Chemical composition effects on the fracture of polystyrene-block-poly(methyl methacrylate)block copolymers
Abstract
The crazing and fracture behaviors of glassy–glassy block copolymers were investigated for polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA) diblock copolymers that had similar overall molecular weights but different poly(methyl methacrylate) (PMMA) molar fractions. A liquid chromatography technique was applied to separate as-synthesized PS-b-PMMA [(1) weight-average molecular weight (Mw) = 94,000 g/mol and PMMA molar fraction = 0.35 and (2) Mw = 65,000 g/mol and PMMA molar fraction = 0.28] into three fractions with different chemical compositions. With a copper-grid technique, the fracture behaviors of 0.5-μm-thick PS-b-PMMA films were studied as a function of the applied strain. For the higher Mw PS-b-PMMA samples, the median strains at crazing and fibril breakdown increased with an increase in the PMMA molar fraction from 0.24 to 0.46, corresponding to an increase in the chain entanglements in the PMMA domains. In contrast, for the lower Mw samples, the two values were not significantly changed even when the PMMA molar fraction was varied from 0.16 to 0.35. Mw of the minor component in PS-b-PMMA played a critical role in controlling the fracture behaviors of the block copolymers. Specifically, Mw/Me of the minor component (where Me is the molecular weight between entanglements) had to be roughly larger than 2 for the block copolymers to sustain sufficient strains before fracture. © 2006 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys 44: 3612–3620, 2006
INTRODUCTION
To design polymeric materials with enhanced mechanical properties, it is important to understand the fracture behavior of polymers in terms of the molecular parameters.1-3 In glassy homopolymers, the entanglements and crosslinks determine whether crazing or shear deformation governs the plastic deformation before the fibril breakdown.4 That this physical and chemical crosslinking effect governs fracture behavior was clearly demonstrated by Henkee and Kramer,4 who used polystyrene (PS) films crosslinked by electron radiation to produce various strand crosslinking densities beyond the physical crosslinking via entanglement. Two different modes of deformation, crazing and shear deformation, exist, depending on the entanglement density for linear glassy polymers. The effects of other parameters, such as the rate of craze initiation5 and molecular heterogeneity, on the growth and breakdown of crazes6-8 have also been studied for homopolymers that exhibit crazing. However, understanding the fracture behaviors of block copolymers (BCPs) in terms of the molecular parameters could be more complicated than understanding the fracture behaviors of homopolymers. BCPs have a wide range of tailorable properties that are strongly affected by microphase separation, chemical composition, and chain orientation. Therefore, the fracture properties of BCPs depend not only on the molecular weight,9 ordered structure,10, 11 and chain architecture12 but also on the macroscopic alignment10, 13 and thermal aging.14
Recent developments in high-performance liquid chromatography (HPLC) for BCPs have enabled the separation of BCPs by chemical composition differences.15, 16 For example, it has been shown that the chemical composition differences of HPLC-fractionated polystyrene-b-polyisoprenes (PS-b-PIs) are as high as 9 wt %.17 HPLC has been successfully applied to fractionate different BCPs such as polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA),18 PS-b-PI,17, 19 and polystyrene- b-poly-2-vinylpyridine.20 In addition to separating BCPs in terms of the chemical composition difference, HPLC fractionation provides a unique opportunity to prepare BCP samples that have narrower chemical composition distributions than the mother BCP samples. Because the overall BCP chain length is very similar for HPLC-fractionated samples, we can focus on two primary factors, (1) the average chemical composition and (2) the chemical composition broadness, for the fracture behaviors of BCPs that have similar molecular weight distributions.
Here we have studied the crazing and fracture behaviors of PS-b-PMMA with similar chain lengths but different average chemical compositions (Fig. 1). We have used a liquid chromatography fractionation technique to fractionate PS-b-PMMA samples with different average chemical compositions with silica gel. Specifically, we have fractionated as-synthesized mother PS-b-PMMA into three fractions with different compositions, including as much as 20 mol % poly(methyl methacrylate) (PMMA). This fractionation technique allows us to elucidate the effect of the chemical composition difference on PS-b-PMMA crazing while keeping the average molecular weight of each fraction constant. Because fractionated PS-b-PMMA has a narrower chemical composition distribution than the corresponding mother sample, we can also study the effect of the chemical composition broadness on the fracture behaviors of PS-b-PMMA BCPs.
Illustrations representing AB diblock copolymers that have the same molecular weight but different chemical compositions.
EXPERIMENTAL
Materials
PS-b-PMMA was synthesized by anionic polymerization. Styrene and methyl methacrylate were added sequentially after the initiation of the polymer with sec-butyl lithium in tetrahydrofuran (THF) at −78 °C. To reduce the reactivity of the polystyryl anion in THF, diphenyl ethylene was added before the addition of methyl methacrylate. Degassed 1-butanol was used to terminate the reaction.21 Two PS-b-PMMAs were synthesized and fractionated by liquid chromatography, and their average molecular weights and chemical compositions are summarized in Table 1.
| Sample | Mw (g/mol) | Mw/Mn | xPMMA | Mn/Me | |
|---|---|---|---|---|---|
| PS | PMMA | ||||
| BCP1 Mother | 94,000 | 1.09 | 0.35 | 3.6 | 2.5 |
| BCP1 F1 | 94,000 | 1.08 | 0.24 | 4.2 | 1.7 |
| BCP1 F2 | 94,000 | 1.09 | 0.31 | 3.9 | 2.2 |
| BCP1 F3 | 94,000 | 1.08 | 0.46 | 3.0 | 3.3 |
| BCP2 Mother | 65,000 | 1.06 | 0.28 | 2.8 | 1.4 |
| BCP2 F1 | 65,000 | 1.05 | 0.16 | 3.2 | 0.8 |
| BCP2 F2 | 65,000 | 1.05 | 0.30 | 2.7 | 1.5 |
| BCP2 F3 | 65,000 | 1.05 | 0.35 | 2.5 | 1.7 |
Fractionation of PS-b-PMMA by the Chemical Composition Difference
PS-b-PMMA solution samples were prepared in a solvent mixture of THF and iso-octane (IO) with 55 vol % THF at a concentration of 3% (w/v). The solution was added to a bare silica gel column (60-Å pore size; Aldrich) to selectively adsorb PS-b-PMMA over PS homopolymer precursors. The adsorbed PS-b-PMMA on the silica surface was separated into three fractions (F1, F2, and F3 in Table 1) by the following procedure. First, fraction F1 was obtained by the rinsing of the PS-b-PMMA-adsorbed silica column with a mixed THF/IO solvent with 58 vol % THF. Then, F2 and F3 were subsequently fractionated via rinsing with 61 and 64 vol % THF, respectively. The molecular weight distributions of the fractionated PS-b-PMMAs were characterized by size exclusion chromatography (SEC) with a LabAlliance model 500 ultraviolet–visible detector. 1H NMR (Varian 500 Unity) was used to characterize the average chemical composition of PMMA in the mother and fractionated PS-b-PMMA samples.
Craze Characterization and Sample Preparation
To quantify the crazing process for polymer thin films, the copper-grid technique22 was employed. Thin films of PS-b-PMMA (∼0.5 μm thick) were spin-cast onto a glass substrate at 1000 rpm from 5.0 wt % toluene and floated on a water surface. Then, the polymer film was picked up by a copper grid, which was previously coated with the same PS-b-PMMA BCP. Finally, the sample polymer film and copper grid were bonded by a short exposure (several seconds) to the solvent toluene and dried at room temperature for 20 h. Figure 4 (shown later) presents a schematic diagram representing uniaxial tensile deformation. By taking advantage of the plastic deformation of the copper grid (annealed at 600 °C for 10 min in vacuo), this technique allowed us to examine many samples in each grid for the statistical analysis of the fracture behavior under uniaxial strains. At strain regular intervals, the strained films on the copper grid were examined with reflective optical microscopy (OM) and atomic force microscopy (AFM). For AFM, a Digital Instrument Multimode atomic force microscope was used in the tapping mode. Transmission electron microscopy (TEM) was also performed to characterize the craze formation and fibril breakdown with a JEOL CM-12 operating at an accelerating voltage of 120 kV.
RESULTS AND DISCUSSION
Chemical Composition Distribution in the BCPs
Because of the polydispersity of each block, as-synthesized AB diblock copolymers should exist as mixtures of polymer chains with different chemical compositions. Experimentally, Tanaka et al.23 showed an approximately 30 mol % compositional distribution of PS-b-PMMA BCP with preparative-scale thin-layer chromatography. Podesva et al.24 also showed divergence in the distribution of chemical heterogeneity in PS-b-PI BCPs synthesized by a modified anionic polymerization procedure. For an AB diblock copolymer, when B is the compositionally minor block, the maximum and minimum chemical compositions in the as-synthesized AB diblock copolymers due to the polydispersity of the molecular weights can be estimated by the calculation of the compositions of a chain with the shortest A blocks and longest B blocks and then a chain with the longest A blocks and shortest B blocks for a given polydispersity index (PDI) of A and B. Figure 2 shows the calculated maximum and minimum chemical compositions (molar fractions) of the B block in AB diblock copolymers containing 700 A units and 300 B units on average (A700–B300) at different PDIs of A and B. For example, when the PDI of A700 and B300 is 1.05, the actual number of repeating units in A and B is 700 ± 50 and 300 ± 35, respectively. Therefore, the maximum and minimum molar fractions of B have been calculated to be 335/(335 + 650) = 0.26 and 265/(265 + 750) = 0.34, respectively. This suggests that the polydispersity of each block will significantly contribute to the broadness of the chemical composition distribution in the as-synthesized BCPs. The polydispersity of each block alone at PDI = 1.1, for example, will produce about a 10 mol % compositional difference in A700–B300 diblock copolymers.
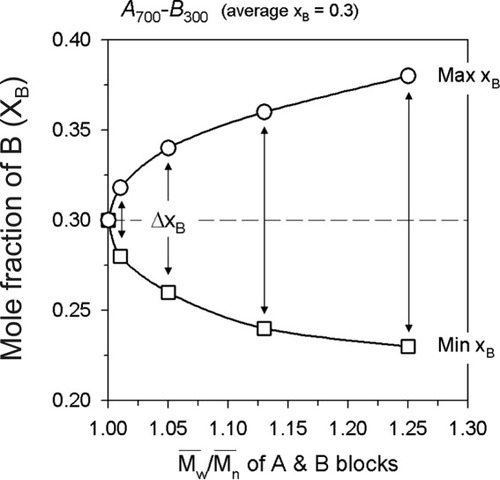
Maximum and minimum chemical compositions in A700–B300 BCPs as functions of the PDI of each block. Each block is assumed to have the same PDI.
Table 1 summarizes the average chemical compositions of three PS-b-PMMA BCPs fractionated from as-synthesized mother PS-b-PMMAs with liquid chromatography. PS-b-PMMA BCP1 [weight-average molecular weight (Mw) = 94,000 g/mol, weight-average molecular weight/number-average molecular weight (Mw/Mn) = 1.09, and molar fraction of PMMA (xPMMA) = 0.35] was separated into three fractions: xPMMA = 0.24, xPMMA = 0.31, and xPMMA = 0.46. Sample BCP2 (Mw = 65,000 g/mol, Mw/Mn = 1.06, and xPMMA = 0.28) was fractionated into three samples: xPMMA = 0.16, xPMMA = 0.30, and xPMMA = 0.35. Therefore, we were able to experimentally fractionate PS-b-PMMA BCP samples, with the PMMA composition differing by about 20 mol %. In addition, the chemical compositional distribution in each fraction must be narrower than that in the mother BCP samples because the liquid chromatography separation was achieved in terms of the chemical composition difference. Although the chemical compositions of the fractionated BCPs are significantly different, SEC profiles (Fig. 3) suggest that the molecular weight distribution of each fraction remains relatively unchanged from that of the mother BCP samples. Therefore, by employing a sample set of a BCP mother, F1, F2, and F3, we can study the effects of the chemical composition difference and broadness on the fracture behavior of PS-b-PMMA while maintaining a similar average molecular weight for each BCP sample.
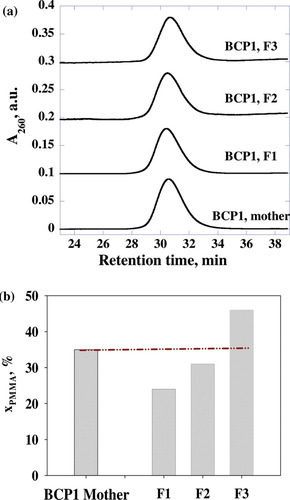
(a) SEC profiles showing absorbance at 260 nm (A260) with respect to retention times, and (b) xPMMA values of BCP1 samples. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fracture Studies of the PS-b-PMMA BCPs
The fracture behavior of BCPs is generally affected not only by molecular factors, such as the homopolymer molecular weight,25 copolymer composition,26 and chain architecture,12, 27 but also by processing parameters, such as the shear alignment13 and thermal aging.14 In particular, the mechanical behavior of BCPs with anisotropic ordered structures is further complicated13, 28 because it depends on the extent of long-range ordering and the direction of macroscopic deformation. Because of such morphological complexity, it is difficult to understand the fracture properties of BCPs in terms of their molecular factors. To avoid such morphological complications originating from processing conditions, we have deliberately limited the ordering of BCPs to a short range by spin-coating BCP films without further thermal annealing.
The copper-grid technique, developed by Lauterwasser and Kramer,22 has been employed to determine the strains for developing crazes or catastrophic failure. Figure 4(b) shows reflective OM images of 0.5-μm-thick PS-b-PMMA BCP films bonded onto copper grids as a function of the applied tensile strain. As shown in Figure 4(c), the development of crazes is identified as dark lines perpendicular to the extension direction with reflective OM. When the sample films are stretched further after the development of crazes, a shadow in the reflective OM images starts to appear, and this is attributed to the catastrophic failures of the film [Fig. 4(d)].22
As shown in Figure 5, both AFM and TEM support the formation of fibril-like structures in the craze region. However, the typical width of the crazed region in BCP1 films does not exceed 0.5 μm, indicating that the films are quite brittle even at Mw = 94,000 g/mol. When BCP2 films (Mw = 65,000 g/mol) were examined, the samples were so brittle that fibril breakdown occurred immediately after the formation of crazes. Therefore, craze-depth characterization of BCP2 films could not be performed by AFM. Because each copper grid contained many polymer films bonded to the copper-grid frame, many sample films were examined at once for the statistical analysis of craze development or fibril breakdown at various strains from a single copper-grid extension experiment. Specifically, the fractions of crazed or failed films were statistically analyzed and plotted as a function of the applied strains (Fig. 6).
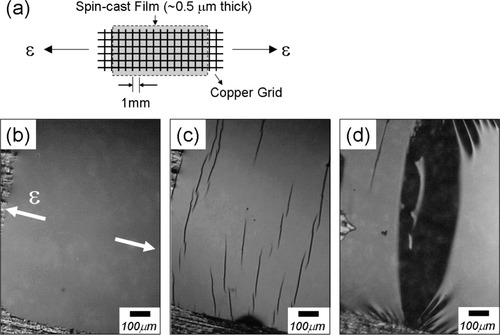
(a) Schematic diagram of the copper-grid setup used to study the fracture of polymer films, (b) OM image representing a BCP1 F3 film with no strain applied, (c) OM image representing a BCP1 F3 film with crazes at ε = 2.4%, and (d) OM image representing a BCP1 F3 film with fibril breakdown at ε = 2.9%.
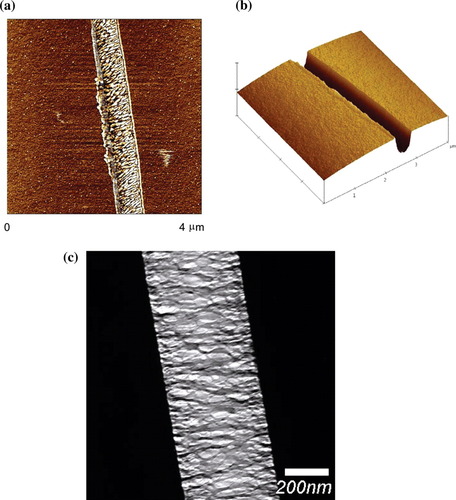
(a) AFM phase image of the crazed region in a BCP F2 film, (b) surface plot of a height image of the same region (height scale = 100 nm), and (c) TEM image of the crazed region in a BCP F2 film.
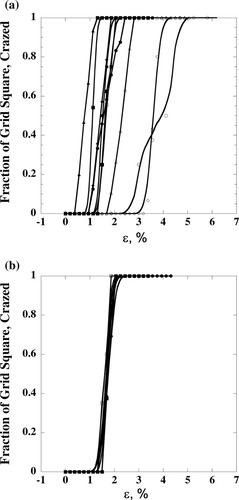
Fraction of copper grids as a function of extension, ε, exhibiting crazing: (a) BCP1 mother (Mw = 94,000 g/mol and xPMMA = 0.35) and (b) BCP1 F2 fraction (Mw = 94,000 g/mol and xPMMA = 0.31). The fractional curves represent more than 10 copper-grid experiments repeated to characterize the statistical development of crazes and catastrophic failure.
Chemical Composition Effects on Crazeand Fibril Breakdown
The median strains for crazing and fibril breakdown were determined when the fraction of square films exhibiting crazing or fibril breakdown reached 0.5. Figure 7(a,b) shows the median strains of crazing and fibril breakdown from several copper-grid experiments for a set of mother and fractionated samples of BCP1 and BCP2, respectively. These sample sets are ideal for us to understand the effects of the average chemical composition difference on the fracture behaviors while keeping the same average molecular weight within each set. As the chemical composition (xPMMA) increases from 0.24 to 0.46 in a series of BCP1 fractionated samples (Mw = 94,000 g/mol), the average median strain for crazing increases from 1 to 2.6%. Such chemical composition dependence can be similarly observed for the fibril breakdown, which increases from 1.1 to 3.3% in the BCP1 fractionated samples. The BCP1 mother sample (xPMMA = 0.35) exhibits median strains for crazing and fibril breakdown at 2.0 and 3.0%, respectively. This suggests that the average chemical composition plays a major role in determining the median strains for crazing and fibril breakdown, regardless of whether the mother or fractionated BCP1 samples (i.e., whether compositionally broader mother or narrower fractionated samples) are used.
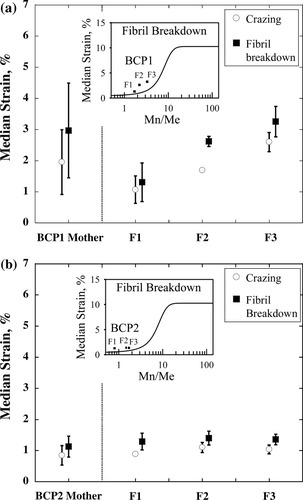
Median strains for (•) crazing and (▪) fibril breakdown for mother and fractionated (F1, F2, and F3) samples of (a) BCP1 and (b) BCP2. The error bars stand for the standard deviation from several copper-grid experiments. The solid lines of the inset figures represent the median strain for the fibril breakdown as a function of Mn/Me from Yang et al.31 using monodisperse homopolymers. The solid squares in the insets indicate the median strain data as a function of Mn/Me of the minor domains in the fractionated samples.
The chemical composition broadness, however, seems to affect the reproducibility of the fraction–strain curves from the repeated copper-grid experiments. We took advantage of the statistical analysis nature of the copper-grid experiment to characterize the fracture behavior, and the fractional curves in Figure 6 show the statistical development of crazes as a function of the applied strain for the BCP1 mother and BCP1 F2 fraction. From 14 copper-grid experiments of the BCP1 mother sample [xPMMA = 0.35; Fig. 6(a)], the standard deviations of the median strains for crazing and fibril breakdown are 1 and 1.5%, respectively. On the contrary, the fractionated F2 sample (xPMMA = 0.31), which has a chemical composition and molecular weight similar to those of the mother sample, exhibits very reproducible median strain values for crazing and fibril-breakdown behavior [Fig. 6(b)]. The standard deviations of the median strains for crazing and fibril breakdown for F2 from several copper-grid experiments are 0.01 and 0.17%, respectively.
Figure 7(b) shows the median strains for crazing and fibril breakdown in a series of BCP2 mother and fractionated samples (Mw = 65,000 g/mol) that have a lower molecular weight than the BCP1 samples (Mw = 94,000 g/mol). Unlike the BCP1 samples, these BCP2 samples have shown little chemical composition dependence on their fracture behaviors. Because the molecular weight of BCP2 is very low, all the samples are already quite brittle, exhibiting fibril breakdown as soon as the craze occurs at a strain of about 1%. It is important to understand why the BCP1 samples show a composition-dependent fracture behavior, whereas the fracture behaviors of the BCP2 samples display little dependence on the chemical composition. Such a contrasting difference in the fracture behaviors of the BCP1 and BCP2 samples should be attributed to the molecular weight differences, which eventually affect chain entanglements in BCPs. Specifically, we have found that the molecular weights of less entangled PMMA domains in the PS-b-PMMA samples play a crucial role in determining the fracture behavior of the BCPs.
As the minor PMMA molecular weight increases for a given molecular weight of a BCP, not only is there an increase in the chemical composition (xPMMA), but there is also an enhancement of the entanglement density within the PMMA domains. Because the molecular weight between entanglements (Me)29, 30 is 17,000 for PS and 13,000 g/mol for PMMA, Mn/Me is lower for the PMMA block, except for BCP1 F3, in our study (see Table 1). From the molecular weight dependence on the fracture behaviors of monodisperse PS, Yang et al.31, 32 have shown that there exists a molecular weight window of Mn/Me (2 ≤ Mn/Me ≤12) in which the fracture properties strongly depend on the molecular weight. In the case of higher molecular weight BCP1 samples, Mn/Me of the PMMA block changes from 1.7 to 3.3 [inset of Fig. 7(a)] when xPMMA increases from 0.24 to 0.46. Therefore, the observed chemical composition dependence on the fracture properties in the BCP1 samples is attributed to the enhancement of Mn/Me in the minor PMMA domains, which are located within the window of 2 ≤ Mn/Me ≤12. On the contrary, Mn/Me of the PMMA block for lower molecular weight BCP2 samples varies from 0.8 to 1.7 [inset of Fig. 7(b)]. Because these ratios are located outside the window, the fracture properties of the PMMA domains in BCP2 do not depend on the molecular weight change of the PMMA blocks.
CONCLUSIONS
The effects of the chemical composition and its broadness on the fracture properties of PS-b-PMMA BCPs have been studied. By the use of a liquid chromatography technique, as-synthesized PS-b-PMMA BCPs [(1) Mw = 94,000 g/mol, PDI = 1.09, and xPMMA = 0.35 and (2) Mw = 65,000 g/mol, PDI = 1.06, and xPMMA = 0.28] have been separated into three fractions with different xPMMA values (as much as 22 mol %) but with the same molecular weight distribution.
The strains for crazing and fibril breakdown have been evaluated by the copper-grid technique for mother and fractionated samples of PS-b-PMMA. For the samples of 94,000 g/mol, the median strain for crazing and fibril breakdown increases from 1 to 2.5% and from 1 to 3.2%, respectively, as xPMMA increase from 0.24 to 0.46. However, for the lower molecular weight PS-b-PMMA with 65,000 g/mol, the fracture behaviors show little compositional dependence. The compositional dependence in the PS-b-PMMA samples with 94,000 g/mol may be attributed to the entanglement density difference of PMMA domains as Mn/Me of the PMMA block, which increases from 1.7 to 3.3. As for PS-b-PMMA samples with 64,000 g/mol, Me of the PMMA blocks is already too low (Mn/Me = 0.8–1.7) to show any significant dependence on the compositional difference. Therefore, we have concluded that the molecular weight of the minor block in the BCPs has to be Mn/Me > 2 to exhibit the compositional dependence on their fracture behaviors. For a given molecular weight and average xPMMA value, the chemical composition broadness of PS-b-PMMA BCPs significantly affects the reproducibility of the fracture behaviors. The fractionated samples exhibit more reproducible fracture behaviors than the mother sample during repeated copper-grid experiments. This is due to the fact that the fractionated samples have a chemical composition distribution narrower than that of the as-synthesized mother BCP sample.
Acknowledgements
This work was gratefully supported by grants from National Science Foundation DMR CAREER (NSF-0449736) and Nanoscale Science and Engineering Center for Directed Assembly of Nanostructures (NSF-0117792).



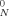 , where G
, where G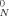 is the plateau modulus, R is the ideal gas constant, T is the temperature, and ρ is the density. Our calculation of Me here used Me = ρRT/G
is the plateau modulus, R is the ideal gas constant, T is the temperature, and ρ is the density. Our calculation of Me here used Me = ρRT/G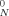 .
.

