′ and 5′-TT GTT GAG TTT GTG CAG ATT CAG GAC TCT CTG CG-3′. To substitute amino acids N175L176 in merlin with E and Q, respectively, PCR-based site-directed mutagenesis was performed using: 5′-CTT CCA AAA AGG GTA ATA GAG CAG TAT CAG ATG ACT CCG GAA-3′ and 5′-TTC CGG AGT CAT CTG ATA CTG CTC TAT TAC CCT TTT TGG AAG-3′. To substitute amino acids N175L176Y177Q178N179 in the N-terminus of merlin with E175Q176H177K178L179, PCR-based site-directed mutagenesis was performed using: 5′-AA TTG CTT CCA AAA AGG GTA ATA GAG CAG CAC AAG CTG ACT CCG GAA ATG TGG GAG GAG-3′ and 5′-CTC CTC CCA CAT TTC CGG AGT CAG CTT GTG CTG CTC TAT TAC CCT TTT TGG AAG CAA TT-3′. PCR products were confirmed by DNA sequencing.
Production and purification of GST fusion of NEP cytoplasmic domain
cDNA fragments containing the cytoplasmic domain starting from Met8 (amino acids 8–26) were subcloned into pESP-1 plasmid (Stratagene) to produce GST fusion proteins (pESP-1/NEP1-19), as described previously.9Schizosaccharomyces pombe yeast cells SP-Q01 were transformed with pESP-1 or pESP-1/NEP1-19 using lithium acetate.34 Synthesis of the GST fusion proteins was induced by incubating transformed yeast cells in essential minimal medium (EMM) broth (ESP Yeast Protein Expression and Purification System; Stratagene) for 20 h, and then sedimenting them by centrifugation at 1000g for 5 min and resuspending in PBST-PI (PBS containing 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μM pepstatin A, 100 μM leupeptin, 1 μg/mL chymostatin). Yeast cells were lysed by vortexing for 10 min at 4°C with glass beads in PBST-PI,35 and the cell debris was removed by centrifugation (12,000g, 5 min, at 4°C). The supernatant was then mixed with G-Sepharose 4B (Amersham) and incubated for 30 min at room temperature, and the pellet was collected by centrifugation (500g, 5 min), washed three times with PBS, and resuspended in GST elution buffer (10 mM reduced glutathione, 50 mM Tris-HCl, pH 8.0). This mixture was incubated at room temperature for 10 min, and the supernatant was collected as a GST fusion protein solution by centrifugation (500g, 5 min). The concentration of GST fusion protein was estimated by SDS-PAGE.
Production and purification of GST fusion of merlin
NEB Express Iq Competent Escherichia coli cells were transformed with pGEX-2T, pGEX-2T/merlin wild-type, pGEX-2T/merlin 2a.a mut, or pGEX-2T/merlin 5a.a mut. Synthesis of the GST fusion proteins was induced by incubating transformed cells in 0.1 mM IPTG for 4 h at 37°C. Then the cells were sedimented by centrifugation at 7700g for 10 min and the cell pellet was completely suspended by adding 50 μL ice-cold 1× PBS per milliliter culture. The suspended cells were disrupted by sonication on ice in short bursts (Triton, Promega Corp.) as described previously.9 The amount of labeled moesin protein used in the binding (20%) was added to a final concentration of 1% and mixed gently for 30 min to aid in solubilizing the fusion protein. Then the cells were sedimented by centrifugation at 12,000g for 10 min at 4°C and the supernatant was transferred to a fresh container. The concentration of GST fusion protein was estimated by BCA protein assay kit (Pierce).
In-vitro binding assay
In-vitro-translated, [35S]methionine-labeled N-terminal wild-type or mutant moesin proteins were generated using TNT Quick Coupled Transcription/Translation Systems (Promega) as previously described.9 The amount of labeled moesin protein used in the binding assays was determined by normalizing the intensity of the autoradiography signal for each labeled protein. In-vitro translation product was incubated with 1–2 μg of GST fusion proteins immobilized on G-Sepharose beads and washed with binding buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% Nonidet P-40) at 4°C for 1 h with rocking. Complexes were pelleted at 10,000g for 2 min, washed three times in 0.5 mL of binding buffer, and subjected to SDS-PAGE. The gels were fixed, dried, and subjected to autoradiography. To assay NEP-merlin binding, 200 μg of WT-5 cell lysate with (+) or without (−) tetracycline or QN-12 was incubated for 2 h with 2 μg of anti-NEP mAb J5 and 30 μL of protein G-Sepharose beads at 4°C. Immune complexes were washed three times with cold PBS. After 25 μg of GST-merlin fusion protein was mixed and incubated for 2 h at 4°C, the complexes were washed three times with cold PBS and subjected to SDS-PAGE.
Computational analysis
- 1
Identification of a potential binding mode of NEP with ERM proteins. We used known structures of ERM protein FERM domain complexes with their cognate peptides, in the following manner: The cytosolic NEP peptide was submitted to Robetta server.36, 37 We obtained putative models of NEP peptide-moesin complex (a) using the moesin C-terminal tail of moesin in complex with moesin structure (PDB: 1EF1)16 as a template and (b) using ICAM-2 peptide in complex with radixin (PDB: 1J19)17 as a template. Models in which the basic K19K20K21 cluster had no interactions with the FERM domain were discarded based on previous experimental data.
- 2
Sequence alignment of the ERM proteins and merlin was performed using ClustalW and is presented using ShadeBox, both tools available at http://www.ch.embnet.org/index.html.
Summary and Conclusions
In summary, based on our previous studies and the work reported here, NEP binds to the FERM domain of ERM proteins but not to merlin. Both ERM proteins and merlin bind the cytoplasmic domain of the hyaluronan receptor CD44 and affect CD44 function, including cell motility and cell adhesion. Moreover, the interaction of NEP with ERM proteins inhibits the association of ERM with CD44 proteins,9 suggesting a possible complex relationship between NEP, ERM, merlin, and CD44 proteins that affects and regulates cell motility. Defining these relationships is crucial for defining merlin and NEPs' tumor-suppressor functions, and may eventually lead to the development of peptidomimetic inhibitors38-42 that can be used for cell-signaling modulation. This is a promising direction in view of the recent advances in peptide synthesis and the fact that inhibition of protein–protein interactions emerges as a novel paradigm for drug discovery.43, 44
Acknowledgements
We thank Drs. Lei Shi and Harel Weinstein for helpful discussions.




