Recombinant chymosin used for exact and complete removal of a prochymosin derived fusion tag releasing intact native target protein
Abstract
Fusion tags add desirable properties to recombinant proteins, but they are not necessarily acceptable in the final products. Ideally, fusion tags should be removed releasing the intact native protein with no trace of the tag. Unique endoproteinases with the ability to cleave outside their own recognition sequence can potentially cleave at the boundary of any native protein. Chymosin was recently shown to cleave a pro-chymosin derived fusion tag releasing native target proteins. In our hands, however, not all proteins are chymosin-resistant under the acidic cleavage conditions (pH 4.5) used in this system. Here, we have modified the pro-chymosin fusion tag and demonstrated that chymosin can remove this tag at more neutral pH (pH 6.2); conditions, that are less prone to compromise the integrity of target proteins. Chymosin was successfully used to produce intact native target protein both at the level of small and large-scale preparations. Using short peptide substrates, we further examined the influence of P1′ amino acid (the N-terminus of the native target protein) and found that chymosin accepts many different, although not all, amino acids. We conclude that chymosin has several appealing characteristics for the exact removal of fusion tags. It is readily available in highly purified recombinant versions approved by the FDA for preparation of food for human consumption. We suggest that one should consider extending the use of chymosin to the preparation of pharmaceutical proteins.
Introduction
Fusion tags are often added to the C- or -N-terminus of hetorologous target proteins to facilitate expression, solubility and/or purification,1-4 however, they are not always acceptable in the final product and may have to be removed during downstream processing. Cleaving the protein sequence between the fusion tag and the native protein could effect efficient (i.e., exact and complete) fusion tag removal. Unfortunately, many cleavage procedures and/or enzymes cleave within their recognition sequence leaving part of the recognition sequence attached to the protein of interest, that is, the protein will not be released in its native form, but rather in a modified form.5-11 Ideally, the cleavage site should be outside the recognition sequence and positioned right at the boundary of the protein of interest as this should lead to the release of any native protein sequence of interest.
Currently, there are only three commercially available endoproteinases, which cleave C-terminally to their recognition sequence allowing N-terminally tagged proteins of interest to be released with intact native N-terminal sequences (for a thorough description see Refs. 12). Two of these “restriction proteases,” Enterokinase and Factor Xa, are derived from animal sources and therefore constitute serious regulatory problems. The third, Tobacco Etch Virus protease, is available recombinantly. It is, however, a cysteine protease requiring addition of thiol containing reagents during cleavage and is therefore not suitable for the cleavage of thiol sensitive proteins.13 Hence, there is a need to discover and develop new methods for fusion tag removal, in particular for the purpose of large-scale production of protein for clinical use.
Abbreviations:
aa, amino acid; HAT, natural histidine affinity tag; IDA, iminodiacetic acid; IMAC, immobilized metal affinity chromatography; MHC, major histocompability complex; PC, prochymosin; TEV, Tobacco etch virus.
Chymosin (EC 3.4.23.4) is available recombinantly as a highly purified, yet inexpensive, bulk product. It is a highly specific aspartic protease cleaving bovine kappa casein between Phe105-Met106.14 It has been examined as restriction protease. Initially, bovine kappa casein sequence (aa 97–113) was used as chymosin cleavage recognition sequence; however, this left 8 aa from this sequence at the N-terminus of target proteins.15 An alternative chymosin cleavage sequence is found within chymosin itself. In vivo, chymosin is produced as a zymogen with a prosegment that stabilizes the inactive form and prevents substrates from entering the active site. In the acidic environment of the stomach, chymosin is activated by disruption of the electrostatic interactions between this prosegment and the active site, followed by proteolytic removal of the 42 aa chymosin prosegment [CP, 42 aa, Fig. 1(A)].14 Recently, a modified CP sequence featuring a deletion of the three aa (YSG) adjacent to the cleavage site [generating CPΔ, 39 aa, Fig. 1(B)], was used as chymosin cleavage recognition sequence. For three of four tested target proteins, the intact protein could be released suggesting that chymosin can act as restriction protease.16 Note, whereas the cleavage of bovine kappa casein was conducted at pH 6.8, the cleavage of CPΔ had to be conducted narrowly at pH 4.0–4.5. Others have noted that chymosin exhibits strong and nonspecific proteolysis at pH 4.515; something that severely would compromise the integrity of target proteins. For some proteins, incubation at a pH of 4.5 could be a problem in itself.

Different prochymosin constructs. (A) Bold letters: amino acid sequence of prochymosin. Bold italic letters: first 10 aa of mature chymosin. (B) CP (previously used as recognition sequence (39 aa, pI value 9.78).16 (C) Amino acid sequence of HAT-C15PΔ-hβ2m. Bold letters: HAT, Bold underlined letters: shortened C15PΔ (15 aa, pI value 5.79). Bold italic letters: First 10 aa of hβ2m. Cleavage site indicated by arrow.
The chymosin/CPΔ restriction cleavage system is potentially very attractive, however, we were concerned that not all proteins would survive chymosin treatment at pH 4.5. In fact, our target protein was completely digested (Supporting information Fig. 1). We reasoned that shortening the chymosin prosegment sequence might reduce the electrostatic interaction between the prosegment and the active site, and allow restriction digestion at more neutral pH. Indeed, we identified a shortened CPΔ recognition sequence, C15PΔ, which could be specifically cleaved by chymosin at pH 6.2 and lead to the release of an intact target protein. Using a panel of further C-terminally shortened analog 9-mer peptides (abbreviated C7PΔXE) as substrates, we further investigated the effect of the identity of the P1′ amino acid, and found that 14 of the 20 naturally occurring amino acids were compatible with specific chymosin mediated cleavage. In conclusion, we have generated a chymosin-based restriction protease system that effectively cleaves off a fusion tag under mild conditions and releases intact native target protein.
Results
Design of a novel chymosin prosegment-derived fusion tag
To reduce the electrostatic interaction between the prosegment and the active site, we analyzed the theoretical pI of the prosegment (http://www.expasy.ch/tools/pi_tool.html), and of any N-terminal truncation hereof. The pI of the entire CPΔ, and of most of its N-terminal truncations, were predicted to be close to 10. N-terminal truncations of 15–24 aa, however, would reverse the pI to below 7, whereas truncating more than 25 aa would return the pI to close to 10. For further work, we selected the shortest possible peptide with a pI below 7 the 15-mer EDFLQKQQYGISSKF (C15PΔ). For purification purposes, we added the natural histidine affinity tag (HAT)2 to the N-terminus of the C15PΔ tag. As a target protein of interest, we added the light chain of the major histocompatibility complex class I (MHC-I) molecule, human beta2 microglobulin (hβ2m), to the C-terminus of the C15PΔ tag. This generated a model fusion construct, HAT-C15PΔ-hβ2m [Fig. 1(C)].
Expression and purification of denatured HAT-C15PΔ-hβ2m
E.coli produced HAT-C15PΔ-hβ2m was deposited in inclusion bodies (IB) similar to previous observations with other hβ2m preparations17 (Fig. 2). After a detergent wash, the IB were extracted into urea, and purified under denaturing, but nonreducing, conditions using IMAC and gel filtration. The yield after purification was 720 mg of 74% pure monomeric HAT-C15PΔ-hβ2m (analyzed by gel densitometry), impurities were mainly dimers of the protein.
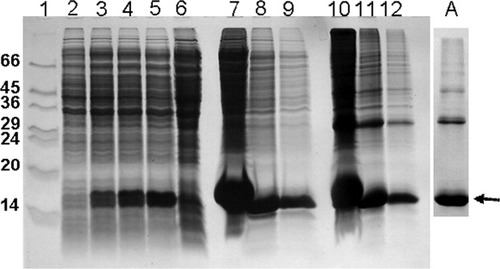
SDS PAGE analysis of expressed HAT-C15PΔ-hβ2m. Lane 1: Molecular weight marker. 2: Fermentation before induction with 1 mM IPTG. 3, 4, and 5: Expression of HAT-C15PΔ-hβ2m 1, 2, and 3 h post induction. Lane 6: Supernatant after cell homogenization. 7, 8, and 9: Two, 10, and 20 times dilutions of urea dissolved inclusion bodies (reduced with β-mercaptoethanol). 10, 11, and 12: Two, 10, and 20 times dilutions of urea dissolved inclusionbodies (nonreduced). Insert (A) shows the purified HAT-C15PΔ-hβ2m after IMAC and Gel Filtration. Arrow indicates the position of monomeric HAT-C15PΔ-hβ2m band.
Refolding of HAT-C15PΔ-hβ2m
The Denatured and purified HAT-C15PΔ-hβ2m (306 mg) was batch refolded in a nonreduced state at room temperature and subsequently loaded on to a Ni2+ loaded IDA column. As shown on Figure 3(A), a shallow gradient of imidazole containing buffer separated the refolded monomers from multimers. The amount of pure monomer was 175 mg giving a refolding yield of 76%.

IMAC separation of refolded HAT-C15PΔ-hβ2m on a Ni2+-IDA column. First Y axis shows the UV absorption while second axis shows the imidazole gradient. Collected fractions from the two peaks were analyzed on a 15% SDS-PAGE gel. Monomers (fraction I) were pooled whereas multimers (fraction II) were discharged.
Analytical scale cleavage of HAT-C15PΔ-hβ2m
Highly purified recombinant chymosin (CHYMAX, a kind gift from Chr. Hansen A/S) was used to cleave the HAT-C15PΔ-hβ2m fusion protein. The cleavage reaction was optimized in analytical batch experiments examining pH, temperature, and enzyme to substrate ratios. A pH scan was initially conducted to determine the optimal pH conditions for cleavage [Fig. 4(A,B)]. Highly specific cleavage, leading to one dominant band representing the expected hβ2m and concomitant removal of nearly all HAT-C15PΔ-hβ2m, was observed when the cleavage reaction was conducted in a narrow pH range between 6 and 6.4. At higher pH values, the cleavage efficiency dropped sharply. At lower pH values, proteolytic activity was increased and specificity lost. Thus, all HAT-C15PΔ-hβ2m was lost when overnight cleavage at 37°C was performed at pH values lower than 5.5 (Supporting information Fig. 1). For subsequent experiments the cleavage reaction was conducted at pH 6.2.
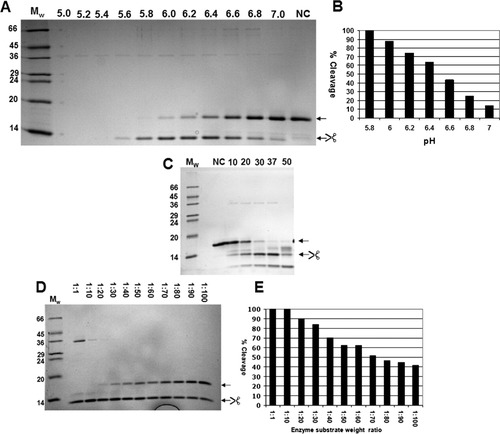
Optimization of cleavage conditions. (A) Cleavage of HAT-C15PΔ-hβ2m at different pH values (6.0, 6.2, 6.4, 6.6, 6.8, and 7) for 18 h at 37°C analyzed by SDS PAGE. MW: Molecular weight markers. NC: Noncleaved, arrows indicate position of noncleaved and cleaved HAT-C15PΔ-hβ2m. (B) Gel densitometry analysis of lanes from A. (C) Cleavage of HAT-C15PΔ-hβ2m at different temperatures (10, 20, 30, 37, and 50°C) (D) Cleavage of HAT-C15PΔ-hβ2m with chymosin at different enzyme to substrate ratios for 18 h at 37°C analyzed by SDS PAGE, Chymosin is seen as band at ∼35 kDa. (E) Gel densitometry analysis of lanes from D.
Both activity and specificity of the cleavage reaction was influenced by temperature [Fig. 4(C)]. At 10°C and 20°C, cleavage was inefficient and slightly unspecific; at 30°C and 37°C, cleavage was faster and specific, whereas cleavage at elevated temperature (50°C) resulted in unspecific cleavage. For subsequent experiments the cleavage reactions was conducted at 37°C.
As expected, the ratio between enzyme and substrate influenced cleavage [Fig. 4(D,E)]. At an enzyme to target protein ratio of 1:20, 90% cleavage was obtained using the specified experimental conditions (37°C, 18 h, pH 6.2). This is the ratio used in the subsequent experiments. Additionally, the influence of salt (sodium chloride) and imidazole was tested at concentrations up to 200 and 80 mM. These concentrations were well tolerated and did not influence cleavage (data not shown).
Influence of the P1′ position of the fusion protein
To test whether the C-terminal amino acid bordering the cleavage site (the P1′ position of the Schechter and Berger nomenclature)18 affected cleavage activity we tested 20 different peptides in which the P1′ position of the prochymosin cleavage site had systematically been replaced with one of the 20 naturally occurring amino acids. For this experiment, the prochymosin sequence with the YSG deletion was further shortened N-terminally and extended C-terminally with XE generating the peptide sequence YGISSKF/XE, where/denotes the cleavage site, X the variable amino acid in the P1′ position and E the naturally occurring P2′ amino acid of the prochymosin recognition sequence. As aspartic proteases are reported to accommodate around 7 aa in the s4-s3′ subsites,19 we reasoned that a short peptide sequence would be sufficient to examine chymosin cleavage specificity. The peptide substrates were digested under the optimal conditions identified above and analyzed by LC-MS. Surprisingly, the enzymatic activity appeared reduced when tested on these peptide substrates. At a chymosin to substrate ratio of 1:20, only peptides with W, Y or F in the P1′ position were successfully cleaved. However, changing the enzyme substrate ratio to 1:1 resulted in more variants at the P1′ position being cleaved, and a hierarchy of preferred amino acids at the P1′ position could be established (Table I). Peptides with P1′ amino acids W, Y, F were still cleaved. Peptides with the P1′ amino acid C was also 100% cleaved; peptides with P1′ amino acids R, I, M, H were 60–50% cleaved; peptide with P1′ amino acids L, V, K, N were 50–30% cleaved; whereas the peptides with P1′ amino acids D, E (acids), S, P, Q and G were not cleaved at all. Note, that digestion of peptides with P1′ amino acids alanine and threonine could not be quantitated because the cleaved product coeluted with noncleaved peptide; nonetheless, the MS analysis revealed that at least some of the peptide was cleaved.
| Residue | Sequence | Da | Cleavage(%) |
|---|---|---|---|
| A | YGISSKFAE | 1001.1 | YES |
| C | YGISSKFCE | 1033.2 | 100 |
| D | YGISSKFDE | 1045.1 | 0 |
| E | YGISSKFEE | 1059.1 | 0 |
| F* | YGISSKFFE | 1077.2 | 100 |
| G | YGISSKFGE | 987.1 | 0 |
| H | YGISSKFHE | 1067.2 | 51 |
| I | YGISSKFIE | 1043.2 | 54 |
| K | YGISSKFKE | 1058.2 | 33 |
| L | YGISSKFLE | 1043.2 | 42 |
| M | YGISSKFME | 1061.2 | 52 |
| N | YGISSKFNE | 1044.1 | 33 |
| P | YGISSKFPE | 1027.1 | 0 |
| Q | YGISSKFYE | 1058.1 | 0 |
| R | YGISSKFRE | 1086.2 | 59 |
| S | YGISSKFSE | 1017.1 | 0 |
| T | YGISSKFTE | 1031.1 | yes |
| V | YGISSKFVE | 1029.2 | 36 |
| W* | YGISSKFWE | 111.62 | 100 |
| Y* | YGISSKFYE | 1093.2 | 100 |
- Peptides were cleaved at an enzyme to substrate ratio of 1:1(pH 6.2 and 37°C, 16 h), following cleavage solutions were subjected to LC/MS analysis. Peptides marked with an asterix were also cleaved 100% at an enzyme to substrate ratio of 1:20; alanine and threonine was cleaved, but the degree of cleavage could not be determined.
Preparative scale cleavage of HAT-C15PΔ-hβ2m
Chymosin was used to cleave HAT-C15PΔ-hβ2m at a preparative scale aiming at generating hβ2m of native sequence. HAT-C15PΔ-hβ2m (37 mg) was cleaved for 46 h with 1.85 mg Chymosin (enzyme:substrate ratio of 1:20) [Fig. 5(A)]. After cleavage, the solution was applied to a Ni2+ loaded IMAC column to separate cleaved fusion product from noncleaved products. Human β2m contains 4% histidines and binds with moderate affinity to a Ni2+ loaded IMAC column [Fig. 5(B), peak II]. Surprisingly, chymosin with only 1.5% histidines also bound to the column and coeluted with hβ2m [Fig. 5(B,C), peak II]. HAT-C15PΔ, either as cleaved tag or uncleaved fusion product eluted later in the imidazole gradient [Fig. 5(B,C), peak III]. The mixture of hβ2m and chymosin was separated on a size exclusion column to yield pure hβ2m [Fig. 5(D)].
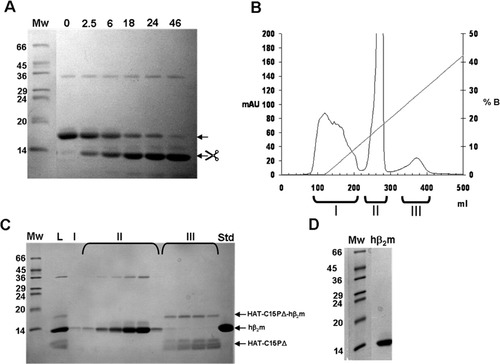
Cleavage of HAT-C15PΔ-hβ2m at preparative scale. (A) Cleavage of 37 mg HAT-C15PΔ-hβ2m with chymosin (20:1) at 37°C for 46 h, MW: Molecular weight markers. Arrows indicate position of noncleaved and cleaved HAT-C15PΔ-hβ2m. (B) Chromatogram showing the UV absorption and imidazole gradient during the IMAC purification of cleaved HAT-C15PΔ-hβ2m. Three distinct peaks are seen. (C) SDS-PAGE analysis of collected fractions from the IMAC purification. L: Load. I, II, and III corresponds to fractions from the three peaks in B. Std: Factor Xa cleaved hβ2m standard (D) Final product after separation of chymosin and hβ2m by gel filtration chromatography.
Mass spectrometry analysis of the cleavage product
The identity of the cleavage product, and the specificity of the cleavage reaction was confirmed by MS analysis. The analyzed hβ2m samples correlated well with the theoretical molecular weights (see Table II).
| Theoretical mass | Determined mass | |
|---|---|---|
| HAT-C15PΔ-hβ2m | 16,140 | 16,138 |
| Chymosin-generated hβ2m | 11,731 | 11,730 |
| Factor Xa-generated hβ2m | 11,731 | 11,729 |
Functional analysis of the cleavage product
Human β2m is also known as the light chain of the major histocompability complex class I complex, which governs the presentation of peptide antigens to CD8 positive T-cells.20, 21 We examined the ability of the hβ2m product obtained by chymosin-mediated cleavage of HAT-C15PΔ-hβ2m to support the binding of an I125 labeled peptide known to be a binder to HLA-B*0702 in a strictly hβ2m dependent fashion. Reassuringly, this hβ2m preparation supported peptide binding as efficiently as a laboratory standard generated from a HAT-Fxa-hβ2m protein cleaved by Factor Xa.20 The concentration needed to half-saturate the HLA-B*0702, the EC50, was 10 and 12 nM, respectively (Fig. 6). In contrast, a metβ2m (carrying an additional N-terminal formyl-methione) and a noncleaved HAT-C15PΔ-hβ2m required 10 and 20 times higher concentrations to obtain half saturation, 100 and 200 nM, respectively.

Functional assay, binding of radioactive peptide to HLA B*0702. Titration of four different hβ2m constructs in a radioactive peptide-binding assay. X-axis shows concentration of β2m, while Y axis shows the percentage of labeled peptide incorporated in an MHC I complex. Binding data was fitted to a sigmoidal model and EC50 values calculated: Chymosin cleaved HATC15PΔG-hβ2m (▪, thin solid line) 10 nMR2 = 0.95, Factor Xa cleaved hβ2m (▴, thick solid line) 12 nMR2 = 0.95, metβ2m (Δ, short dotted line) 100 nMR2 = 0.97 and noncleaved HAT-C15PΔG-hβ2m (□, long dotted line) 200 nMR2 = 0.97, respectively.
Discussion
We have developed a new 15-mer prochymosin fusion tag, C15PΔ, which can be specifically cleaved by chymosin under mild conditions allowing release of intact target protein both at the analytical and preparative scale. Our C15PΔ fusion tag is an N-terminally truncated version of the 42-mer prochymosin fusion, CPΔ, tag reported recently by Kuhnel et al.16 For removal, the original CPΔ fusion tag requires acidic cleavage conditions (pH 4.0–4.5). This is known to cause strong and nonspecific chymosin activity,15 which could threaten the integrity of target proteins and limit the utility of the original fusion tag (e.g., at acidic pH, our chosen target proteins are completely digested by chymosin, Supporting Information Fig. 1). In contrast, chymosin is known to exhibit highly specific cleavage of its casein substrate at pH 6.8. Thus, the requirement for acidic cleavage conditions of the original CPΔ is not inherent to chymosin; rather, we speculated that it could be due to the nature of the CPΔ fusion tag. This bears strong resemblance with the chymosin prosegment. At neutral pH, the prosegment binds to the active site of chymosin and blocks activity; whereas at acidic pH, it leaves the active site, is cleaved off and activates chymosin. We speculated that the CPΔ fusion tag might specifically bind to and inhibit chymosin, and that the requirement for acidic cleavage conditions of the original CPΔ tag technology might be a requirement for the CPΔ to dissociate from and enable chymosin. We further reasoned that removing some of the positively charged amino acids from the N-terminus of the original CPΔ fusion tag could reduce electrostatic interactions between the CPΔ and the active site, and enable chymosin activity at more neutral pH. The original CPΔ fusion tag has a calculated pI of around 10. Many of the positive residues reside in the N-terminus of the CPΔ. Removing the first 24 amino acids of the original CPΔ, we generated the C15PΔ fusion tag with a calculated pI of 6.17. Indeed, we have demonstrated that chymosin can specifically cleave C15PΔ fusion tag at a much more neutral pH (around pH 6.0–6.4), and that under those conditions our proteins of interest remained intact. We propose that chymosin in conjunction with this novel C15PΔ fusion tag might be a valuable and versatile restriction protease system.
Ideally, a “restriction protease” should cleave outside its recognition sequence, that is, it should be indifferent to the N-terminal amino acid sequence of the target protein. We also used synthetic peptide substrates to investigate how the P1′ position influences cleavage. For this purpose, the recognition sequence was further shortened to 7 aa followed by a variable P1′ position and the P2′ glutamic acid adopted from native chymosin. At an enzyme to substrate ratio of 1:20, only peptides carrying F, W, or Y in the P1′ position was cleaved. The preference for bulky aromatic residues in the +1 position has been previously described for chymosin and pepsin relatives.22 Raising the enzyme to substrate ratio to 1:1 resulted in increased, yet specific, cleavage, and a hierarchy could be established. Aromatic residues in P1′ still cleaved extremely well followed by C, R, I, M, H, L, V, K, and N. By mass spectrometry, T and A were also cleaved, but the cleavage efficiency could not be estimated since by HPLC the cleaved and noncleaved peptides coeluted. In contrast, D, E, S, P, Q and G were not cleaved at all. The inability of chymosin to cleave substrates with S in the P1′position was also noticed by Kuhnel et al. Thus, chymosin is not indifferent to the identity of the P1′ amino acid, and this somewhat limits its utility as a restriction protease. An indication of the magnitude of this problem can be obtained from the Signal P prediction server (http://www.cbs.dtu.dk/services/SignalP and personal communication Henrik Nielsen, CBS, DTU), which predicts the presence and location of signal peptide cleavage sites in amino acid sequences from different organisms.23 About 56, 65, and 62% of all eukaryotic, gram-negative and gram-positive procaryotic proteins, respectively, have one of the acceptable amino acid residues in P1′ where chymosin is likely to be effective as a restriction protease in the generation of native heterologous target proteins. These limitations are not unique to chymosin; other proteases used as restriction proteases have disallowed P1′ amino acid residues (e.g., a P1′ proline residue is not allowed for Factor Xa, enterokinase and TEV13 and a P1′ acidic residue is disfavored for Factor Xa, and TEV. Currently, enterokinase and Granzyme B seems to be the only solutions for cleaving substrates with an acidic P1′ residue.24 Using chymosin as restriction protease, Kuhnel et al. proposed a work-around for this problem. Inserting a methionine in front of the protein of interest, something that is often done for expression purposes anyway, would change the P1′ residue seen by chymosin to the permissible methionine, and that this nonnative methionine could subsequently be removed with methionyl aminopeptidase.25, 26
We propose that chymosin in conjunction with the C15PΔ fusion tag is an excellent alternative to existing fusion tag protease technologies. The modified prochymosin fusion tag is easily inserted in front of suitable target proteins (i.e., avoid those with E, D, G, P, Q, or S at the N-terminus), and expressed. Chymosin is available as a cheap, yet highly purified, recombinant bulk product, which is approved by the FDA for food consumption with a GRAS (generally recognized as safe) status. Even at the high enzyme:substrate ratio used in this study, chymosin activity did not compromise yield and purity of the target protein, and the price of chymosin would not compromise the cost-efficiency of prochymosin fusion tag removal. An additional advantage of the suggested prochymosin fusion tag removal system was observed. Chymosin bound with moderate affinity to the IMAC column and this could conveniently be used to separate chymosin from the target protein during downstream processing (obviously this step would also remove the HAT-C15PΔ fusion tag).
In conclusion, chymosin is a valuable restriction protease for research applications, and we suggest that it should be considered to extend its use to the preparation of pharmaceutical proteins.
Methods
Chymosin
Chy-Max™, a solution of affinity purified recombinant bovine chymosin expressed in Aspergillus niger and was kindly provided by Christian Hansen A/S.
Cloning of HAT-C15PΔ-hβ2m
The construct used by Ferré et al.17 comprising MG-HAT-GS-FXa-hβ2m was inserted into the pET28a+ vector containing the kanamycin resistance gene (Novagen). Using standard molecular biology techniques, the FactorXa recognition sequence (IEGR) was changed to C15PΔ [Fig. 1(C)], and subsequently transformed into E. coli strain BL21(DE3). Clones, which produced the fusion product upon induction with IPTG, were identified and the insertion was verified by DNA sequencing (ABI310, Perkin Elmer). The complete DNA and amino acid sequence of the HAT-C15PΔ-hβ2m construct is shown in supporting information Figure 2.
Expression and purification of denatured HAT-C15PΔ-hβ2m
BL21 cells expressing HAT-C15PΔ-hβ2m were expanded overnight and 1-mL culture used to seed a 2.5-L fermentor (Labfors). Cells were grown to an OD of 25 where expression was induced with IPTG. After 3 h, cells were opened at 2.3 kBar in a cell disrupter (basic Z, Constant Systems Ltd Daventry.UK) After a centrifugation step the pellet was washed twice in 0.5% NP40, 0.1% DOC in PBS. The washed pellet was dissolved O.N. in 200 mL 8 M Urea, 25 mM Tris, pH 8 and remaining DNA precipitated with streptomycin sulphate (10 g/L). After a centrifugation step (17,000 g, 30 min), the denatured protein solution was applied to an Äkta FPLC chromatography system (GE Healthcare) fitted with a 250 mL Ni2+ loaded IDA Sepharose column [immobilized metal affinity chromatography (IMAC)]. The column was washed with 8 M Urea, 25 mM Tris, 100 mM NaCl, pH 8 (Buffer A) developed with a three segment gradient of Buffer B (A + 250 mM Imidazole, 0–20% in 500 mL, 20–50% in 250 mL, and 50–100% in 100 mL). Fractions containing the protein of interest was pooled and concentrated to 40 mL using a tangential ultrafiltration concentrator (Vivaflow 200, Vivascience AG, Göttingen, Germany). Twice, 20 mL concentrate was applied to a 840 mL Superdex 200PG column (XK50, GE Healthcare) and eluted with 8 M Urea, 25 mM Tris, 150 mM NaCl, pH 8. Fractions containing primarily denatured monomer was pooled and used for refolding experiments.
Refolding of HAT-C15PΔ-hβ2m
Denatured and purified HAT-C15PΔ-hβ2m was refolded in 10 L 300 mM Urea, 150 mM NaCl, 25 mM Tris, 20 mg/L PMSF, 0.7 mg/L pepstatin, pH 8. At RT and under magnetic stirring, denatured protein was added drop wise to a final concentration of 30.6 μg/mL. The solution was left for 2 h and subsequently filtered (0.45 μm). The filtrate was applied to a 250 mL Ni2+ loaded IDA Sepharose column (XK50, GE Healthcare) and the column was washed with 25 mM Tris, 100 mM NaCl, pH 8 (A) and developed by a two segment gradient (0–20% B (A + 250 mM Imidazole) in 3 L and 20–50% B in 0.5 L) to separate monomers from multimers and higher aggregates. Fractions containing refolded monomer (as analyzed by SDS-PAGE) was pooled and concentrated by tangential ultrafiltration and consecutively diluted with PBS until the imidazole content was reduced by a factor 70 (this was done to rule out any effect from imidazole on cleavage). Finally, glycerol was added to 50% and the concentrated protein was stored at −20°C until further use.
Cleavage of HAT-C15PΔ-hβ2m with chymosin: pH, temperature, and ratio optimization at analytical scale
The refolded HAT-C15PΔ-hβ2m was cleaved in PCR tubes for 18 h at 37°C in 50 mM Tris/MES, 0.02% azide buffer at five different pH values in the range 6–7, and five different temperatures ranging from 10 to 50°C. The concentration of HAT-C15PΔ-hβ2m was 200 μg/mL and the weight ratio between enzyme and substrate was 1:20. For the ratio optimization studies, the refolded HAT-C15PΔ-hβ2m was cleaved at pH 6.2 and 37°C while the weight ratio between enzyme and substrate was varied from 1:1 to 1:100. For all reactions, incubation time was 18 h, reaction volume 100 μL, and temperature control was done using a DYAD Peltier Thermal Cycler (MJ Research). Following cleavage, samples were analyzed by SDS-PAGE and gel densitometry.
Influence of the +1 position of the fusion protein
Twenty different peptides (YGISSKFXE, C7PΔXE), where X indicates the variable position covering all twenty amino acids, were produced by standard FMOC chemistry and cleaved at 37°C for 36 h in 100 μL 50 mM Tris/MES, pH 6.2, 0.05% Azide. The peptide concentration was 200 μg/mL and the peptide to chymosin ratio (w/w) was either 20:1 or 1:1. To remove chymosin after the cleavage reaction, the solution was passed through an ultrafiltration spinfilter (nanosep, Cut-off 5 kDa). The ultrafiltered peptide solution was analyzed by LC-MS.
Cleavage of HAT-C15PΔ-hβ2m with chymosin: preparative scale
The refolded HAT-C15PΔ-hβ2m (37 mg) in 27 mL PBS, 50% Glycerol was diluted 1:1 with 100 mM TRIS/MES, 0.05% azide, pH 6.2 added 1.9 mg chymosin. The solution was adjusted to pH 6.2 and incubated 46 h at 37°C in a shake incubator. After cleavage, the solution was loaded onto an 80 mL NiIDA column, and the column was washed with 25 mM Tris, 100 mM NaCl, pH 8 (A). Bound protein was eluted with a gradient of 25 mM Tris, 200 mM NaCl, 250 mM Imidazole, pH 8 (B). The collected fractions were analyzed by SDS-PAGE and fractions containing cleaved HAT-C15PΔ-hβ2m were pooled and concentrated to 10 mL using a 20 mL spinfilter with a cut-off 5 kDa (Vivascience AG, Göttingen, Germany), and loaded to a 1000 mL Superdex200 PG and eluted with PBS.
LC-MS analysis
Human β2m constructs and peptides were injected on an Agilent 1100 HPLC system consisting of degasser, pump, auto sampler, column compartment, and Diode Array Detector (DAD). Separation was done on a RP-HLPC C8 column (hβ2m-constructs, ZORBAX Eclipse XDB-C8: 4.6 × 150 mm2, 5 μm) or a RP-HLPC C18 column (peptides, GRACE VYDAC: 2.1 × 150 mm2, 5 μm) using a gradient of Acetonitrile in water 0.02% TFA (0–100% in 30 min, 30°C, flow rate 0.25 mL/min)
Mass spectrometry was performed with an Esquire-LC ion-trap mass spectrometer (Bruker Daltonics), equipped with an Electro Spray Ionization (ESI) source. Drying gas flow rate was 9.0 L/min, nebulizer was 50 psi, ion source temperature was 330°C and capillary voltage was 4.0 kV. The mass spectrometer was scanning between 300 and 2000 Da, with an ion trap target of 10,000 ions, maximum accumulation time of 50 ms and with positive ion mode. Data acquisition from HPLC system and from the mass spectrometer was performed using Chemstation (Agilent) and EsqiureControl (Bruker Daltonics), respectively. Analysis of data was performed using DataAnalysis (Bruker Daltonics).
Functional analysis, MHC I radioactive immuno assay
The ability of hβ2m to support binding of an immunogenic peptide to a MHC class 1 heavy chain was tested in a peptide-binding assay. Four different hβ2m variants were compared: (a) chymosin cleaved HAT-C15PΔ-hβ2m, (b) a laboratory reference hβ2m produced according to17 (i.e., Factor Xa cleaved HAT-FXa-hβ2m), (c) methβ2m (hβ2m preceded N-terminally by the bacterial initiation amino acid, formyl-methionine), and (d) noncleaved HAT-C15PΔ-hβ2m. Briefly, titrations of the different hβ2m's were added to fixed amounts of urea denatured HLA B*0702 (final concentration 3 nM, produced according to Ferre et al.)27 and an I125 labeled B*0702 restricted peptide (APRTLVYLL, final concentration 2 nM) in a reaction buffer consisting of 100 mM Tris-maleate, pH 6.6 supplemented with 1 mg/mL pluronic copolymer Lutrol F-68 (BASF, Germany). The reaction mixtures (100 μL) were incubated for ∼24 h at 18°C. Duplicate samples (15 μL) were subsequently analyzed by Sephadex G-50 spun column chromatography as described.28 The radioactivity of the excluded “void” volume, containing formed MHC-I complexes, and that of the retained volume, containing unbound peptide, was measured by gamma spectrometry (Packard Instruments). Binding values were calculated by dividing excluded radioactivity with the total amount of radioactivity offered. The peptide binding values were plotted as a function of the concentration of β2m in the folding reaction, and the data was fitted to a sigmoid dose response curve using GraphPad Prism.
Acknowledgements
The authors thank Malene Nielsen and Anne Caroline Schmiegelow for expert technical assistance. They also thank Chr Hansen, Denmark for supplying ChyMax, and Henrik Nielsen, CBS, DTU, Denmark for information on the P1′ frequencies in proteins.




