Intrasurgical Protein Layer on Titanium Arthroplasty Explants: From the Big Twelve to the Implant Proteome
Abstract
Purpose
Aseptic loosening in total joint replacement due to insufficient osteointegration is an unsolved problem in orthopaedics. The purpose of the study is to obtain a picture of the initial protein adsorption layer on femoral endoprosthetic surfaces as the key to the initiation of osseointegration.
Experimental design
The paper describes the first study of femoral stem explants from patients for proteome analysis of the primary protein layer. After 2 min in situ, the stems are explanted and frozen in liquid nitrogen. Proteins are eluted under reducing conditions and analyzed by LC-MS/MS.
Results
After exclusion of proteins identified by a single peptide, the implant proteome is found to consist of 2802 unique proteins. Of these, 77% are of intracellular origin, 9% are derived from the plasma proteome, 8% from the bone proteome, and four proteins with highest specificity score could be assigned to the bone marrow proteome (transcriptome). The most abundant protein in the adsorbed total protein layer is hemoglobin (8–11%) followed by serum albumin (3.6–6%).
Conclusions
A detailed knowledge of the initial protein film deposited onto the implants, as demonstrated here for the first time, may help to understand and predict the response of the osseous microenvironment to implant surfaces.
1 Introduction
Total hip replacement is a common and highly standardized procedure in the Western world. Compared to other surgical interventions, it is safe and effective, improving mobility and quality of life in the elderly and younger population. Whereas polymethylmethacrylate fixation was the gold standard (“bone cement”) in the 1970s and 1980s, cementless fixation has become more and more popular in femoral stem fixation since the 1990s. Especially, titanium alloys are frequently applied and have shown superior clinical results compared to other biomaterials.1 However, still-remaining challenges in arthroplasty are early implant failure due to insufficient osteointegration of the biomaterial and long-term aseptic loosening caused by wear debris particles. Exemplary, in the national German registry,2 122 961 primary cementless total hip and 97 034 primary cementless total knee implants are documented for 2016, illustrating the overall importance of these therapeutic interventions in the health care of the population.2 About 11–12% of these cases underwent surgical revisions and 38.2% of these were caused by implant loosening.2 These latter cases illustrate the need for basic and clinical research during the initial implantation phase to reduce these early complications.
In cementless fixed total arthroplasties, the implant contacts the bone and its marrow directly. Here, the physicochemical composition and the surface structure of an implant control the interactions to the microenvironment in vivo. These are characterized by initial protein adsorption followed by cellular attachment and adherence leading to migration, differentiation, and proliferation of local cells. Mediated by cytokines and biomechanical forces, subsequent biomineralization and bone remodeling occur.3 Since rapid osteointegration is a prerequisite for early weight bearing after total hip replacement, detailed knowledge of the initial protein film deposit onto the implant may help to understand and predict the latter response of the osseous microenvironment to implant surfaces.
In the 1970s and 1980s, it was hypothesized that plasma proteins are adsorbed as the first step following prosthetic implantation4, 5 (see also ref.6: “The Big Twelve”). Subsequent models of implant integration were thus based on fracture healing with the periimplant compartment containing blood.7 Thus, it has become current doctrine that “Blood is invariably the first tissue that the implant will contact when introduced into the bone.”8 Accordingly, the initial phase of periimplant healing is said to begin with the adsorption of extracellular proteins from native blood.9 Based mainly on in vitro experiments, protein adsorption is followed by the first cells, that is, platelets, appearing after 15 min on a titanium surface10 adhering to surface-adsorbed fibrinogen by glycoprotein GPIIb/IIIa receptors.11 Thus, coagulation is initiated, followed by inflammation and migration of many cell types, especially macrophages, into the periimplant compartment.12 Biomineralization and periimplant bone healing then commences via contact and distance osteogenesis7, 13 until the integration is finalized by bone bonding to the implant.7, 14
From the biomaterial point of view, the initial and sequenced protein adsorption onto titanium surfaces is influenced by its surface topography and chemistry. Typical parameters are porosity, roughness, the size, distribution, and type of surface geometries, the surface energy, contact angle, the thickness of passivation layer.15, 16 Moreover, the type of protein solution (osmolarity, pH, temperature, type of proteins and salts) strongly influence the binding kinetics to surface structures. In contrast to experimental in vitro settings, where defined parameters are controlled, the in vivo scenario is much more complex. It is characterized by a hardly defined local microenvironment which may differ from individual to individual (e.g., the osmolarity in serum physiologically can vary from 280 to 295 mOsm per L). In addition, the surgical procedure itself may have an impact on local protein release and binding.
This paper describes the first clinical study of obtaining explants from patients for proteome analysis of the primary protein layer 2 min after femoral stem implantation. It will be shown that the current doctrine of protein layer formation on implants cannot be upheld. It has so far escaped scientific notice, that a large number and amount of intracellular proteins are incorporated into the primary adsorbed protein layer on hip implants, whose periimplant functions remain to be elucidated.
2 Experimental Section
2.1 Materials
2.1.1 Implants and Rasps
To compare the in situ protein adsorption onto two different surface structures in the same patient and for safety purposes, the Bicontact stem (Braun Aesculap, Tuttlingen, Germany) was chosen, a long-term established Ti-6Al-4V implant which is characterized be a large anchoring distance and high implant survival rates.17, 18 The proximal part of the implant is covered by a 0.35 mm plasma spray layer of commercial pure Ti (cpTi) and has a microrough surface structure (Plasmapore, porosity 35%, pore size 50–200 µm).
Clinical Relevance
Total hip replacement is a common procedure with more than 120 000 primary cementless total hip implants in Germany in 2016. Still-remaining challenges in arthroplasty are early implant failure due to insufficient osteointegration of the biomaterial and long-term aseptic loosening caused by wear debris particles. About 12% of these cases underwent surgical revisions with 38% due to implant loosening. They illustrate the need for basic and clinical research during the initial implantation phase to reduce these early complications. In cementless fixed total arthroplasties, the implant directly contacts the bone and its marrow. Here, the physicochemical composition and the surface structure of an implant control the interactions to the microenvironment in vivo. These are characterized by initial protein adsorption followed by cellular attachment and adherence leading to migration, differentiation, and proliferation of local cells. Mediated by cytokines and biomechanical forces, subsequent biomineralization and bone remodeling occurs. Since rapid osteointegration is a prerequisite for early weight bearing after total hip replacement, detailed knowledge of the initial protein film deposit onto the implant may help to understand and predict the latter response of the osseous microenvironment to implant surfaces. This paper describes the first clinical study of obtaining explants from patients for proteome analysis of the primary protein layer on the implant after femoral stem implantation. Obviously, the current doctrine of protein layer formation on implants cannot be upheld since a large number and amount of intracellular proteins are incorporated into the primary adsorbed protein layer on hip implants, whose periimplant functions remain to be elucidated.
The distal part of the implant is much smoother and was treated by glass-pearl blasting. Some in vivo data from the literature have shown that this uncoated part of the implant is not or less covered with bone compared to the proximal microrough surface.19 The bilateral flanges guide the stem into the intermedullary and improve rotational stability (Figure 1A–C).

Implant Surface Characteristics: Scanning electron microscopy (SEM) (EVO 50, Carl Zeiss AG, Oberkochen, Germany) with energy dispersive X-ray (EDX) (X-Max, Oxford Instruments plc, Abingdon, United Kingdom) was used to characterize the two different surface areas of the hip stem. At least three images from the proximal plasma sprayed layer of cpTi and the distal glass pearl–blasted Ti-6Al-4V surface were taken with the secondary electron (SE) detector for surface topography and the back scattered detector for surface composition (Figure 1D,E).
2.1.2 Biochemical Materials
Amicon Ultra Centrifugal Filters (Ultracel, Merck Millipore, Darmstadt, Germany), C18 nano trap column (Dionex Ultimate 3000 RSLS nano flow system, Dionex, Camberly, UK), Acclaim PepMap C18 nano column 75 µm × 50 cm (Dionex), Orbitrap Velos FTMS (Thermo Finnigan, Bremen, Germany).
2.1.3 Reagents
The following reagents were used: sodium dodecyl sulfate (SDS) (reagent grade), dithiothreitole (DTT), Tris (premium) trypsin (proteomics grade), and acetonitrile, all (Sigma Aldrich, Taufkirchen, Germany); iodoacetamide (grade bc, Applichem, Darmstadt, Germany); NaCl (grade p.A) and Folin Reagent (Merck Millipore); bovine serum albumin (BSA; high quality reference sample, Thermo-Fischer, Rockford USA); trichloroacetic acid (TCA) (Roth, Karlsruhe, Germany); commercial distilled water was employed for preparative methods and ddH2O for analytical methods.
2.2 Methods
2.2.1 Clinical Methodology
Patient Characteristics
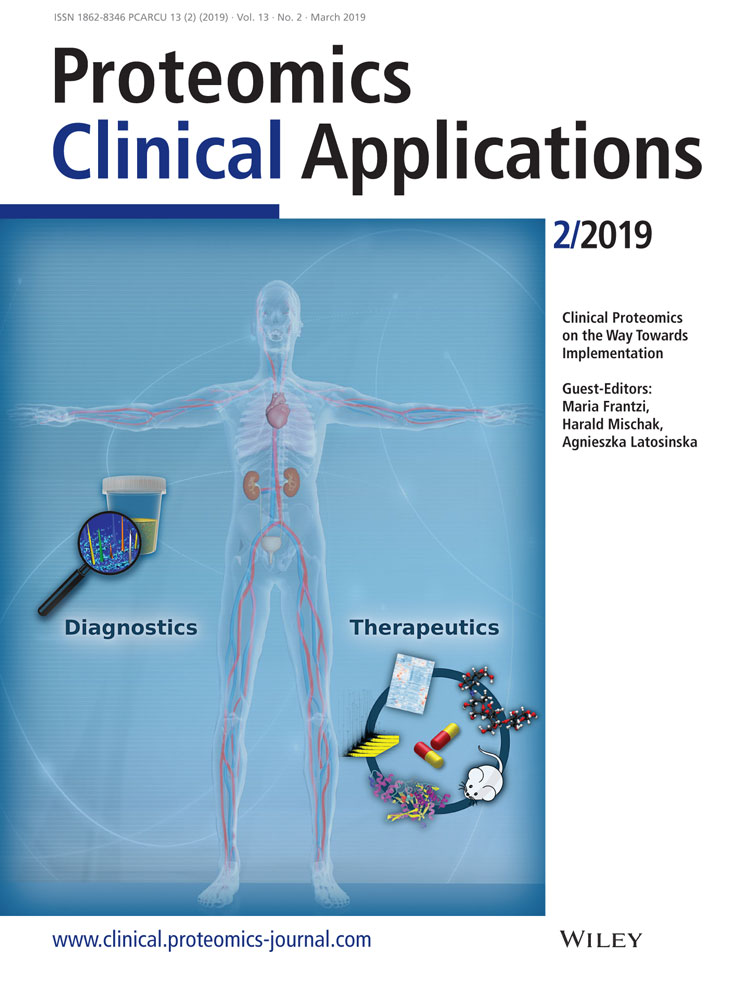
Only adult patients in advanced stages of osteoarthritis who showed normal medullary canal conditions in preoperative standard X-rays were included. Indication for total hip replacement was independently and not influenced by the study. Septic conditions, active neoplasm, or other consuming diseases (autoimmune diseases) and coagulopathy were exclusion criteria. In this single surgeon study (M.J.) two patients were operated after written informed consent. The study has been approved by the Ethical Commission of the University Duisburg-Essen (AZ 17-7844-BO). Figure 2A gives detail information on the patients. The preoperative laboratory findings are given in Table 1 and were in the normal range of such a procedure.
| Plasma/blood parameter | Day | Male | Female | Normal values |
|---|---|---|---|---|
| Sodium [mmol L−1] | −1 | 141 | 143 | |
| 0 | 139 | 136 | 136–145 | |
| 2 | 142 | 133 | ||
| Potassium [mmol L−1] | −1 | 4.4 | 4.4 | |
| 0 | 4.3 | 4.1 | 3.5–5.1 | |
| 2 | 4.2 | 4.1 | ||
| Leucocytes [1 per nL] | −1 | 7.3 | 7 | |
| 0 | 12.2 | 12 | 4.0–11.0 | |
| 2 | 9.4 | 10 | ||
| Hemoglobin [g dL−1] | −1 | 14.3 | 13 | |
| 0 | 12.8 | 11.3 | 11.6–16.3 | |
| 2 | 11 | 9.5 | ||
| Thrombocytes [1 per nL] | −1 | 207 | 307 | |
| 0 | 198 | 234 | 140–320 | |
| 2 | 166 | 209 | ||
| Albumin [%] | −1 | 47.7 | 47.4 | 35.0–52.0 |
| Protein [g L−1] | −1 | 73.8 | 74 | |
| 0 | 68 | 62 | 64–83 | |
| 2 | 58.2 | 56.5 | ||
| Quick [%] | −1 | 119 | 98 | |
| 0 | 107 | 101 | 70–130 | |
| 2 | 94 | 73 | ||
| pTT [s] | −1 | 26 | 29.5 | |
| 0 | 22.4 | 26.1 | 24–32.2 | |
| −1 | 29.5 | 31.8 | ||
| Fibrinogen [mg dL−1] | −1 | 368 | 300 | |
| 0 | 368 | 261 | 180–350 | |
| 2 | 878 | 606.0 |
Surgical Procedures
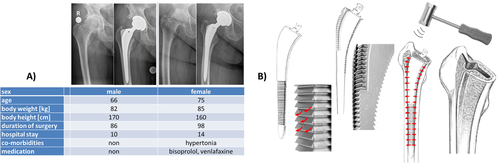
An anterolateral approach to the right hip joint was performed20, 21 (see Figures 2B and 3). After femoral neck resection, the femoral canal was prepared by two different rasps in accordance to the manufacturer's manual (A-/B-Osteoprofiler) (Figure 3A). These instruments and the mechanical procedure (Figure 2B) compress and compact the spongious bone, instead of removing it, form a sheath for implant interception, allowing a maximum of stability, preservation and protection within the existing bone substance. To this end, the rasps (Figure 2B) were inserted and driven inside the femoral canal by controlled mallet stokes. As a muscle sparing measure, the trochanteric wing was prepared. Since both rasps determine the dimension of the implant, an original Bicontact stem was implanted and controlled by fluoroscopy image intensifier (see Figure 2A).
Retrieval of Explants
After 2 min in situ the stems were explanted in a no tough technique (Figure 3). After removal from the pith of the femur (Figure 3C), the titanium implant was washed twice for 1 min with saline (Figure 3D) removing any non-adherent substances (Figure 3E), packaged and quick frozen in liquid nitrogen for transport and storage at −80 °C.
2.2.2 Preparative Methods
Removal and Collection of Adsorbed Protein Layer from Implants
In total, three implants were analyzed: implant 1 from patient 1 (size 12 mm), implant 2 (size 10 mm), and implant 3 (size 11 mm) from patient 2.
On the first implant, the insertion depth in the femur was marked with a marking pen, which was checked for non-interference with the proteome analysis in case of leaching. For protein removal, the implants were thawed at room temperature for 20 min.
Proteins were eluted from the surface in a three step process by incubation and washing with elution buffers: i) buffer A (4% SDS, 1 M DTT, 0.1 M Tris-HCl pH 7.6), ii) buffer B (4% SDS, 1 M DTT, 1 M NaCl, 0.1 M Tris-HCl pH 7.6), and iii) buffer C (4% SDS, 0.1 M DTT, 1 M NaCl, 0.1 M Tris-HCl pH 7.6). The elution process was performed consecutively for both surfaces starting with the rough surface. The elutions were performed by positioning the implant horizontally over a sterile petri dish Ø ≈10 cm by applying the buffer solution with a 1 mL syringe and a 27G cannula.
The general workflow for elution of a single implant with the timescale is detailed in Table 2. The upper and bottom sides of each implant (rough, smooth) are consecutively incubated and eluted with 1 mL of the referred buffers A–C, respectively, for a total time of ≈24 min. The buffer eluates are then pooled theoretically yielding ≈6 mL for later analysis. The procedure necessitates two persons and consequently the yields are not 100% but can vary quite widely. As can be seen in Table 3, the final harvested sample volumes vary from 2.4 to 6.0 mL. In some cases, some volume was lost and in others, a higher volume was necessary for follow-up elution. However, these volume variations appear to have little effect on the protein composition and relative abundance for the subsequent proteome analysis.
| Single implant | Buffer volume | Incubation time | ||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| [mL] | [mL] | [mL] | [min] | [min] | [min] | |
| Rough surface: upper side | 1 | 1 | 1 | 2 | 2 | 2 |
| Rough surface: bottom side | 2 | 2 | 2 | |||
| Smooth surface: upper side | 1 | 1 | 1 | 2 | 2 | 2 |
| Smooth surface: bottom side | 2 | 2 | 2 | |||
| Sum | 2 | 2 | 2 | 8 | 8 | 8 |
| Patient No | Implant No | Implant size | Surface | Sample number | Sample volume | Protein Concentration | Total proteina |
|---|---|---|---|---|---|---|---|
| [mm] | [mL] | [μg mL−1] | [μg] | ||||
| 01 | Implant 01 | 12 | Rough | 01A | 4.60 | 30 | 138.0 |
| Smooth | 01B | 3.70 | n.d. | ||||
| 02 | Implant 02 | 10 | Rough | 02A | 3.48 | 136 | 473.3 |
| Smooth | 02B | 2.28 | 34 | 77.5 | |||
| Implant 03 | 11 | Rough | 03A | 6.00 | 72 | 432.0 | |
| Smooth | 03B | 2.40 | 21 | 50.4 |
- a The calculated total protein is only an estimate because of the high variation of sample volume. n.d. = not determined.
2.2.3 Analytical Procedures
Protein Quantification
The eluted samples could not be analyzed directly because the elution buffers contained high concentrations of SDS (4%), DTT (≈0.7 M) and iodoacetamide (0.2 M), which interfere strongly with all protein assays. Therefore prior to protein quantification by the Lowry method22 (which allows SDS), the eluted protein samples of the hip implant were purified and concentrated by TCA precipitation. In this step, 200 µL of samples 01A and 02A and 400 µL of the samples 03A, 02B, and 03B were mixed with the same volume of 10% TCA. By adding TCA to the samples, the color changed from yellow to rosy. The samples were kept on ice for 15 min and centrifuged at 46 000 × g for 2 min. The supernatant was discarded and the white pellet was washed again with 100 µL of 5% TCA. After 15 min on ice, 5 min of centrifugation followed. The supernatant was discarded and the pellet of the samples 01A, 02A, 03A, and 02B was dissolved in 200 µL 0.1 M NaOH containing 1% SDS. For 03B 150 µL were used.
Afterward, protein quantification by the Lowry method was performed. A BSA standard series of five concentrations was prepared by diluting a stock solution of 200 µg mL−1 BSA with ddH2O (100, 80, 60, 40, 20 µg mL−1). All four purified samples were diluted 1:2, 1:3, and 1:4 for the assay. Into a 48-well plate 40 µL of standard/sample were incubated with 200 µL of the Lowry reagent A+B for 10 min at room temperature (RT). For the standards, a double determination and for the samples a triple determination was used. Then, 20 µL of 1:2 diluted Folin-Ciocalteu's phenol reagent was added and mixed. After 30 min of incubation at RT, absorbance was measured at 750 nm on a plate reader (Multiscan Ascent, Thermo-Fischer, Rockford, USA).
Sample Processing for Proteome Analysis
Fifty µL of the implant eluate was processed with the filter-aided sample preparation protocol, as previously described.23 Reduction was performed with 0.1 M dithioerythritol (DTE) for 20 min at RT and the samples were loaded into 30 kDa Amicon Ultra Centrifugal Filters, Ultracel (Merck Millipore). The filters were centrifuged at 16 000 g for 15 min and washed twice with 0.2 mL of urea buffer. Afterward, the concentrates were mixed with 0.1 mL of 50 mM iodoacetamide in urea solution and incubated in the dark, at RT for 20 min (alkylation), followed by centrifugation for 10 min. Then, the filters were washed once with 0.1 mL of urea solution and twice with 0.1 mL of 50 mM NH4HCO3, pH 8.5. Finally, samples were digested with trypsin (proteomics grade) overnight, in the dark at RT (trypsin/protein ratio: 1/100) and the peptides were collected by centrifugation of the filter units for 10 min at 16 000 g in clean tubes. The filters were washed once more with 40 µL of 50 mM NH4HCO3 and eluted peptides were collected in the same tube. The eluates were lyophilized and stored at −20 °C until further processing.
LC–MS/MS Analysis
Analysis was performed as described previously.23-25 Protein digest corresponding to 10 µL eluate, was loaded onto a 0.1 × 20 mm 5 µm C18 nano trap column (Dionex Ultimate 3000 RSLS nano flow) at a flow rate of 5 µL min−1 in 0.1% formic acid and 2% acetonitrile. Subsequently, samples were applied onto an Acclaim PepMap C18 nano column 75 µm × 50 cm (Dionex), 2 µm 100 Å at a flow rate of 0.3 µL min−1. The trap and nano flow column were maintained at 35 °C. The samples were eluted with a gradient of solvent A: 0.1% formic acid and 2% acetonitrile versus solvent B: 0.1% formic acid and 80% acetonitrile starting at 1% B for 5 min rising to 5% B at 10 min then to 25% B at 360 min and 65% B at 480 min. The column was then washed and re-equilibrated prior to injection of the next sample. The eluent was ionized using a Proxeon nano spray ESI source operating in positive ion mode into an Orbitrap Velos FTMS (Thermo Finnigan). Ionization voltage was 2.6 kV and the capillary temperature was 275 °C. The mass-spectrometer was operated in MS/MS mode scanning from 380 to 1600 amu. The resolution of ions in MS1 was 60 000 and 7500 for higher-energy collisional dissociation (HCD) MS2. The top 20 multiply charged ions were selected from each scan for MS/MS analysis using HCD at 40% collision energy. AGC settings were 1 000 000 for full scan in the FTMS and 200 000 for MSn. Dynamic exclusion was enabled with a repeat count of 1, exclusion duration of 30 s. The mass spectrometry (MS) proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository26 with the dataset identifier PXD012614.
MS Data Processing
Protein identification was performed with Proteome Discoverer 1.4 (Thermo Scientific) using the SEQUEST search engine. Protein search was performed against the SwissProt human protein database. The following search parameters were applied: i) precursor mass tolerance—10 ppm and fragment mass tolerance 0.05 Da; ii) full tryptic digestion; iii) maximum missed cleavage sites: 2; iv) static modifications—carbamidomethylation of cysteine; v) dynamic modifications—oxidation of methionine and proline; vi) event detector mass precision 2 ppm; vii) precursor mass, 390–6000 Da; and viii) collision energy, 0–1000 eV.
After completing the analysis of individual RAW MS files, proteomics data were exported at the peptide level using the following filters: i) peptide confidence: high, medium, and low; ii) peptide rank up to 5; and iii) peptide grouping was enabled and protein grouping was disabled. Of note, protein grouping was disabled to allow for consistent assignment of the proteins across multiple MS runs.
These peptide lists were used as an input for an in-house software and were processed using a clustering approach, as described in ref.24 Briefly, to assure of the comparability of the data between different MS runs, peptides’ retention time (rt) was calibrated using LOWESS (locally weighted scatterplot smoothing) non-parametric regression method using one sample (01B) as reference. Then peptides from different MS runs were grouped (“clustered”) based on the predefined window of mass (± 5 ppm) and rt (± 5% of the peptides’ rt). Specifically, during the grouping/clustering, peptides (pairs of mass and rt) were placed in 2D space, and rectangles of predefined size were drawn and moved until optimal capturing of most data points was achieved. Such groups (“clusters”) were used to generate a peptide reference list (“cluster list”—unique list of features) for the study, in which each cluster is described by the center of the rectangle (defined by pair of mass and rt). Each cluster contains a group of peptides (minimum two members), for which the sequence (belonging to the cluster) with the highest sum of Xcorr was reported as reference. When the same Xcorr sum was reported, the frequency of sequence was considered as a second criterion). Once each group (cluster) had a reference sequence selected, protein matching was performed. Since the protein grouping was initially disabled, possible protein IDs, as defined in Proteome Discover peptide exports, corresponding to the certain sequence were assigned. However, in the next step, for peptides that were assigned to multiple proteins, one protein ID was kept. The protein ID that occurs more frequently in the reference list (“cluster list”) was assigned. This means that the selected protein ID was explained by the higher number of peptides. Therefore, there is no conflict that the certain peptide is used multiple times for protein identification and quantification. As such, the number of proteins identified is not affected by the disabling of protein grouping option. Subsequently, based on the reference list (peptide–protein), information on the peptide abundance (peak area) from individual samples was retrieved.
Quantification of Proteomics Data
Quantification was based on the quantitative values obtained by Proteome Discoverer (Precursor Ions Area Detector node). Specifically, the peak area quantification was applied, that uses the precursor ions for estimation of relative abundance. After retrieval of peak areas, for a limited number of peptides for which area could not be retrieved by Proteome Discoverer (this is a well-known, but not yet corrected problem of this software), the missing values were replaced by the mean area values of all samples. If the peptide was not identified in the particular sample, the missing values were replaced with zero. Subsequently, the individual peptide peak areas were normalized against the total peak area per samples using part per million (ppm)-normalization: normalized peak area = (peptide peak area/total peak area in a sample) × 106. Protein abundance in each sample was calculated as the sum of all normalized peptide areas for a given protein, as described previously.24 This analysis was used as a base for the investigation of the implant proteome (Table S1, Supporting Information).
MS Data Processing Using MaxQuant
Furthermore, RAW mass spectrometry data were also analyzed with MaxQuant (version 1.6.0.16)27 using more stringent criteria for peptide/protein identification, as an alternative to the abovementioned approach. Analysis was performed as previously described28 with some minor modifications. List of identified proteins is presented in Table S2, Supporting Information.
Matching of Implant Proteome with Previously Published Profiling Datasets
Proteome profiling delivers important information on the affiliation of other proteomes to proteins in the implant proteome. For information on the plasma proteome, data were retrieved from Plasma Proteome Database (PPD), which contains 10 546 proteins, see ref.29 The PPD database was downloaded in June 2018. Unfortunately, the online database is now temporarily unavailable. It can therefore not be excluded, that new additions were not considered. Along these lines, the implant proteome was compared with 838 proteins identified using plasma proteome profiling24 as well as with 1175 proteins included in a reference paper on plasma proteome by Anderson et al.30 Furthermore, the implant proteome was also compared with whole red blood cell proteome comprising 2090 proteins.31 The bone proteome as published in ref.32 with 1213 proteins was also employed for matching.
Compared to the bone proteome, the bone marrow proteome, from a tissue with one of the lowest number of genes (see ref.33 and https://www.proteinatlas.org/humanproteome/bone+marrow), is based on molecules that are preselected on the basis of transcript abundance in bone marrow (transcriptome). It appears very extensive, since it includes 58% (n = 11 395) of all human proteins (n = 19 613), most of which are of so-called “housekeeping functions.” After their exclusion and other corrections, only 302 genes with an elevated expression in bone marrow remain as specific. Of these, the 12 proteins noted for their highest tissue transcription specificity score of > 86 were included for comparison.
The protein isoforms of all human genes are annotated using the three categories i) secreted, ii) membrane, and iii) intracellular proteins.33 Therefore, the authors have also distinguished between intracellular and extracellular proteins. For more information on intracellular and extracellular proteome proteins, see refs.,34, 35 respectively.
Gene Ontology Enrichment Analysis
Gene ontology (GO) enrichment analysis was conducted using ClueGO (Cytoscape plug-in).36 Annotation of the implant proteome was focused on Gene Ontology Biological Processes. Analysis was conducted for 2802 proteins that were identified based on at least two peptides. Two-sided (enrichment/depletion) tests was applied, followed by the correction of the p-values using Bonferroni step-down method. GO Term fusion and GO Term grouping was applied, considering GO level = 4. Individual terms were grouped based on the kappa statistics (kappa score threshold of 0.4). Only significantly enriched findings were considered (Bonferroni step-down p-value < 0.05). Subsequently, for each functional group, a leading term was selected based on the highest term coverage (%). Term coverage is defined as percentage, and is calculated based on molecules from the input file that were mapped to the term in comparison with all the molecules mapped with term.
2.3 Statistical Procedures
Mean values and standard deviations were calculated by row analyses using GraphPad Prism (v4.0, Graphpad Software Inc., San Diego, CA, USA). It should be noticed that there are two patients. In the first patient, one Bicontact artificial hip was implanted and retrieved. In the second patient, two separate artificial hips (=implant repeat) were implanted and retrieved for this study (i.e., two patients, three implants and six surfaces must be considered). This was accounted for in the calculation of means and standard deviations by row analysis. Statistical tests for significance were not performed.
3 Results
Time of surgery was comparable in both patients and the pre- and postoperative serum and blood parameters where uneventful and comparable (Table 1).
3.1 Surface Implant Characterization
SEM and EDX as demonstrated in Figure 1D,E show both surface structure and chemical composition of the implant prior to implantation. For the smooth distal part of the hip stem, the chemical composition of the Ti-6Al-4V was confirmed. However, some traces of Ca, Si, Na, and Mg (Figure 1D) were found. Also, EDX confirmed the chemical composition of the proximal rough part of the implant (Plasmapore coating) which is covered by cpTi. The SEM of the latter surface structure demonstrated the high micro-roughness with cuboid, cobblestone, and amorphous geometries (Figure 1E).
3.2 The Implant Proteome
3.2.1 Quantification and Identification of Proteins Adsorbed
On receiving the first data analyses by LC-MS/MS of our implant eluates, we were greatly surprised by two facts: i) first by the large number of proteins detected and ii) second by the fact that the protein with the overall highest abundance on the implant surfaces was not a classical extracellular plasma protein but apparently cell-free hemoglobin (CFH). Only the second protein was one of the big twelve,6 that is, serum albumin. In fact, the abundance value of hemoglobin is ≈twofold higher than the value for albumin on both surfaces. For further work all proteins (of the 7301 detected), which were identified based one peptide (#peptide number = 1), were excluded from interpretation because of uncertainty, leaving 2802 unique proteins identified with at least two peptides (Table S1, Supporting Information). An analysis of the origin of the 2802 proteins (see Table 4) led to the result, that ≈2156 proteins (i.e., 77%) on the implant surface were of intracellular origin and only 211 (≈8%) were classified as secreted or of extracellular origin. In 435 cases (≈15%), a clear classification was not possible.
| n | Secreted | Intracellular | No data | ||||
|---|---|---|---|---|---|---|---|
| 2802 | 211 | 2156 | 435 | ||||
| 7.5% | 76.9% | 15.5% | |||||
| Cell membrane | Cytoplasm | Nucleus or nucleus membrane | Mitochondria | Other | |||
| n | 437 | 857 | 733 | 54 | 75 | ||
3.2.2 Description of the Implant Proteome on Two Different Surfaces
As stated in Section 2.2, the Bicontact hip prosthesis has two different surfaces: i) a cp pure titanium rough surface manufactured by plasma pore coating (Plasmapore) and ii) a relatively smooth glass pearl–blasted matt Ti-6Al-4V alloy stem. Both surfaces were separately eluted and analyzed (see Table 2). The results of this analysis are shown in Figure 4. Although there are some interesting differences (see further) between the adsorptive properties of these two surfaces, the overall picture is very similar. However, due to the limited number of implants analyzed, comparison of protein adsorption to different surfaces lacks statistical power, and requires further investigation. Based on the similarity of proteins identified on both surfaces, no major differences are expected to be detectable.
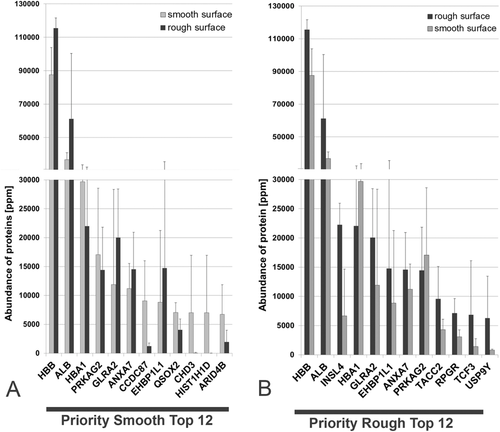
For both surfaces, the abundance of the hemoglobin subunit beta is the highest followed by serum albumin. It is of interest, that annexin 7, which anchors and scaffolds proteins to the surface of cells, is among the first seven proteins on both surfaces. In Figure 4A, the proteins are sorted according to the abundance on the smooth surface. In Figure 4B, they are assorted according to the abundance on the rough surface. The quantitative aspects of the two surfaces are shown in greater detail in Table 5. Only 5 of the 12 proteins on the two surfaces are identical, indicating that their adsorptive properties are different. If all 2802 proteins in the implant proteome are evaluated together, one finds that 21 proteins are distinctly adsorbed only on the rough but not on the smooth surface and that 167 proteins are only adsorbed on the smooth but not on the rough surface, indicating possible surface differences. However, those proteins represent rather the proteins of low abundance and their distinct adsorption should be further validated using other methodologies.
| A: Smooth surface | B: Rough surface | |||||||
|---|---|---|---|---|---|---|---|---|
| Abundance [ppm] | Abundance [ppm] | |||||||
| Number | Protein name | Symbol | mean | SD | Protein name | Symbol | mean | SD |
| 1 | Hemoglobin subunit beta | HBB | 87 595 | 16 257 | Hemoglobin subunit beta | HBB | 115 526 | 6059 |
| 2 | Serum albumin | ALB | 36 694 | 4180 | Serum albumin | ALB | 61 160 | 39 222 |
| 3 | Hemoglobin subunit alpha | HBA1 | 29 705 | 3897 | Early placenta insulin-like peptide | INSL4 | 22 227 | 3733 |
| 4 | 5′-AMP-activated protein kinase subunit gamma-2 | PRKAG2 | 17 061 | 11 521 | Hemoglobin subunit alpha | HBA1 | 22 026 | 10 221 |
| 5 | Glycine receptor subunit alpha-2 | GLRA2 | 11 891 | 16 472 | Glycine receptor subunit alpha-2 | GLRA2 | 20 027 | 84 148 |
| 6 | Annexin A7 | ANXA7 | 11 206 | 4331 | EH domain-binding protein 1-like protein 1 | EHBP1L1 | 14 752 | 20 816 |
| 7 | Coiled-coil domain-containing protein 87 | CCDC87 | 9090 | 6947 | Annexin A7 | ANXA7 | 14 561 | 6367 |
| 8 | EH domain-binding protein 1-like protein 1 | EHBP1L1 | 8852 | 12 406 | 5′-AMP-activated protein kinase subunit gamma-2 | PRKAG2 | 14 449 | 73 748 |
| 9 | Sulfhydryl oxidase 2 | QSOX2 | 7070 | 1689 | Transforming acidic coiled-coil-containing protein 2 | TACC2 | 9536 | 5607 |
| 10 | Chromodomain-helicase-DNA-binding protein 3 | CHD3 | 7036 | 9894 | X-linked retinitis pigmentosa GTPase regulator | RPGR | 7091 | 2555 |
| 11 | Histone H1.3 | HIST1H1D | 7022 | 9910 | Transcription factor E2-alpha | TCF3 | 6814 | 9288 |
| 12 | AT-rich interactive domain-containing protein 4B | ARID4B | 6763 | 5088 | Probable ubiquitin carboxyl-terminal hydrolase FAF-Y | USP9Y | 6265 | 7194 |
3.2.3 Matching of Plasma Proteome with the Implant Proteome
Following the seminal paper by Andrade and Hlady on the “Big Twelve,”6 we searched for plasma proteins on the implants. To this end, we matched the plasma proteome to our protein profile (see Figure 5A,B). Only 248 implant proteins (≈9% of the 2802 proteins) could be assigned to proteins from the plasma proteome. Because hemoglobin is an intracellular, non-secreted protein and is counted as a trace component in the plasma of healthy humans, it was excluded from the analysis (see Table 6). Under these conditions, serum albumin is the most abundant plasma protein on the implant surface. The presence of the two enzymes carbonic anhydrase 1 and catalase in the first six proteins is of interest. Fibrinogen is also present, as expected, and interestingly also a defensin. Conspicuously the expected plasma proteins for cell adhesion, that is, fibronectin is fully absent in the list. In addition, we have compared implant proteome data with the reference manuscript on the plasma proteome by Anderson et al.30 that provides a combination of four different data resources. A total of 142 proteins identified in implant proteome could be matched with the non-redundant list of plasma proteins by Andersen et al. (5% of 2802 implant proteins). This included also 54 proteins that have been reported by Anderson et al. as found in more than one (out of four) resources included in this investigation. In addition, we have also compared the data with previous investigations of the plasma proteome using similar methodology as applied in this study.24 Of the 838 proteins identified with high confidence by Lygirou et al.,24 361 were detected in the implant proteome (13% of 2802 implant proteins). When examining the top 100 abundant proteins from plasma and implant proteome, only eight proteins were common (ALB, CCDC87, IGHG1, SERPINA3, TTN, FGB, TF, FGG). This indicates a limited contribution of plasma proteins to the implant-associated proteome.
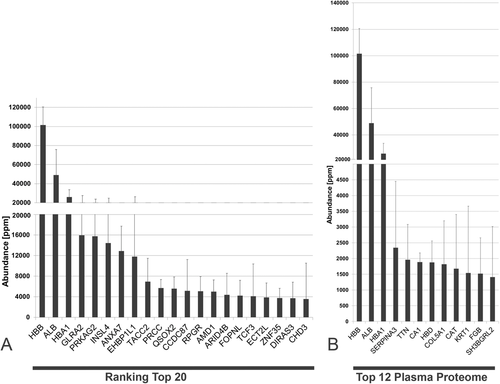
| Abundance [ppm] | ||||
|---|---|---|---|---|
| Nr | Name | Symbol | Mean | SD |
| 1 | Serum albumin | ALB | 48 927 | 17 300 |
| 2 | Alpha-1-antichymotrypsin | SERPINA3 | 2342 | 1174 |
| 3 | Titin | TTN | 1961 | 98 |
| 4 | Carbonic anhydrase 1 | CA1 | 1888 | 21 |
| 5 | Collagen alpha-1(V) chain | COL5A1 | 1820 | 1486 |
| 6 | Catalase | CAT | 1678 | 947 |
| 7 | Keratin, type II cytoskeletal 1 | KRT1 | 1540 | 2002 |
| 8 | Fibrinogen beta chain | FGB | 1518 | 529 |
| 9 | SH3 domain-binding glutamic acid-rich-like protein 2 | SH3BGRL2 | 1411 | 1946 |
| 10 | Keratin, type I cytoskeletal 10 | KRT10 | 1359 | 811 |
| 11 | Fibrinogen gamma chain | FGG | 1161 | 248 |
| 12 | Neutrophil defensin 1 | DEFA1 | 1055 | 342 |
3.2.4 Matching of Bone Proteome and Bone-Marrow Proteome with the Implant Proteome
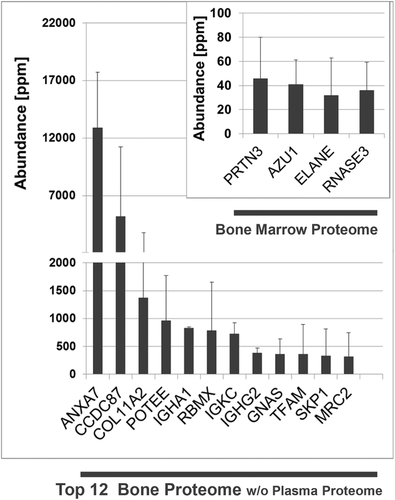
Finally, we wanted to see if bone proteins are also components of the initial protein layer on hip implants. The profiling analysis is shown in Figure 6. Altogether 229 proteins of the implant proteome could be assigned to the bone proteome. Again, there is an overlap with the high amounts of the non-bone protein hemoglobin and also serum albumin, so that these proteins were excluded from the graph in Figure 6. In this proteome, annexin 7 became the most abundant bone protein on the surface. As shown in the insert of Figure 6, four proteins of the implant proteome could also be assigned to the bone marrow proteome. Of the 12 proteins within the bone marrow proteome recognized with high fidelity, 33% are present in the implant proteome.
3.2.5 Matching of Red Blood Cells Proteome with the Implant Proteome
To further investigate if red blood cells adhere to implants, the implant proteome was compared with whole red blood cells proteome.31 Only 405 of the identified implant-associated proteins (14% of the total 2802 proteins) were reported in the red blood cell proteome. Importantly, this does not correspond to the highly abundant red blood cell proteins. Because, when examining the distribution of the top 100 proteins with the highest abundance in implant versus the top 100 proteins with the highest abundance in whole red blood cells proteome, the overlap is even lower (7 common proteins out of 100). The latter includes hemoglobin (HBB, HBA1, HBD), carbonic anhydrase 1, catalase, and keratins (KRT1, KRT10). Among those, HBB and HBA1 belong to the top 10 most abundant implant associated proteins. These results do not support non-specific adherence of red blood cells to the implant's surface.
3.2.6 Functional Annotation of Implant Proteome
Although many publications describe how proteins condition bone healing, a deeper and detailed knowledge of cellular function including inflammation and osteointegration remains unclear for some of the identified proteins. Especially there are only a few studies trying to understand how defined types of biomaterials can influence the protein adsorption onto surfaces.37-41
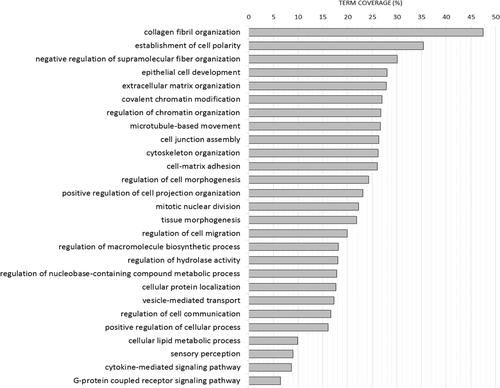
To provide an overview on the biological relevance of the identified implant associated proteins, enrichment analysis was conducted based on Gene Ontology Biological Processes.36 A total of 27 functional groups were identified (Bonferroni step-down p-value < 0.05), and for each group a GO term (biological process) with the highest term coverage was selected as leading term. Overview on the representative terms indicative of the processes represented by the implant proteome is provided on Figure 7. Among them, several biological processes related to bone healing have been identified including collagen fibril organization, extracellular matrix organization, or vesicle-mediated transport.
4 Discussion
In this first report (pilot study) on the protein layer of a hip prosthesis explanted 2 min after implantation, we aim to describe initial protein adherence derived from the surrounding tissue under in situ conditions. The relatively short exposure time of 2 min has been chosen based on ethical reasons. To prevent harm for the patient a longer time was not acceptable since this would prolong the surgery and increase the risk of complications (e.g., amount of blood loss, infection rate).
The obtained results question all previous hypotheses concerning the initial protein layer on implants and their function in initiating bone healing and implant integration. In their report of 1987 on the big twelve (i.e., the twelve plasma proteins are calculated to arrive at the implant surface first), Andrade and Hlady6 discussed the state of the art at that time. There 2D electrophoresis showed over 150 bands from plasma (the proteome of that time) and that it was estimated that a cell contains ≈5000 proteins.
More than 15 years later other investigators such as Pieper,42, 43 Shen,44 and Anderson30 gave deeper insights in the plasma proteome, as the soluble component of the human blood. Based on technical progress, these pioneers of the human proteome have demonstrated that the results of proteomes found in the plasma is strongly dependent to the method applied (e.g., multidimensional chromatography of proteins followed by 2D electrophoresis and the MS identification of resolved proteins, tryptic digestion, and multidimensional chromatography of peptides, followed by the MS identification, tryptic digestion, and multidimensional chromatography of peptides from low-molecular-mass plasma components followed by MS identification). At these days, cytokines and protein hormones were almost completely absent from the proteomics data. However, as noted in the Section 2.2.3, the latest given number of proteins in the plasma proteome actually exceeds 10 000 and the total number of human proteins now lies in the range of 70 000,45 due to splice variants. So now, with a first glance at the implant proteome at hand, we have a tremendous mission in implantology before us.
In the study presented herein, proteomics data were analyzed by applying a peptide-centric approach, which is based on a reference list of peptides/proteins identified when combining all data from the study (as described in Section 2.2.3). Such an approach was developed as an alternative to traditional analysis of MS-based proteomics data, aiming at increasing consistency when combining data from multiple MS runs. In the traditional approach, each sample is analyzed separately considering the stringent criteria for peptide-spectrum match and protein grouping, followed by the comparison. This can result in the loss of information. For example, a peptide, characterized by similar mass and calibrated retention time, that was identified based on the spectrum of lower quality in some of the samples will be by default excluded from analysis. Further, the same peptide might have a different sequence assigned (and as such likely different protein ID) across MS runs, introducing inconsistency in the data. Moreover, at the protein level, grouping of the proteins is applied in the traditional approach, as a result of which different master protein may be selected across different MS runs, which will hamper the proper mapping across multiple datasets. These facts indicate a need for harmonization of the data. To account for these aspects, as presented here, we initially apply lower stringency criteria for peptide-spectrum match, and disable the protein grouping option. In this way, a reference list of peptides (with corresponding proteins) considering all MS data is generated, allowing for a unified assignment of peptide sequences and corresponding protein IDs in the study, improving consistency in sequence/protein assignment.
To further support the validity of the approach, proteomics data were analyzed using MaxQuant. More stringent criteria were considered for peptide/protein identification (i.e., false discovery rate (FDR) 1% at peptide and protein level). After merging of the individual files, a total of 352 proteins were identified (Table S2, Supporting Information). A good overlap was observed between proteins identified using MaxQuant and the clustering approach, with a total of 162 common identifications (46%). For the latter, significant correlation of the average abundances was evident (p-value < 0.0001). Of note, an in-depth comparison of the two solutions for data evaluation is obviously not the scope of the paper. The validity of the approach that has been applied in this study is also supported by a previous publication24, in which the analysis resulted in obtaining biologically meaningful results in the context of cardiovascular diseases. This is reflected by the identification of numerous differentially expressed proteins that have been previously reported to be associated with cardiovascular diseases.
Based on the conducted analysis, one of the major findings is the large amount of hemoglobin in the implant proteome (see Figure 4 and Table 5). It is well known that cell free hemoglobin is elevated in blood plasma during surgery and especially in primary hip arthroplasty.46 In fact, it was reported that the CFH increased ≈tenfold from the preoperative value of 0.02 to 0.2 g L−1 at the end of the operation.46 The decrease in preoperative red cell hemoglobin level (which does not correspond to CFH) from 14 to 11 g/L for the male patient and from 13 to 9.5 g L−1 for the female patient on the second postoperative day (see Table 1) could explain a strong increase in CFH by hemolysis in addition to blood loss. The presence of CFH can be explained by the mechanical destruction of the local tissue during surgical reaming and impaction of the stem and/or by the hemolytic effects due to the friction of the surface structure of the titanium implant. The exact cause-and-effect relationship for the extreme values of hemoglobin on the surface of implants has to be studied further in future experiments. Such studies are crucial, because of the known hemoglobin induced cell trauma on endothelial cells47 leading to endothelial cell permeability changes.48 Thus, hemoglobin on the implant surface may have negative effects on initial bone-healing after implantation. Alternatively, it could be argued that erythrocytes are bound to the surface and later lysed by the protein solubilization steps. However, if erythrocytes were on the surface of the retrieved implant, they would only be loosely bound and are easily washed off the surface with physiological saline as demonstrated in Figure 2D,E. In addition, one would expect a high abundance of the membrane markers CD24, CD45, CD235a, and TER119 for which there is no indication.
The matching of the implant proteome with the plasma proteome, with the bone proteome and with the bone marrow proteome demonstrated, that intracellular proteins from these tissues and probably from all tissues damaged during the operation can be rapidly adsorbed on the titanium implant surfaces.
Except for serum albumin and fibrinogen, the plasma proteome profile adsorbed onto the titanium surface did not correspond to the classical plasma protein layer on implants. Plasma proteins which show a high concentration in the human plasma such as gamma immunoglobulins (i.e., IgG, IgA, IgM), were not found in relevant amounts in the top proteins. The presence of the two described enzymes and a defensin (Table 6) indicate a possible protective potential of the plasma proteome within the implant proteome.
The very coherent proteomic results, that is, the overall very similar protein compositions within the samples, support the conclusion that although the sample volume varied, this had little effect on the protein composition within the samples. Practically nothing is known about the biological effects and interactions between the other adsorbed proteins orchestrating implant integration in cementless total hip replacement. This is in contrast to other experimental studies emphasizing the relevance of other proteins such as fibronectin (Fn), fibrinogen or thrombin as a molecular glue, anchoring osteoblasts to the implant.16, 49-52 Some in vitro data indicate that fibronectin showed excellent adsorption on titanium surfaces even at low concentrations in vitro.49, 53 Other in vitro investigations document that fibronectin adsorption to titanium surfaces is superior to albumin, which is believed to have cell adhesion-inhibiting properties.54 These data cannot be confirmed by our in situ data. One reason could be that the most studies used human plasma but not whole blood as a liquid.
So where are the plasma proteins? Quite a bit is known about the mechanisms underlying the Vroman effect55 and the protein displacement of adsorbed proteins56 by proteins of higher affinity to the surface, for example, through adsorption hysteresis.57 It may be that the amount of 77% of intracellular proteins is the result of having displaced nearly all of the plasma proteins down to 9% (proteome displacement), which are generally held responsible for initiation of bone healing and implant integration. Concerning the 2 min of in situ interaction and the possibility of competitive displacement of proteins, this phenomenon has been shown to occur within the first 2 min of a competitive protein adsorption system (see ref.56). All of these questions will have to be answered in detail in the coming years.
However, the higher protein adsorption rate onto Plasmapore-coated Ti surface compared to smoother surface geometries in our study correspond to the study of Kohavi et al.51 In their experiments they found four times higher protein adsorption for sand-blasted Ti surfaces compared to machined surfaces. Most studies emphasize the significance of fibronectin, albumin, fibrinogen, and IgG, but do not consider hemoglobin as demonstrated for the proteome in spine titanium rods.
One question concerning experimental controls is quite interesting, but methodologically unresolved. In a sense, we have an internal implant control formed by the smooth surface. The rough surface was plasma sprayed onto the original smooth surface, which in that case would be the control for the rough surface. The results in Table 3 strongly indicate that the rough surface adsorbs more protein than the smooth surface, which is in agreement with other studies.51, 58 Moreover, the different physical–chemical composition of both surfaces might have an impact on protein binding. Data from the literature show that abundant protein albumin and hydrogen peroxide (H2O2) can influence corrosion and metal release in Ti6Al4V implants.59 Especially V4+/V5+ ions might influence osteoclasts,60 have an insulin and growth factor mimicking action,61 and can modify inflammatory responses.62 The strong consistency of our data demonstrate that the implant proteome is different from the plasma proteome. However, based on the low number of explants our pilot study does not allow to draw final conclusions on a single protein-binding level.
Some other investigators have looked into the effect of blood onto titanium. Here it is suggested that blood preincubation of implant surfaces mimics a more physiological situation, eventually providing a more predictive in vitro model for the evaluation of novel bone implant surfaces.63
There are only limited data on investigating protein adsorption onto titanium surfaces after in vitro exposition: After incubation of dental titanium implants with human serum, Romero-Gavilan et al. identified 30 out of 218 human serum proteins, which are associated with bone metabolism. The authors found Apo E, antithrombin, and protein C predominantly on sand-blasted or acid-etched Ti whereas proteins of the complement system (complement system factor 3) prefers adsorption onto smooth Ti surfaces.64
We hypothesize that hemoglobin is released by local hemolysis as a side effect during surgical preparation or by the biomaterial itself. The high amount of the beta subunit which was more than sixfold higher than the alpha subunit was surprising since the tetramer human hemoglobin A consists of each two alpha and two beta chains. Furthermore, the significance of highly concentrated iron (Fe protoporphyrin IX, Fe2+) as a major element the prosthetic hem group b to the titanium surface was not investigated yet. Here several interactions are possible (e.g., replacement of Ti2+ of formation of crystals such as pseudobrookite, Fe2TiO5). However, the detailed sequence and kinetics of competitive protein adsorption in vivo remains unclear.
Considering the increasing trend to develop functionalized Ti implant-coatings which promote the adsorption of adhesive proteins such as fibronectin and collagen to create an “osteogenic” implant excluding hemoglobin, iron could disturb osteoblast growth and biomineralization.54, 65 Iron oxyhydroxide is able to generate free radicals and can lead to cell damage. Moreover, a local overload may induce pathological iron biomineralization.66, 67 Iron was also found as the major non-implant related element in dental titanium explants (0.13–0.15%).68 On the other side, coculture experiments with primary human bone marrow and mesenchymal stem cells suggest that a local overload of iron may accelerate osteoblast proliferation and differentiation accelerated S phase entry.66 Here, the investigators observed neither apoptotic signs nor any up-regulation of reactive oxygen species. In addition, it is not known if the high concentration of hemoglobin including Fe will change the corrosion resistance of cpTi surface in situ. However, some new titanium biomaterials include iron as an element, such as Ti–Nb–Zr–Ta–Si–Fe (biomedical beta titanium).69
Our study has some limitations. We are aware that low numbers of samples have been analyzed within this pilot study. Therefore, a statistical comparison of the proteome adhering to the two different surfaces could not be performed. However, one should consider that the samples were obtained in situ during implant surgery. This is an invasive procedure that cannot be repeated multiple times, as it could raise ethical concern (especially when collecting multiple samples from the same patient). In addition, due to the use of low stringency criteria for the peptide-spectrum match, the false sequence assignment may occur, especially when the peptide has low quality of spectra across all samples. By excluding proteins identified based on only one peptide, the risk of false identification is reduced. When analyzing the data with more stringent criteria (1% FDR), the number of identified proteins is decreased, as expected. Further validation of the findings in a larger cohort is required. Our data clearly show a high degree of consistency: independent from the surface structure or the physical–chemical composition of the implant, in that the implant proteome is different from the plasma proteome. In addition, in our study we only documented a snapshot of a condition. We are aware that protein adsorption is a thermodynamically irreversible process characterized by different molecular conditions (fold/unfold) during surface adsorption. This also includes desorption phenomena and metastable conditions (absorption–desorption–hysteresis).58, 70, 71 Moreover, some animal experiments indicate that low protein intake impaired titanium osteointegration.72-74 We also cannot exclude that some blood cells may adhere onto the implant surface, even after buffer treatment. However, a comparison of the implant proteome to red blood cells proteome may indicate that adherence of red blood cells is rather weak. To develop new reliable methods simulating and predicting in situ protein binding onto titanium implant surfaces and implant survivorship, the combination of clinical and in situ proteome analysis is useful. Based on these data, deep learning and neuronal networks might help to find new strategies in personalized medicine in the future.
5 Conclusions
Through this investigation, we have demonstrated that the proteome adhering onto a titanium implant is not the same as the plasma proteome. Titanium endoprosthetic surface structures bind relevant amounts of hemoglobin when exposed to the femoral canal in situ. Moreover, the implant proteome showed a relative high consistency for both Ti surfaces investigated. Being aware of the descriptive character of this pilot study, further clinical and experimental investigations have to demonstrate the relevance of this finding for implant osteointegration.
Acknowledgements
M.J. and H.P.J. contributed equally to this work. The authors thank Prof. Harald Mischak, Chair of Proteomics and Systems Medicine, University of Glasgow and CEO, and Dr. Agnieszka Latosinska of Mosaiques-diagnoscis GmbH, Hannover, Germany for excellent discussions and great advice. The authors thank Heike Rekasi of the Department of Orthopaedics and Trauma Surgery, University Hospital Essen, for her technical input. Finally, the authors also thank the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; Je84/15-3) and B. Braun Aesculap AG for their support and funding.
Conflict of Interest
The authors declare no conflict of interest.