From the discovery of sodiumoxyorganoalkoxysilanes to the organosilicon dendrimers and back
Abstract
Upon being discovered 20 years ago, sodiumoxyorganoalkoxysilanes became the key to the world of organoelement dendrimers. Even considering the great variety of objects that had appeared in this area during the last 20 years, the organosilicon dendrimers are still one of the most actual objects in this class. Above all, this is fair concerning the carbosilane systems. The high reactivity of the functional groups, the well controlled chemistry of their transformations, and the inertness of the molecular skeleton are the reason for making these systems highly actual in two main regards: as model objects for the deep research of the dendrimers' properties and as polyfunctional matrixes for numerous derivatives. In this review, we were mainly focusing on the importance of the former part. In the conclusion, we showed the motivation for further development of this area both in regard of synthesis of new carbosilane systems and further development of siloxane dendrimers. © 2008 Wiley Periodicals, Inc. J Polym Sci Part A: Polym Chem 46: 4935–4948, 2008
INTRODUCTION
One of the founders of silicon chemistry Professor K. A. Andrianov in the final period of his life created the Scientific Council on Synthetic Materials that was later transformed into the Institute of Synthetic Polymer Materials of the Russian Academy of Science. The synthetic group of this organization was based on the graduates of K.A. Andrianov's Chair of the Lomonosov Moscow Institute of Fine Chemical Technology. At that time we were young, self-confident and eager to seek for the new and unusual things in the field of organosilicon chemistry. Since 1997 one of us has headed this laboratory. Now it presents the alloy of experience and the youth in the 1:2 golden proportion. The laboratory is operating and developing dynamically, and we consider it as the main achievement of our life for the moment.
It is of interest to review our results on the synthesis of dendrimers according to the chronology of their appearance in the focus of our attention. Strange as it may seem but there is certain logic in our movement from one object to another. The offer to write the review caught us at the new turn of the spiral that explains its heading.
Our way to dendrimers was rather unusual. The idea of their synthesis was caused not by the aim of creation of the layer structures themselves, but by the development of synthetic possibilities of organosilicon reagents. Being not satisfied by the empirical and statistical character of most condensation reactions of siloxane synthesis we dreamt about the selective processes enabling preparation of siloxane polymers with predetermined structure. Without being aware, we tried to realize one of the ideas of our teacher1 which had not been developed properly due to the absence of the appropriate reagents. Since the middle of 70s our attention focused on sodium salts of silanols and siloxanediols, and we experienced both success and disappointment concerning this class of reagents. Being insoluble in common organic solvents and usually containing traces of water or alcohol, they did not justify our hopes.
After several years of intense search we managed to choose a couple of disodium salts which led us to a series of polyfunctional oligomers with rather promising set of properties. To some degree it prevented us from publishing our findings in this field. A small review was made much later.2 However these results did not give us any satisfaction as these reagents lacked universality. While most people took this synthetic limitations for granted one of us continued the search of more efficient reagents. It was E.A. Rebrov. He systematically studied the synthesis of sodium salts and one day we saw a strikingly simple reaction—dissolution of sodium hydroxide powder in the excess of alkoxysilane (Fig. 1).
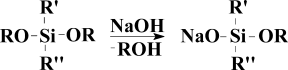
Scheme of sodiumoxyorganoalkoxysilanes synthesis.
This achievement was reported in 1988 for the first time,3 but the details of RnSi(OR')3-nONa synthesis and the reasons behind have appeared recently.4
VOLUME GROWING POLYORGANOSILOXANES
The obtained reagents were completely dissimilar to the known sodium salts. The unique balance of polar and nonpolar units provided good solubility in organic solvents. Because of the appearance of these reagents, unusual for organosilicon chemistry, the synthetic possibilities of the siloxane structure control increased dramatically. The first applications of sodiumoxyorganoalkoxysilanes were in the field of high functional oligomers because all the previous research was undertaken in this area. A number of compact high functional “spiders” of predetermined structure was prepared,2, 5 and as it was found later most of them could be regarded as the first generation of dendrimers. However the perception of this fact would come much later.
At that moment we considered that our main success was the independence of functional groups because the interaction with sodiumoxy group did not touch alkoxysilyl groups. The next question was: “What should be done with them further?” Alkoxysilyl groups are not the too active functional groups. Most of their typical reactions need catalyst and rarely end in full conversion. The only way was to transform alkoxysilyl groups into chlorosilyl ones that could enable further fast and controlled transformations, and the most attractive one seemed to be repeated treatment with the same sodiumoxyorganoalkoxysilanes (Fig. 2).
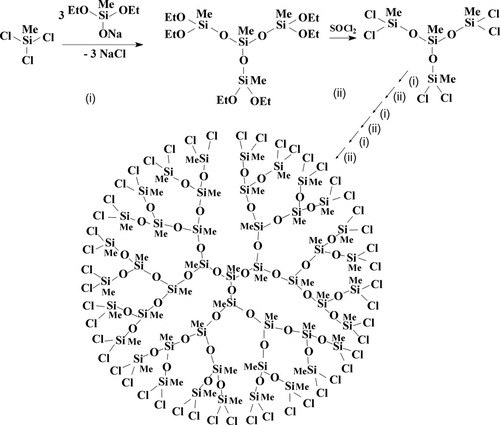
Scheme of polymethylsilsesquioxane dendrimers preparation.
Thus a short one silyl group build-up cycle was born, and it gave a chance of further structure growing. We liked the idea of the combining of various branching centers with salts of different functionality. The simple repeating of the same steps was rather boring, and only the prospect of creation of the new form of polymethylsilsesquioxanes (completely acyclic polymer systems) rather than the known polycyclic structures damped blaze to combining. The possibility of the formation of three-dimensional structures (called by us as the “volume growing”) seemed to be rather interesting. Unfortunately the English word reflects in less degree the peculiarity of the new structure.
We did not join at once the world dendrimer community which was not numerous at the moment. The citing of our work in CA with heading “Organosilicon dendrimers” and the search on the new key word revealed that we were not alone in our desire to control the structure. Strange it may seem but this fact did not upset us, on the contrary it pleased us. On one side we became a part of a new infant area of polymer chemistry that provided us the instantaneous support of our government, and on the other side no one disputed our priority in the field of organosilicon structures. The possibility of structural combinatorics was demonstrated as well but it did not get further development.6
CARBOSILANE DENDRIMERS
The switch to carbosilane dendrimers was caused by objective factors. The most important thing at the moment was the evaluation of the dendrimer properties, but it was rather difficult task for siloxane systems. Moreover the necessity to achieve the full conversion of functional groups and keep siloxane network untouched at the same time demanded a new approach. It was found quite soon and presented a combination of the hydrosilylation reaction and organomagnesium synthesis7 (Fig. 3).
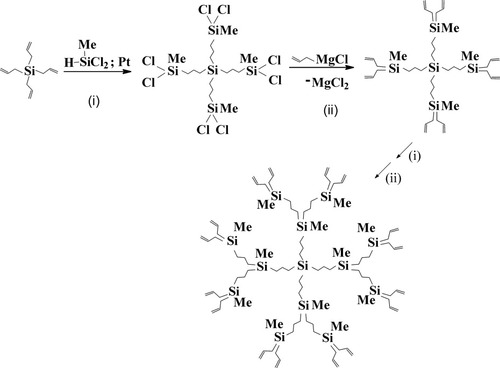
Scheme of polyallylcarbosilane dendrimers preparation.
Carbosilane dendrimers were obtained as branching centers for multiarm stars in group of Professor Roovers. Siloxane systems were not appropriate for this aim.8, 9 Our intentions were different. We would like to have something more stable compared to alkoxysilane derivatives of dendrimers. It was a period of the random search for optimal conditions of synthesis and isolation, and sometimes the whole number of homologs were lost during the storage as the methods of purification had not been worked through yet.10
Our interest in carbosilane systems coincided with the beginning of our collaboration with the group of Professor Martin Moeller from University of Ulm. We owe this man the new level of our work with these objects, of their identification, and more systematic character of our publications. He put at our disposal the first class equipment of his laboratory in the framework of collaboration on INTAS program, and enthusiastically proposed new ideas for joint research. It helped us to overcome without loss the most difficult period in the middle of 90s, and to get the feeling of belonging to the world polymer community.
At that time the main tendency in the synthesis of dendrimers was the study of various derivatives11, 12 (Figs. 4 and 5).
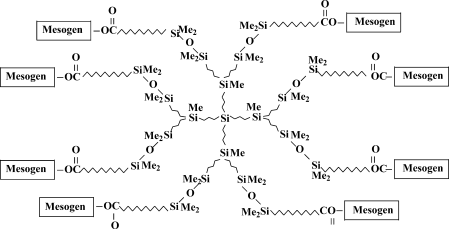
Scheme of dendrimer with mesogen groups on exterior layer.

Planar projection of dendrimer with hydroxyl groups in exterior layer of molecular structure.
It was the display of possibilities and estimation of influence of exterior layer nature on the property of the system as a whole. And still the main driving force was the interest in understanding how the dendrimers were arranged and what could be expected from the development of the area. The experiments on dendrimer synthesis with fluorescent probe in focal point were illustrative13-16 (Fig. 6).
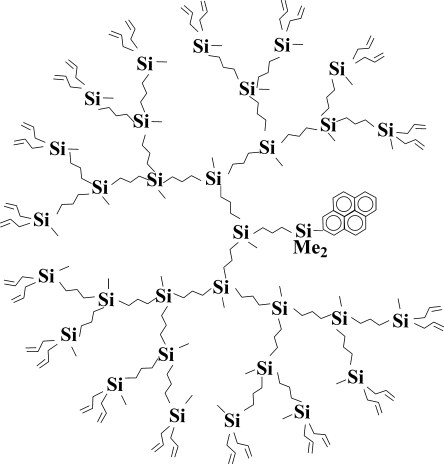
Scheme of dendrimer with fluorescent group in focal point.
We managed to “feel” the density of the molecular structure comparing quenching of luminescence of pyrenyl center of dendrimers of various generations with molecules of quencher.16 The experiment carried out at very low concentrations allowed us to relate unambiguously the rate of quenching to the change in density of dendrimer molecular structure (Table 1). It became more “tangible” only by the fourth generation, as the quenching coefficients differed by an order of magnitude when passing from 3rd to 4th generation.
| Sample | TMPS | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| Diffusion coefficient D (m2 s−1) | 5 × 10−9 | 2.3 × 10−9 | 2.7 × 10−9 | 1.47 × 10−9 | 1.18 × 10−9 |
After these first attempts to “touch” dendrimers, the new ones were undertaken. Synthesis and preparative chromatographic purification of the first five generations seemed to be enough for estimation of the homologous series as a whole.17 The obtained samples (Fig. 7) were used for quite fundamental research on demarcation of interior and exterior spheres.18 The GPC traces demonstrate, that availability (B) or not (A) of hydroxysilyl groups within the same dendritic structures for the intermolecular interactions depends on their distance from the dendrimer core.
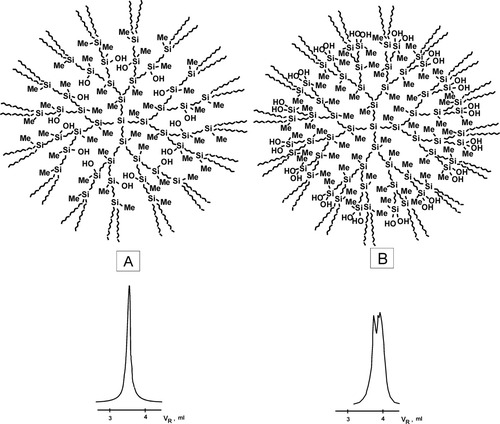
Examples of dendrimers with HO-groups in interior (A) and exterior (B) spheres and their SEC curves.
Their comprehension enabled the realization of the molecular membrane effect and the preparation of unassociated polylithium initiators (Fig. 8) for anionic polymerization19, 20 in particular.
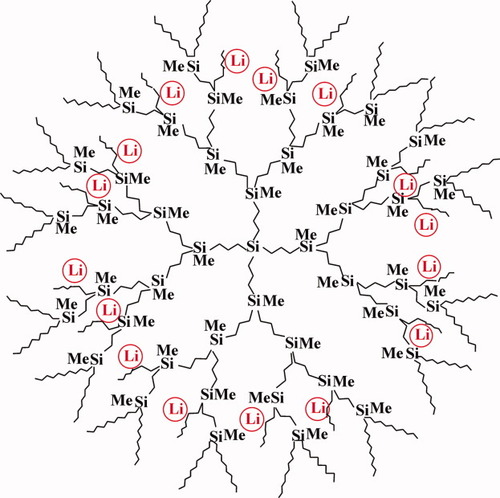
Scheme of dendrimer with Li atoms in interior spheres.
It was the time when everything done was new and unknown. We were eager to try everything at once. Thus the increase in a number of arms on passing from low generations to high ones gave us a chance to determine the boundaries of the characteristic change in the behavior of multiarm stars (Fig. 9) in solutions.21, 22
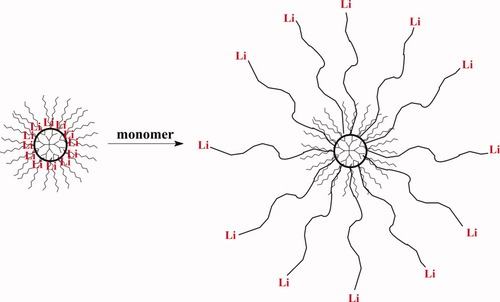
Scheme of multiarm stars preparation starting with dendritic polylithium initiators.
Fortunately just at that time the young students joined our group and we were able to realize some fundamental research. So E. Tatarinova got a task to repeat the synthesis of the first five generations of carbosilane scheme both in functional and nonfunctional version (Fig. 10) and find a new way for higher generations.
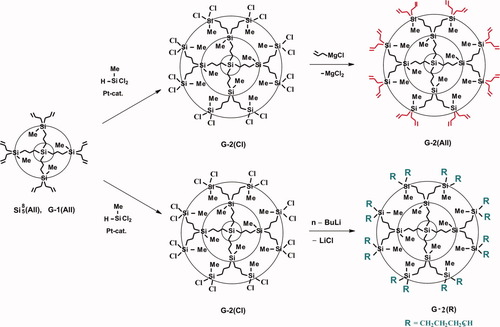
Synthetic routes to carbosilane dendrimers with functional and nonfunctional exterior.
Synthesis and isolation of nonfunctional polybutylcarbosilane dendrimers enabled the systematic investigation of dendrimer properties. The most important results turned out to be the data on dependence of characteristic viscosity and density on the number of generation.23 Along with those obtained on capacity of dendrimer interior sphere,24-27 the new results allowed us to characterize unambiguously carbosilane dendrimers as polymer objects not only according to formal features—presence of a great number of repeating units in molecular structure, but also to the ability to swell and shrink depending on a solvent quality. Figure 11 illustrates the operation done based on SANS contrast variation experiment results indicating the amount of solvent within hydrodynamic volume of carbosilane dendrimer.
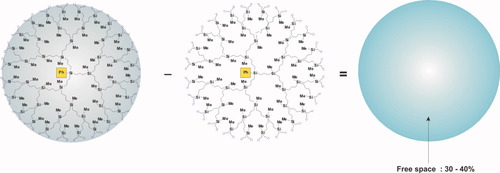
Determination of dendrimers' capacity of solvent uptake.
The study on synthesis of the networks based on dendrimers was illustrative. The task easy at first glance turned out to be much more complicated if you tried to control the conversion of functional groups. Two issues were to be developed here. The problem of determining of conversion degree of functional group was solved first, while that concerning the ratio of intra- and intermolecular reaction in the process of network formation stayed still open.28 The most unusual answer was found later. The network was formed from the mixture of dendrimers of various sizes (Fig. 12) in heterofunctional variant. A small dendrimer appeared to be a perfect bifunctional spacer between the molecules of larger dendrimer.29
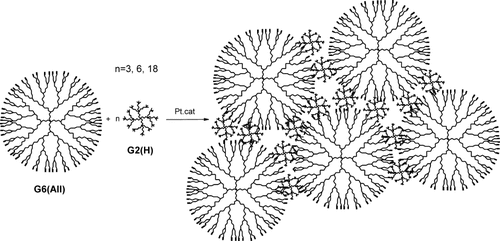
Scheme of network formation based on dendrimers of two sizes heterofunctional interaction.
In this case the control over net structure was almost ideal. The intramolecular cyclization processes did not take place and the unstrained net structure was obtained where dendrimers retained their ability for swelling and shrinking depending on the quality of the solvent. Such “lyrical” digression from the main stream of the research of dendrimer behavior as synthesis of multiarm stars or dendrimer networks gave more food for thought than just evaluation of properties, though no one would have set us free from the necessity to characterize new objects exhaustively and in detail.
The glass transition temperature was the most significant and complicated manifestation of polymer nature of dendrimers. In the case of classical polymers two main factors determine its value—chain flexibility and the level of intermolecular interactions.30 The existence of interior and exterior spheres in the dendrimer structure made possible to reveal the influence of intermolecular interaction on the value of glass transition temperature from the very beginning. The consecutive analysis of facts and synthesis of the key compounds with more typical features provided more deliberate investigation of glass transition temperature peculiarities.
Summary of the results obtained is given in the Table 2. Two conclusions were made from the above data. (1) The chemical nature of exterior layer determined the glass transition temperature, which could vary in the rather wide range. (2) The devitrification of interior structure took place simultaneously with exterior one. It became especially apparent for the dendrimer with siloxane outer-shell.31, 32 Though the influence of outer-shell was expected, that of interior architecture was to be studied further.
| Dendrimer | Tg (°C) |
|---|---|
 |
−93 ± 1 |
 |
−87 ± 1 |
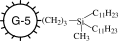 |
−64 ± 1 |
 |
−76 ± 3 |
 |
−50 ± 3 |
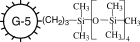 |
−106.5 ± 1 |
It should be noted there was a result of the research that could be even more important for understanding of polymer nature of dendrimers than the data obtained on Tg and their interpretation. The second transition (Fig. 13) found for dendrimers of high generations in a process of the consecutive measurements of the heat capacity dependence on temperature for the great number of various generations dendrimers (made with high precision in the Center of Calorimetry of the RAS headed by Professor N.N. Smirnova in the Institute of Chemistry of University of N. Novgorod).33, 34
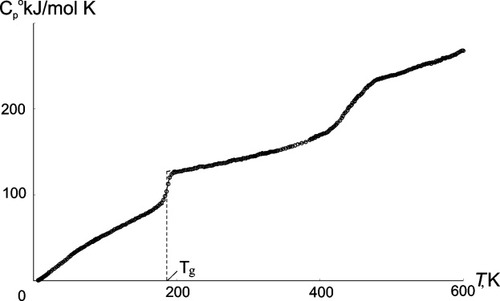
Heat capacity-temperature relationship for G7(Bu) dendrimer.
It is important that the appearance of the second transition coincides with the change of the physical state of a dendrimer samples. If till the fifth generation they are viscous transparent liquids, starting from the sixth one they behave as wax-like substances. The nature of the second transition is the main question of our current study. Probably the growth in generation changes the character of intermolecular interaction in dendrimers similar to the classic polymers where the increase in the molecular mass leads to the appearance of network of entanglements, though the mechanism of the physical knots formation is evidently different and could be connected with changes in dendrimers interpenetration extent with increase of generation number. However it is just a guess-work, but the fact of the second transition after its confirmation for the next generations is beyond any doubt and requires further investigation and comprehension.35
So the switch to carbosilane structure was quite reasonable because as model series this type of dendrimers turned out to be very convenient and their study rather informative.
HYBRID STRUCTURES
Combination of siloxane and carbosilane fragments in the structure of dendrimers appeared to be more fruitful for increasing of synthetic possibilities.36 For example, it was trimethylsilyl protection that allowed us to carry out a systematic investigation of carbosilane dendrimers with hydrophilic surface layer.37 Fluorocontaining dendrimers belong to the hybrid siloxane carbosilane dendrimers.38, 39
All the library of LC dendrimers synthesized in collaboration with the group of Professor Shibaev from Moscow State University possesses flexible siloxane spacer17, 40, 41 (Fig. 4).
Perhaps, the “double” hybrid system is that obtained according to so-called universal protocol (Fig. 14) which is the combination of a divergent center and convergent monodendrons and at the same time it was combination of carbosilane and siloxane structures.42

Universal protocol of hybrid dendrimer synthesis.
Hybrids within the family were prepared as well. So the use of two different organometallic reagents in the frames of one synthetic route gave the possibility to design a purely carbosilane structure with inert exterior and active functional (Fig. 15) interior layers.43
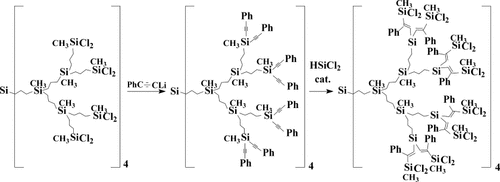
Scheme of synthesis of dendrimer with inert exterior and active interior sphere.
It was made as special molecular container and proved its effectiveness. Nanocluster formations of cobalt oxide were generated in its interior layer (Fig. 16) while dendrimer possessed good solubility in hexane and other organic solvents.44
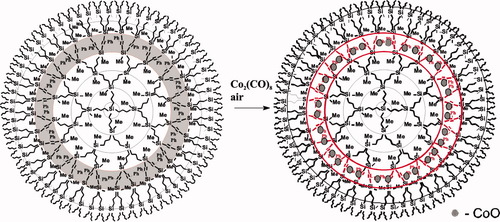
Scheme of the cobalt oxide generation within dendrimers' interior layer.
However it was this system that demonstrated certain limitations of carbosilane dendrimers. Namely, temperature of 200 °C in oxidative atmosphere is the upper limit for the integrity of molecular network, while the catalytic applications demand more oxidative stability.
The difference in thermostability of carbosilane and methylsilsesquioxane units of dendrimer molecular structure enabled nanoporous material with potential application as dielectric underlayer in a process of the controlled thermodestruction. The intense study of the formation of the networks based on dendrimers28, 29 allowed us to choose a more optimal hybrid structure providing the even distribution of nanopores in the material.45
Two ways of the solution of the higher thermostability problem were laid down. (1) Switch to more oxidatively stable aromatic bridges between silica atoms (Fig. 17). Sergei Ponomarenko along with his youth group displayed enormous combinatoric abilities of the similar systems and their prospective use as materials mainly for photophysical applications.46-49 (2) Return to the dendrimer synthesis with siloxane network. The last one appears to be a step back but really it is a new turn of the helix. Carbosilane systems as models for the study of dendrimer nature for the theoretical concept turned out to be beyond competition. However, the development of the new synthetic methods of siloxane dendrimers is in need for the whole range of potential applications and realization of some peculiarities of siloxane systems.
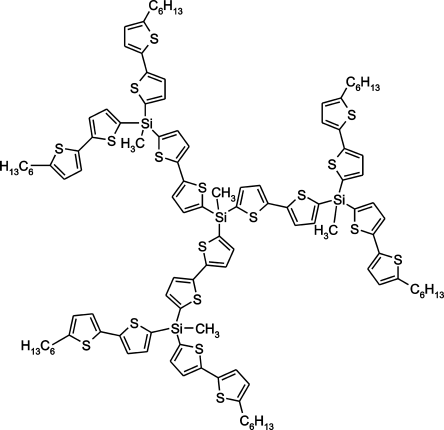
Projection of Oligothiophenesilane dendrimer molecular structure.
Despite the fact that we switched to carbosilanes and hybrid systems in the field of regular systems the use of sodiumoxyalkoxy-derivatives potential went on in other dimension. So the hyperbranched polyethoxysiloxanes (Fig. 18) were prepared and their inorganic structure was characterized as a polymer object.50-52

Scheme of hyperbranched polyethoxysiloxane preparation.
The transition from hyperbranched polymer to nanogel carried out as reaction of intramolecular polycyclization provided an absolutely special form of silica which is called molecular silicasol (Fig. 19). This inorganic molecular system could be used as a solution in dry polar solvents.
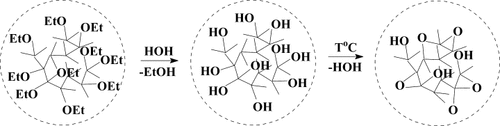
Preparation of molecular silicasol starting from polyethoxysiloxane.
The use of both hyperbranched polyethoxysiloxane and nanogel (Fig. 19), based on it, for the preparation of polymer nanocomposites is an evident application of these products. The possibility of design of more complex molecules and control over their structure, transformation of these objects into the “core-shell” systems in comparison with alternative methods of preparation of similar particles53 let us regard such macromolecules as universal nanoscale fillers for various polymer matrices (Fig. 20).
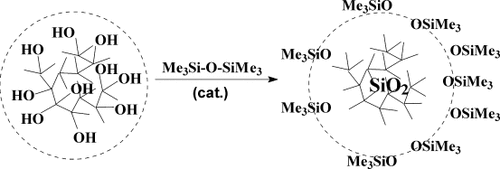
Formation of modified nanogel core-shell systems.
VIEW INTO THE FUTURE
Some other chemical transformations were invented on the base of sodiumoxyalkoxysilanes. Their application in process of synthesis of functional metalsiloxanes was always in mind but only recently they have been used for the preparation of functional metalosiloxane oligomers54, 55 (Fig. 21).

Example of synthesis of functional metalsiloxanes with Fe atoms.
Another very important step in widening of synthetic ability of sodiumoxyalkoxysilanes is the change of order of independent functional groups. If earlier the scheme with “sodiumoxy first” and “alkoxy second” at the end was used, recently another protocol for linear polymethylsilsesquioxane (Fig. 22) has been proposed where the reverse order of functional group usage was realized—“alkoxy first” and “sodiumoxy second.”56

Scheme of linear polymethylsesquioxane terminated by trimethylsilylgroups.
The linear polymethylsilsesquioxane with sodiumoxy groups distributed along the chain was obtained on the intermediate stage. It allowed us to synthesize rather unique polymer objects. The ordinary neutralization of polysodium salt resulted in polyhydroxyl derivative, and blocking with trimethylsilyl groups of polymer led to an isomer of known polydimethylsiloxane. Moreover the polymer synthesized was nothing more but a linear analog of polymethylsesquioxane dendrimer blocked with trimethylsilyl groups.
So it can be affirmed that the real prerequisites for the Second Advent of siloxane dendrimers have been created. They may be summarized in this way. (1) Analysis of carbosilane and hybrid dendrimers as well as their organic analogs provided rather clear notions concerning the relationship between their structure and properties. (2) The unique flexibility of siloxane bond in dendrimers is even more pronounced than in linear systems. (3) Synthetic prospects in design of reactive siloxane oligomers and dendrons are essentially widened. (4) The areas where siloxane dendrimers are especially unique were specified. On this basis we dare to make the forecast that the appearance of new generations of siloxane dendrimers is the matter of the near future.
Acknowledgements
The authors are grateful to all their coworkers and Ph.D. students listed in references with special thanks to V.S. Papkov, N.F. Bakeev for fruitful discussions. They also thank RFFI, Russian Academy of Science and Ministry of Education and Science for financial support.
Biographical Information
AZIZ M. MUZAFAROV
Professor A.M. Muzafarov graduated from Andrianov Chair of Moscow Institute of Fine Chemical Technology in 1973, obtained his Ph.D. in 1981 with Professor A.A. Zhdanov, on the subject of high functional organosilicon oligomers and thermostable polymers. He achieved his Dr. of Chemistry degree in 1997 on the subject of Organosilicon dendrimers and hyperbranched systems. In 1998 together with E.A. Rebrov, he received S.V. Lebedev Award of Academy Sciences of Russia for the investigation of organosilicon dendrimers and hyperbranched systems. From 1996 up till now he is the Head of the Organoelement Polymers Synthesis Laboratory in Enikolopov Institute of Synthetic Polymer Materials of the Russian Academy of Sciences. Since 2000 he is a Corresponding Member of the Russian Academy of Sciences. His major scientific interests include molecular nano-objects (dendrimers, hyperbranched polymers, multiarm stars, nanogels, etc) and general chemistry of organosilicon polymers. He published over 120 papers and has more than 30 Russian and international patents.
Biographical Information
EVGENIJ A. REBROV
Dr. E.A. Rebrov graduated from Andrianov chair of Moscow Institute of Fine Chemical Technology in 1977, obtained his Ph.D. in 1990 with Dr. A.M. Muzafarov and Professor A.A. Zhdanov, on the subject of “High functional branched organosilicon oligomers based on sodiumoxyorganoalkoxysilanes.” In 1998 together with A.M. Muzafarov he received S.V. Lebedev Award of Academy Sciences of Russia for the investigation of organosilicon dendrimers and hyperbranched systems. At present, he is a leading researcher in the Enikolopov Institute of Synthetic Polymer Materials of the Russian Academy of Sciences. His major scientific interests include general organosilicon chemistry molecular design of organosilicon polymers and dendrimers. He has published over 60 papers and has over 10 Russian patents.






