Potential therapeutics of Alzheimer's diseases: New insights into the neuroprotective role of trehalose-conjugated beta sheet breaker peptides
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease with no cure. The conversion of the amyloid-β (Aβ) peptide into soluble oligomers has recently become recognized as playing a key role in AD pathogenesis. Thus, prevention and therapeutic strategies against AD focus on modulating Aβ levels aiming also at stabilizing Aβ's monomeric status or inhibiting the peptide's self-assembly. Peptide-based inhibitors may provide a reasonable alternative to chemical small molecules. We report herein further insights into the neuroprotective action of two trehalose-conjugated peptides able to counteract the Aβ's oligomers associated neuronal toxicity. In addition, the Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2) and Ac-LPFFD-Trehalose (Ac-LPFFD-Th) derivatives protect neuronal cells from excitotoxic insult mediated by NMDA and evoke by themselves pro-survival signal pathways as an additional cell protective action. UHPLC-HRMS experiments suggest that a possible step associated to the neuroprotective mechanism involves the cell uptake and/or a membrane interaction of the studied peptides.
Graphical Abstract
1 INTRODUCTION
In the next decades, a significant increase of social and health care costs will affect the public health system due to the mounting incidence of Alzheimer's disease (AD) and other neurodegenerative diseases. It is estimated that by 2050 more than 131 million people will suffer from AD.1 Unlike other human pathologies, AD has so far proved to be extraordinarily refractory to any attempt aimed at halting or slowing the progression of the disease. Indeed, current approved drugs provide some relief of the AD symptoms, but do not contrast the causes of disease neither delay its progression.2, 3 Therefore, the identification of pharmacologically effective targets is urgently needed for AD. Because the underlying pathogenesis of AD is multifactorial and still under investigation, the design of novel drugs acting according to multiple mechanisms may be an appealing and promising approach. Moreover, if Aβ monomers are crucial for the survival of neurons,4 the selective targeting of cytotoxic oligomers is fundamental for the development of effective therapeutic strategies against AD.5
As concerns the field of small molecule therapeutics, current research efforts focus on the inhibition of Aβ's self-assembly process that may be achieved either by promoting the clearance of aggregated Aβ or by lowering aggregate-induced neurotoxicity.6-9
Other complementary approaches may act via the activation of prosurvival pathways,10-12 or through the regulation of the Aβ monomer levels by the amyloid-degrading enzymes (ADEs).13-16
In terms of general safety, peptide-based amyloid inhibitors exhibit a comparatively lower toxicological profile with respect to various small molecules inhibitors. This could be an advantage especially for illnesses requiring prolonged therapy like AD. A variety of small peptides that inhibit aggregation of Aβ and reduce its toxic effects have been already described and some of them showed to be effective in AD rodent animal models.17, 18 Moreover, Aβ binding peptides have been proposed for suitable uses in in vivo imaging and early diagnosis of AD.19, 20
An apparent problem when using peptides derived from the amyloid core of full-length Aβ, is that these fragments may show a high propensity to form amyloid structures as well.21, 22 Different peptide engineering strategies can now be employed successfully to reduce the intrinsic amyloid-forming properties of these peptide fragments and to improve their inhibitory effect toward Aβ peptides self-assembly. The introduction of proline residues,23 the chemical modifications of the peptide backbone24, 25 such as N-methylation or the conjugation of the peptide sequences with suitable biological moieties (oligosaccharides, porphyrins, etc.),5, 26-30 can be listed as examples of the above mentioned engineered strategies.
In addition, modern formulations and peptide drug design can suppress the rapid enzymatic breakdown of peptides in biological systems, making them eligible drug candidates.31
We have previously reported that three new trehalose (Th) conjugated peptides were able to affect Aβ fibrillogenesis and protected pure culture of rat cortical neurons from Aβ oligomers cytotoxicity.26
Thioflavin-T (Th-T) kinetics and dynamic light scattering (DLS) data supported the hypothesis that these peptide derivatives inhibited the early nucleation phase of Aβ aggregation where toxic oligomers start to assemble.5
Trehalose was introduced at different region of the pentapeptide LPFFD that is, at the N- or C-terminus of the aminoacid sequence as well as at the side chain carboxyl group of the Aspartic acid.26
Interestingly, trehalose conjugation endowed these peptides with enhanced resistance toward proteolytic degradation in rat brain homogenate, in vitro.26
Trehalose, a nonreducing disaccharide that generally exists in yeast, bacteria and invertebrates, is known to inhibit the aggregation of Aβ40 and Aβ42.32, 33 Trehalose may act as chemical chaperone in Huntington disease as shown by in vitro and in vitro assays.34, 35 In other neurodegenerative disorders, it prevents protein misfolding into toxic aggregates thereby reducing the disease pathology.36-39
Among its multiple biological activities, trehalose is able to resist stress such as hydration and to avoid oxidation by protecting the protein from denaturing.40 It has been issued by the FDA with a “generally recognized as safe status.”41
Trehalose has been shown to suppress inflammation, inhibiting expression of different cytokines (interleukin-1β, interleukin-6, tumor necrosis factor-α) and nitric oxide in the conditioned medium released from lipopolysaccharide (LPS)-stimulated BV-2 cells. Furthermore, trehalose alleviated PC12 neuronal death, blocking LPS-induced nuclear transcription factors of NF-κB and AP-1 activation.42 The disaccharide suppresses oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage in rabbits,43 and inhibits protein aggregation due to ischemic insults, preserving proteasome activity.44
- induce chaperone molecules such as HSP90 and SigmaR1 in a mouse model of Lewy body disease, increasing the autophagosomal protein LC3, especially a lipidated form LC3-II;45
- ameliorate dopaminergic and tau pathology in parkin deleted/tau overexpressing mice;46
- delay the progression of amyotrophic lateral sclerosis in motor-neurons.47
Trehalose treatment slows down the pathology in a chronic MPTP-induced Parkinson's disease (PD) mouse model and protects against A53T α-synuclein-mediated dopamine degeneration in an adeno-associated virus serotype 1/2 (AAV1/2)-based rat model of PD.48, 49
The disaccharide enhanced autophagy in the striatum by increasing formation of LC3-II (at concentrations of 5 and 2% in drinking water) and decreased the A53T-AS overexpression and toxicity in transduced PC12 cells, at concentrations lower than 1 mM.50
Recently, the ability of trehalose to induce autophagy and to stimulate lysosomal biogenesis, improving neuronal survival upon exposure to alpha-synuclein preformed fibrils, has been questioned.51 Concerns have been raised also towards the hypothesized action of trehalose as an autophagic inducer and controller of alpha-synuclein aggregation.52
The use of trehalose as a therapeutic agent for AD has been proposed,53 because the disaccharide was able to induce conformational changes in the Aβ peptide,54 and inhibit the self-assembly and neurotoxicity of the polypeptide in cell culture.32 Trehalose administration significantly reduced the amyloid deposits in APP/PS1 mice.55
Alterations of the subcellular trafficking and metabolism of APP (Aβ Precursor Protein) have been imputed to trehalose.56 However, other studies argued on this trehalose's activity.57
A report on transgenic Tg2576 mouse model of AD revealed that trehalose treatment caused a significant increase in the hippocampus of both synaptophysin, a synaptic vesicle protein and surrogate marker of synapses, and doublecortin, a reliable marker of neurogenesis. The growth factor progranulin has also been found significantly increased in the hippocampus and cortex without, in any case, an effect on autophagy, as assessed by western blot of the LC3-1 to LC3-2 protein ratio.58
These findings suggested a new neuroprotective role of the disaccharide that has been related to its ability to control cellular proteostasis via Akt modulation and nuclear translocation of transcription factor EB (TFEB), a master regulator of lysosomal pathways.58
Interestingly, the TFEB over-expression alleviates AD progression by reducing Aβ accumulation. Such an effect is achieved by modulating the autophagy-lysosome pathway and reducing Aβ-induced ROS production and cell apoptosis.59 The relevance of trehalose in activating TFEB translocation to nucleus is further stressed by the recent evidence indicating that TFEB also induces APP degradation with concomitant decrease of Aβ levels.60, 61
It is apparent from the above, that trehalose may exert a series of complex functions in cellular protein homeostasis, and its application, in terms of therapeutic approaches in neurodegenerative diseases, deserves particular consideration.
To obtain multi-target agents against Aβ neurotoxicity and to preserve peptide integrity, we conjugated the active beta sheet breaker peptide LPFFD with trehalose.
In our previous studies, we have been able to establish the ability of the trehalose-conjugated LPFFD peptide derivatives to counteract the Aβ's oligomers associated neuronal toxicity.5 However, in order to be used as a preventive anti AD agent, a molecule should be devoid of toxic effects at the pharmacological doses employed.
To investigate the neuroprotective effect of the Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2) and Ac-LPFFD-Trehalose (Ac-LPFFD-Th) derivatives, we used primary rat cortical neurons and the retinoic acid differentiated human neuroblastoma cell line SH-SY5Y, a neuronal-like model to study AD pathology. Beside further insights into the interaction of the Th-Succ-LPFFD-NH2 derivative with Aβ42, we propose a neuroprotective mechanism of the Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th derivatives. In particular, the studied derivatives not only exert a neuroprotective action against Aβ42 oligomers’ toxicity but they also protect mature neurons against excitotoxic insults mediated by NMDA and promote AKT phosphorylation as a possible pro-survival cell signaling.
2 EXPERIMENTAL
2.1 Materials
The MALDI matrices 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid, SIN) and α-cyano-4-hydroxycinnamic acid (α-CHCA), were purchased from Sciex and were used without further purification. Peptide Mass Standard Kit was obtained from Sciex. Acetonitrile (ACN) and Trifluoroacetic acid (TFA) for mass spectrometry grade were purchased from Fisher Scientific. Dimethylsulfoxide (DMSO) was purchased from Carlo Erba. All aqueous solutions were prepared using Barnstead NanoPure system with a 0.2 μm membrane filter (Thermo Scientific). Alpha-chymotrypsin and 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP) were purchased from Sigma/Aldrich. Aβ42 was obtained from Bachem (Switzerland). All the other materials were of the highest available purity and were used without further purification.
2.2 Sample preparation
Aβ42 monomerization was accomplished using the following procedure. The peptide was dissolved in TFA (1 mg mL−1) and sonicated in a water bath for 10 min. Then the solvent was evaporated under a gentle stream of argon and 1 mL of hexafluoroisopropanol (HFIP) was added to the resulting peptide film. After 1 hr incubation at 37°C, the peptide solution was dried under a stream of argon and the residual was dissolved in 2 mL of HFIP. The solution was dried with argon stream to remove remaining trace of TFA and the sample was again dissolved in 1 ml HFIP and frozen at −30°C. The iced solution was lyophilized overnight. The lyophilized sample was dissolved in DMSO, to obtain a concentration of 2.2 × 10−4 M (Aβ42 stock solution). The Ac-LPFFD-NH2, Ac-LPFFD-Th and Th-Succ-LPFFD-NH2 peptides were dissolved in 5 mM phosphate buffer pH 7.4 to obtain for each peptide a stock solutions of 5.5 × 10−5 M. Aβ42 sample and the equimolar mixtures of Aβ42/Ac-LPFFD-NH2, Aβ42/Ac-LPFFD-Th, and Aβ42/Th-Succ-LPFFD-NH2 were prepared from stock solutions to a final concentration of 5.5 × 10−6 M.
For limited proteolysis experiments, a fresh stock of alpha-chymotrypsin (1 mg mL−1) was prepared with HCl (1 × 10−3 M), then an appropriate volume of the enzyme stock solution was added to Aβ42 and Aβ42/Ac-LPFFD-NH2, Aβ42/Ac-LPFFD-Th, and Aβ42/Th-Succ-LPFFD-NH2 samples to obtain a final enzyme/substrate ratio of 1:20 w/w and 1:200 w/w. Solutions were incubated at 25°C for 1 hr. The digestion reactions were stopped by adding 1 μL of 0.3% aqueous TFA.
For MALDI-TOF measurements, samples were analyzed with α-Cyano-4-hydroxycinnamic acid (α-CHCA) and sinapinic acid (SIN) matrices. SIN was prepared dissolving 4–8 mg/vial of matrices in 1 mL of 50% ACN in 0.3% aqueous TFA. The α-CHCA was prepared dissolving 4–8 mg/vial of matrices in 1ml of 30% ACN in 0.3% aqueous TFA. An internal Standard (I.S.), corresponding to the peptide fragment PrP23–50 (KKRPKPGGWNTGGSRYPGQG SPGGNRYP), was also added to the matrix solution for a final concentration of 5 × 10−7 M. Samples were spotted on the plate using the “thin-layer” preparation method. According to this method, a 1 µL aliquot of the α-CHCA matrix solution was spotted onto the target, then 1 µL of the sample solution was deposited onto the thin matrix layer. All the samples were spotted in three different wells of the plate (triplicate) and five mass spectra were acquired for every spot.
2.3 Thioflavine-T (Th-T) assay
The kinetic features of the amyloid aggregation were studied by using the Th-T binding assay. A multiwell-plate reader (Varioskan Flash, Thermo Scientific) was used for the fluorescence measurements. Readings were performed for 28 hr, every 10 min. The excitation and emission wavelengths were set at 440 and 480 nm, respectively. Aβ42 and the trehalose-peptide conjugates were freeze-dried into the well plate in order to minimize errors for the sample preparation. The mixtures were incubated at 37°C in phosphate buffer (10 mM, pH 7.4). The Aβ42 and Th-T concentrations were 15 and 45 μM, respectively. The Aβ42/tested compound ratios spanned from 1:1 to 1:20.
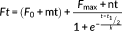
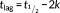
The kinetic parameters are expressed as mean (± SD.) three independent experiments.
2.4 MALDI-TOF mass spectrometry
Standard kits were used to calibrate mass scale of MALDI mass spectrometer. The peptide mass standard kit includes des-Arg1-Bradikynin, Angiotensin I, Glu1-Fibrinopeptide B, ACTH (Clip 1–17), ACTH (Clip 18–39), and ACTH (Clip 7–38) and it was used to cover a mass range from 800 to 4000 Da. Lyophilized samples were dissolved in 100 μL of 1:1:0.01 acetonitrile/water/TFA. Bovine insulin, E. coli thioredoxin and horse apomyoglobin were used to cover a mass range from 4 000 to 20 000 Da. Lyophilized samples of bovine insulin, E. coli thioredoxin and horse apomyoglobin were dissolved in 3:7:0.01 acetonitrile/water/TFA at a final concentration of 0.5 pmol µL−1, 2.75 pmol µL−1, and 4.0 pmol µL−1, respectively.
MALDI mass spectra were obtained using a 5800 MALDI-TOF/TOF mass spectrometer (Sciex) equipped with an automated single-plate sample-loading system, 1 kHz OptiBeam™ On-Axis Laser Nd:YAG 349 nm wavelength, delayed-extraction (DE), two acceleration regions, QuanTis™ Precursor Ion Selector, CID cell, two-stage reflector mirror and a 1000 MHz digitizer. The instrument was operated in Reflectron mode (m/range: 800–4 000) by applying the following voltages: 15 kV source 1, 12 kV Grid 1, 2.25 kV Source 1 Focus, 7.5 kV source 1 lens, 0.153 kV Y1 deflector, 0.095 kV Y2 deflector, −0.011 kV X2 deflector, 5.25kV lens1, 3kV linear detector. The same voltages were applied when operating in linear mid molecular weight mode (m/range: 3 000–15 000) with the exception of source 1 lens where a 4.25 kV voltage was used. Power resolution was improved by using the Delayed extraction approach. The delay time was set according to the molecular weight of the analytes. Mass spectra were acquired by averaging 300 to 600 shots. Laser pulse energy was adjusted according to the various MALDI matrices.
MS data were imported into a freely available open-source software, mMass (http://www.mmass.org). To reduce signal variability, mass spectra acquired for each sample were averaged and monoisotopic peaks were automatically picked. The m/z signal intensities were normalized to an internal standard (PrP23–50). Theoretical m/z values of Aβ42, PrP23–50 (I.S.), Ac-LPFFD-NH2, Ac-LPFFD-Th, Th-Succ-LPFFD-NH2 and peptides resulting from in silico digestion of Aβ42 were compared with the m/z values assigned to experimental mass spectra. Peptides matched successfully, within a tolerance of 0.2 Da, were annotated. The Compare Peak Lists tool was used to compare annotations between multiple spectra.
2.5 Culture of pure cortical neurons and treatments
Animal care and experimentation was in accordance with institutional guidelines. Pure cortical neurons were obtained from E15 rat embryos. Cortical cells were dissected, mechanically dissociated and maintained in Neural Q™ Basal Medium supplemented with GS21 (Globalstem, Rockville, Maryland, US) as previously described.62 Cortical neurons were seeded on 24-well plates pre-coated with 0.1 mg mL−1 poly-d-lysine and incubated at 37°C with 5% CO2 in a humidified atmosphere. To avoid the proliferation of glial cells, Cytosine-β-d-arabinofuranoside (3–10 µM) was added to the cultures 18 hr after seeding. The ability of LPFFD derivatives to inhibit the formation of Aβ oligomers, was assessed by incubating 100 µM solutions of un-aggregated Aβ42 for 48 hr at 4°C, either in the presence or in the absence of each peptides at 1:5 molar ratio. The LPFFD peptide was also used as a control. After the incubation period, coincubated peptides were applied for 48 hr to mature cortical neurons at a final concentration of 100 nM. To test the neuroprotective activity of Ac-LPFFD-Th and Th-Succ-LPFFD-NH2, mature pure cortical neurons were starved from insulin and treated with the Aβ42 peptide or LPFFD derivatives for 48 hr. Cell viability was assessed by the 3-(4,5- dimetiltiazol −2- il)-2,5- difeniltetrazolio treatment (MTT) (0.5 mg mL−1, SIGMA) for 2 hr at 37°C. Formazan production was measured in a plate reader at 560 nm. Mixed cortical cells, containing both neurons and glia, were obtained from E17 rat embryos and grown as described previously.4 Mature cultures (14–16 days after plating) were exposed to 300 µM NMDA for 10 min at room temperature in a HEPES-buffered salt solution. NMDA was added in combination with Aβ42 monomers or LPFFD derivatives. At 24 hr after treatments, neuronal toxicity was tested by trypan blue exclusion (5 min, 0.4%). Three random fields/well were considered.
2.6 Differentiated neuroblastoma SH-SY5Y and treatments
SH-SY5Y cell line were cultured in Dulbecco's DMEM F12 (Gibco, Thermofisher) supplemented with 10% heat inactivated fetal calf serum (FCS) (Gibco, Thermofisher), penicillin and streptomycin (100 mg mL−1 each) and 2 mM L-glutamine. To obtain differentiation in neuronal-like cells, SH-SY5Y cells (5 × 103) were seeded on 6-well plate in medium containing 5% FCS. Then, serum was gradually reduced to 1% and 5µM all-trans-retinoic acid (RA) (SIGMA, Saint Louis, MO) was used. Neuronal like cells were obtained roughly 7 days after RA supplementation. Trehalose or LPFFD derivatives were applied to differentiated SH-SY5Y cells at a final concentration of 5 µM for 15 min in PBS buffer.
2.7 Western blot analysis
Differentiated SH-SY5Y cells, exposed to LPFFD derivatives or trehalose, were harvested in RIPA lysis buffer (Alfa Aesar, GmbH Co KG) in the presence of protease- and phosphatase inhibitors cocktails (both from Pierce, Thermofisher). BCA protein Assay Kit (Pierce-Thermofisher) was used to determinate protein concentration. Proteins (30 µg) were separated by electrophoresis on precast 4–12% gradient gels (Bolt, 4–12% Bis-Tris Plus Gels, Invitrogen, Thermofisher) and electro-transferred onto a nitrocellulose membrane. Membranes were incubated overnight at 4°C with the following primary antibodies: p(ser473)-Akt and pan Akt (all used at 1:1000 dilution, Cell signaling Technology, Beverly, MA). Secondary goat anti-rabbit labelled with IR dye 800 and goat anti-rabbit labelled with IR dye 680 (all used at 1:20 000 dilution, LI-COR Biosciences). Hybridization signals were detected under the Odyssey CLx Infrared Imaging System (LI-COR Biosciences).
2.8 Aβ oligomerization (aβo)
Aβ42 oligomers (Aβos) were obtained by incubating 100 µM Aβ42 monomers (DMSO 5mM stock solution) in ice-cold phenol-red free medium (Ham's F12, Life Technologies) for 48 hr at 4°C. Aβ42 preparations (100 µM) were allowed to oligomerize in the presence of Ac-LPFFD-NH2 peptide derivatives (Aβ42/peptide ratio 1:5), in order to assess the ability of the analyzed peptides to inhibit the formation of Aβos.
2.9 Quantitation of the Ac-LPFFD-NH2 and related trehalose derivatives upon cell interaction
Cell lysates were diluted with methanol to promote protein precipitation. After centrifugation, repeated chloroform addition and extraction were performed in order to achieve complete organic solvent and lipid removal. The aqueous solution was diluted (50–100 fold) and analyzed by means of ultra-high performance liquid chromatography (UHPLC) system (Ultimate 3000, Thermo). The analytical separation was performed on an Easy-spray® column (PepMap C18 – 100 Å, 3 µm, 150 mm × 75 µm) by using a solution of 0.1 formic acid (FA) in water and 80:20 ACN:H2O containing 0.1% FA as the eluents. The flow rate was 300 nL min−1. A high-resolution mass spectrometer (HRMS) was the detector (Q-Exactive, Thermo). The spray voltage and capillary temperature were 2 kV and 300°C, respectively. Ac-LPFFD-NH2 and its trehalose derivatives (Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th) in the samples were quantified by means of calibration curves obtained after the analyses of solutions containing known concentrations of the standard compounds.
3 RESULTS AND DISCUSSION
3.1 Inhibition of Aβ42 fibrillization: Th-T fluorescence assays
The ability of Th-Succ-LPFFD-NH2 and their parent compounds to inhibit the fibrillar aggregation of Aβ42 was studied by Th-T fluorescence spectroscopy. The most relevant kinetic parameters (Table 1) were obtained by properly fitting the experimental curves to the theoretical ones. The fluorescence curves reported in Figure 1 show a typical sigmoidal profile indicating the presence of a lag phase (9.6 hr for Aβ, Table 1) followed by the exponential elongation phase and a linear fluorescence increment (Fmax 9.3 for Aβ).
| Aβ | Aβ:Th-Succ-LPFFD-NH2; 1:1 | Aβ: Th-Succ-LPFFD-NH2; 1:5 | Aβ:Ac-LPFFD-NH2; 1:1 | Aβ:Ac-LPFFD-NH2; 1:5 | Aβ:Th; 1:1 | Aβ:Th; 1:5 | |
|---|---|---|---|---|---|---|---|
| Fmax | 9.3 ± 0.4 | 7.5 ± 0.3 | 6.6 ± 0.4 | 9.4 ± 0.2 | 9.2 ± 0.4 | 9.8 ± 0.3 | 9.7 ± 0.3 |
| tlag | 9.6 ± 0.3 | 8.8 ± 0.3 | 7.7 ± 0.5 | 8.8 ± 0.2 | 10.0 ± 0.3 | 8.9 ± 0.2 | 8.8 ± 0.2 |
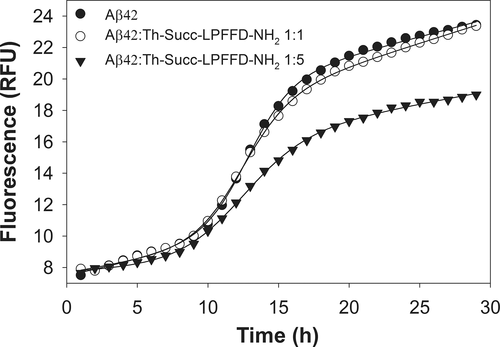
Time dependent Th-T fluorescence curves for pure Aβ42 (15 μM) and mixed Aβ42/Trehalose-Succinyl-LPFFD-NH2 (Aβ42/Th-Succ-LPFFD-NH2) at different molar ratios (1:1 and 1:5). Experimental conditions: phosphate buffer solution (10 mM), pH 7.4, 37°C
It is clear from Figure 1 and Table 1 that Th-Succ-LPFFD-NH2 has an effect on Aβ42 fibril formation. In particular, what is observed is a reduction of Fmax, proportional to the concentration of Th-Succ-LPFFD-NH2 (7.5 and 6.6, which means 81 and 71%, respectively, to that produced by Aβ42 alone). On the contrary, the lag phase (tlag) is shortened (8.8 and 7.7) compared to that of Aβ alone. The antifibrillogenic activity of Th-Succ-LPFFD-NH2 follows a dose dependent response similar to that seen for the Ac-LPFFD-Th derivative in our previous work,5 up to an Aβ:compound ratio 1:5. Higher concentrations of Th-Succ-LPFFD-NH2 did not significantly outperform the antiaggregant activity (Supporting Information Figure S3).
As for the parent compounds of Th-Succ-LPFFD-NH2, neither Th nor Ac-LPFFD-NH2 have an effect on the amyloid aggregation of Aβ. Both Fmax and tlag are not significantly different to those related to the fibril formation of Aβ (see Table 1). Overall, the covalent conjugation of trehalose clearly affects the dose-dependent antiaggregant activity of the LPFFD moiety, as previously found in the case of other peptide systems.63, 64
3.2 Aβ42/peptide interactions: MALDI-MS investigations
We have previously reported that the C-terminally trehalose conjugated Ac-LPFFD-Th preferentially interacts with the KLVFF amino acid sequence of Aβ42 monomers.5 Nevertheless, the question as to whether the trehalose, bonded at the N or C terminus sides of the LPFFD amino acid sequence, could differently affect the interaction with Aβ42, has still to be elucidated. Therefore, MALDI-MS measurements were carried out to evaluate adducts formation when Ac-LPFFD-NH2, Ac-LPFFD-Th or Th-Succ-LPFFD-NH2 were added to the Aβ42 sample solution. Unfortunately, MALDI-MS spectra recorded using samples containing equimolar concentrations of Aβ42 with Ac-LPFFD-NH2 and the trehalose conjugated derivatives, did not give any visible adducts. This is probably caused by co-crystallization of the analyte with the organic matrix used in MALDI experiments, which limited the hydrophobic interactions of the peptides with Aβ42. Nevertheless, it is interesting to note that m/z values corresponding to Aβ42, reveals different signal intensity when Ac-LPFFD-NH2 or the trehalose-conjugated derivatives were added to the Aβ42 sample solutions. The lowest signal was observed in mass spectrum of Aβ42 alone where only sodium adducts were detected (Figure 2, black line).
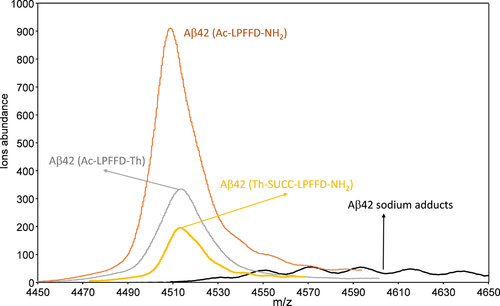
MALDI-MS spectra of Aβ42(CAβ42 = 5 × 10−6M) in aqueous solution at pH 7.2 acquired in linear mode (m/z range = 4450–4650) at different conditions: Aβ42 alone (black line); equimolar solution of Aβ42/Ac-LPFFD-NH2 (orange line); equimolar solution Aβ42/Ac-LPFFD-Trehalose (Aβ42/Ac-LPFFD-Th) (grey line); equimolar solution Aβ42/Trehalose-Succinyl-LPFFD-NH2 (Aβ42/Th-Succ-LPFFD-NH2)(yellow line)
In these conditions, the Aβ42 aggregation process enhances the formation of oligomers and fibrils, reducing the amount of monomers species in solution. On the other hand, upon addition of the Ac-LPFFD-NH2 peptide to a prepared Aβ42 monomer sample, the m/z signal corresponding to Aβ42 turns very prominent (Figure 2, orange line). This is in agreement with other studies that indicate the ability of the Ac-LPFFD-NH2 peptide to reduce Aβ association in the oligomer forms, driving the distribution of Aβ42 species towards monomers.65 We recently demonstrated that our trehalose-conjugated LPFFD peptides have some inhibitory effect on Aβ42 aggregation as well.26 Interestingly, the MALDI-MS measurements of the Aβ42 samples, mixed with the C- or N-terminally linked trehalose peptides, showed well defined m/z signals corresponding to the Aβ42 monomer (Figure 2, grey and yellow lines). These findings are in keeping with the hypothesis that the Ac-LPFFD-NH2 and the trehalose-conjugated derivatives may interfere with Aβ aggregation by means of the formation of a noncovalent adduct with the amyloid peptide. Experimental evidence of this was obtained by means of limited proteolysis experiments. We supposed that proteolysis process rates of Aβ42 could be affected by interactions with other molecules. These interactions might occur at the peptide bonds involved in the proteolytic cleavage in turn affecting enzyme's accessibility to the cleavage sites. Therefore, we analyzed the Aβ42 peptide fragments generated after 1 hr of α-chimotrypsin digestion. This enzyme selectively catalyzes the hydrolysis of peptide bonds at the C-terminal side of tyrosine, phenylalanine, tryptophan, and leucine residues.66 Some of these cleavage sites are located in the Aβ42 region (KLVFF) involved in the hydrophobic interactions with the Ac-LPFFD-NH2 and the trehalose-conjugated derivatives, making the α-chimotrypsin a suitable enzyme for the limited proteolysis investigations.
The MALDI-MS spectrum of Aβ42 carried out after 1 hr of α-chimotrypsin digestion with an enzyme/substrate ratio of 1: 20 w/w, is reported in Figure 3.
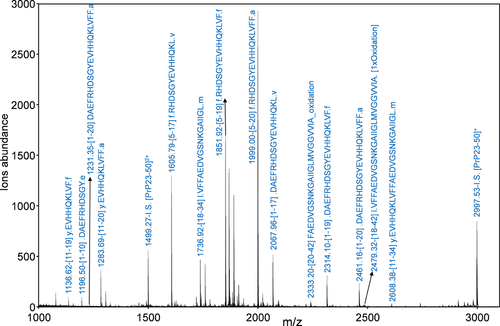
MALDI-MS spectrum of Aβ42 (CAβ42 = 5 × 10−6M) after digestion with α-chymotrypsin at an enzyme/substrate ratio of 1: 20 w/w. Measurements was carried out using α-CHCA matrix containing the peptide fragment encompassing aminoacid residues 23–50 of prion protein (PrP23–50) as Internal Standard (I.S.) (see materials and methods section)
The identified peptide fragments are indicated in Scheme 1.
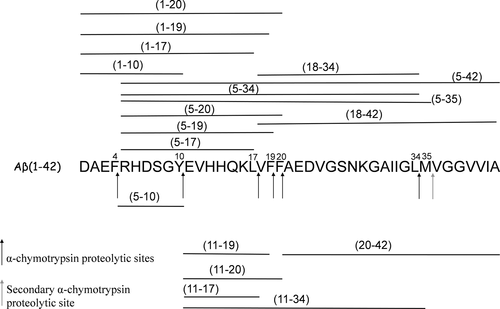
Aβ42 proteolytic pattern after 1 hr of α-chimotrypsin digestion at an enzyme/substrate ratio of 1: 20 w/w observed in the MALDI mass spectrum reported in Figure 3
It is clear from Scheme 1 that almost all of the proteolytic fragments are not completely digested after 1 hr of α-chimotrypsin digestion. This suggest that the hydrolysis of Aβ42 is only partially accomplished. Quite similar proteolytic patterns were observed when Ac-LPFFD-NH2 or the trehalose-conjugated derivatives were added to the Aβ42 samples solutions (data not shown).
Because no significative changes were observed, we decreased the α-chimotrypsin hydrolysis rate by reducing the enzyme concentration (enzyme/substrate ratio of 1: 200 w/w). In these conditions, Ac-LPFFD-NH2 or the trehalose conjugated derivatives should be able to limit the accessibility of α-chimotrypsin to the cleavage sites of Aβ42. Therefore, small differences in the peptide interactions with Aβ42 would be more pronounced generating different proteolytic patterns.
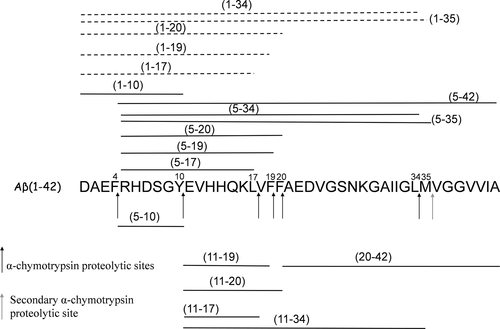
The main outcomes of the enzymatic concentration changes, during Aβ42 digestion, are reported in Scheme 2 (see caption for more details) where m/z signals, observed after 1 hr of α-chimotrypsin digestion at the enzyme/substrate ratio of 1:20 and 1:200, were compared.
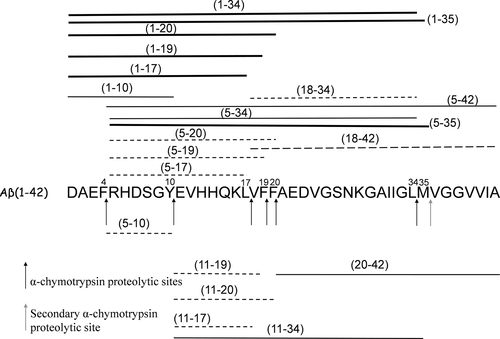
Effect of enzyme/substrate ratio on Aβ42 proteolytic pattern after 1 hr of α-chimotrypsin digestion. When the α-chimotrypsin concentration was reduced (changing the enzyme/substrate ratio from 1:20 w/w to 1:200 w/w), the m/z signal intensity of the peptide fragments represented by thick lines increased, whereas the m/z signal intensity of peptide fragments indicated by the dashed lines decreased. The m/z signal intensity of peptide fragments indicated by the thin lines did not change. Only m/z signals intensities ratio, observed at an enzyme/substrate ratio of 1:20 w/w and 1:200 w/w, higher than 2 are considered
In particular, when the α-chimotrypsin concentration was reduced, new partially digested fragments, Aβ(1–34) and Aβ(1–35) were observed, while the peptides fragments Aβ(18–34) and Aβ(18–42) disappeared. Scheme 2 also indicates an increase of the signal intensity corresponding to the Aβ(1–20), Aβ(1–19), and Aβ(1–17) fragments while the signal intensity of Aβ(5–17), Aβ(5–19), Aβ(5–20), Aβ(11–17), Aβ(11–19), and Aβ(11–20) fragments decreased. The results obtained indicate that low hydrolysis rate occurs at the Phe4-Arg5, Tyr10-Glu11, and Leu17-Val18 peptides bonds of Aβ42 when very limited cleavage conditions are used. These differences can be related to the conformational features of Aβ42 that could affect the accessibility to the cleavage sites.
The processing of Aβ42 by α-chimotrypsin in the presence of the trehalose-conjugated derivatives, at an enzyme/substrate ratio of 1:200, produces only negligible changes of the proteolytic patterns (data not shown). On the other hand, sensible changes in the proteolytic pattern of Aβ42 α-chimotrypsin digestion, in the presence of the Ac-LPFFD-NH2 peptide, are observed. In particular, the signals intensity of the m/z values corresponding to the peptide fragments Aβ(1–34), Aβ(1–35), Aβ(1–20), Aβ(1–19), and Aβ(1–17), formerly reported to increase when the enzyme/substrate ratio were changed from 1:20 to 1:200, were reduced again in the presence of Ac-LPFFD-NH2 (dashed lines of Scheme 3 and Figure 4).
Effect of Ac-LPFFD-NH2 on Aβ42 proteolytic pattern after 1 hr of α-chimotrypsin digestion (enzyme/substrate ratio of 1:200 w/w). In the absence of Ac-LPFFD-NH2 the peptide fragments observed in the MALDI mass spectrum are indicated as dashed and thin lines. In the proteolytic pattern observed in the presence of Ac-LPFFD-NH2, the m/z signal intensity of the peptide fragments represented by the dashed lines decreased, whereas the m/z signal intensity of peptide fragments indicated by the thin lines did not change. Changes are considered significant when the intensities ratio of the m/z signals compared is higher than 2
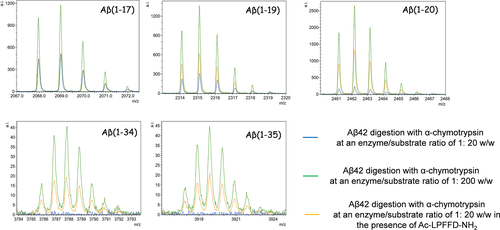
MALDI-MS signals corresponding to peptides fragments obtained by α-chymotrypsin digestion of Aβ42 at an enzyme/substrate ratio of: 1:20 w/w (blu lines); 1:200 w/w (green lines); 1:200 w/w in the presence of Ac-LPFFD-NH2 (orange lines)
The reduction of these signals intensity could be related to higher hydrolysis rate at the cleavage sites of Phe4 and Tyr10. These changes could be also related to a lower hydrolysis rate at the cleavage sites of Leu17, Phe19, and Phe20 but, in these case, a lower signal intensity of the peptide fragments Aβ(5–17), Aβ(5–19), Aβ(5–20), Aβ(11–17), Aβ(11–19), and Aβ(11–20) should be expected. Considering that interaction of Ac-LPFFD-NH2 with amyloid peptide may induce conformational changes to Aβ42, it is reasonable to suppose that these structural changes may promote the proteolysis of Phe4 and Tyr10 cleavage sites.
3.3 The neuroprotetcive Th-succ-LPFFD-NH2 and Ac-LPFFD-Th are devoid of toxicity
The pentapeptide LPFFD firstly synthesized by Soto and coworkers, was reported to inhibit Aβ42 fibril formation and Aβ42 induced neuronal death as well as to significantly reduce Aβ deposition in vivo.67, 68 We found that trehalose conjugation does not affect the protective properties of the LPFFD, yet improves its stability in biological fluids.26 Most interestingly, in primary cortical neurons, we found no toxicity associated to the application of even massive concentration (250 µM) of a series of trehalose-conjugate penta-peptides for 4 days which instead resulted in neuroprotection against Aβ induced toxicity.26 Although, a significant number of publications have confirmed these data, still to our best knowledge, no mechanisms have been provided to give more insight into the neuroprotective function of beta-sheet breakers. Because we found that monomeric Aβ42, as well as the penta-peptide KLVFF, (corresponding to Aβ (16–20) sequence, also known as Tjernberg's peptide) are able to support neurons against various insults through the activation of the prosurvival Akt signaling pathway, we reasoned that the neuroprotective features of trehalose conjugate LPFFD derivatives, might as well involve Akt activation.4, 11, 12 To confirm our hypothesis, we exposed differentiated neuroblastoma SH-SY5Y cells to 5µM of Ac-LPFFD-NH2 or Ac-LPFFD-Th or Th-Succ-LPFFD-NH2. This concentration was chosen as the lowest resulting in a neuroprotective ability provided by the monomeric Aβ in this cellular model (not shown). As shown in Figure 5, either the Ac-LPFFD-NH2 or Ac-LPFFD-Th or Th-Succ-LPFFD-NH2 produced a significant phosphorylation of Akt achieved by 15 min exposure, suggesting that the prosurvival ability of both unconjugated and trehalose conjugated LPFFD underpin the activation of the prosurvival Akt pathway. To strengthen the aforementioned findings we also checked for additional prosurvival markers belonging to the same pathway. GSK-3 is a pivotal downstream element of the PI3K/Akt prosurvival pathway whose activity can be modulated by Akt-mediated phosphorylation at Ser9 (inhibition) of GSK-3β. Here we found that ser9 phosporylation of GSK-3β is increased upon exposure to both our conjugated and unconjugated compounds, confirming their prosurvival properties. These data suggest at least two hypothesis, (i) the modified Aβ 16–20 sequence included in LPFFD derivatives is endowed by its self with neuroprotective features involving the activation of the Akt pathway, (ii) as beta sheet breakers, LPFFD derivatives hinder Aβ42 self-aggregation, increasing the amount of Aβ42 monomers, which have been demonstrated to support neurons upon different toxic insults, via the activation of PI3K/Akt pathway. This latter hypothesis is also supported by our MS data, showing that more Aβ42 is available for cleavage when the digestion is carried out in the presence of Ac-LPFFD-Th or Th-Succ-LPFFD-NH2.
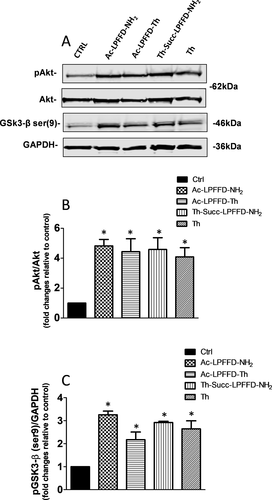
Effects of Ac-LPFFD-NH2, Ac-LPFFD-Trehalose (Ac-LPFFD-Th) and Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2) on pAkt and pGSK3-β (ser9) expression levels. A, Representative immunoblot of pAkt and pGSK3-β (ser9) from differentiated SH-SY5Y treated with 5 μM Ac-LPFFD-NH2, Ac-LPFFD-Th or Th-Succ-LPFFD-NH2 for 15 min. B, Densitometric values (means ± SEM) of pAkt/Akt and C, pGSK3-β (ser9)/GAPDH ratio. Values are expressed as fold changes relative to respective control. *P < 0.05 versus Ctrl by One-Way Anova + Tukey's test
We previously showed that monomeric human Aβ protects cultured neurons against excitotoxic death and support neurons upon insulin withdrawal.4
Here we find that, the neuroprotection both against excitotoxic death and insulin deprivation is also produced by synthetic Ac-LPFFD-NH2 and its derivatives, namely Ac-LPFFD-Th and Th-Succ-LPFFD-NH2 (Figures 6 and 7). We have also reported about the Ac-LPFFD-Th ability to hinder the formation of Aβ42 oligomeric assemblies, resulting in neuroprotection against the Aβ42 oligomers’ toxicity.5 To assess whether or not the Th-Succ-LPFFD-NH2 derivative is also able to prevent the formation of Aβ42 toxic species, a preparation of un-aggregated form of 100 µM Aβ42 was incubated in the absence or in the presence of Th-Succ-LPFFD-NH2 (48h at 4°C; Aβ42: Th-Succ-LPFFD-NH2 ratio tested was 1:5). Primary cortical neurons were exposed for further 48 hr to the incubated Aβ42 samples diluted to 100nM (in presence or absence of Th-Succ-LPFFD-NH2). This concentration produced a 40% of neuronal death as assessed by MTT assay. The coincubation of Aβ42 with Th-Succ-LPFFD-NH2 reduced the neuronal death induced by toxic Aβ42 samples as shown in Figure 8. Thereafter we demonstrate that the Th-Succ-LPFFD-NH2 also share this anti Aβ42 oligomers activity.
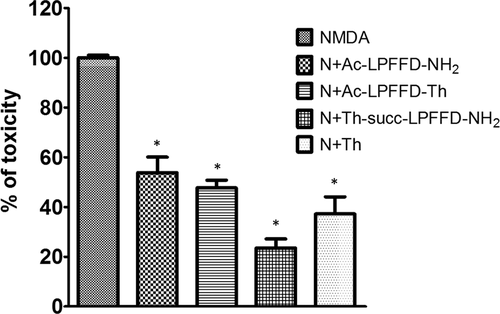
Mixed cortical cultures at maturation exposed to 300 μM NMDA for 10 min at room temperature in a HEPES-buffered salt solution. A 100 nM of Ac-LPFFD-NH2 or Ac-LPFFD-Trehalose (Ac-LPFFD-Th) or Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2) were added in combination with NMDA. Neuronal toxicity was examined 24 hr later by light microscopy and quantified after staining with trypan blue (0.4% for 5 min). Stained neurons were counted from three-random fields/well. Bars are Means ± SEM of three different experiments *P < 0.001 versus NMDA by Fisher LSD method
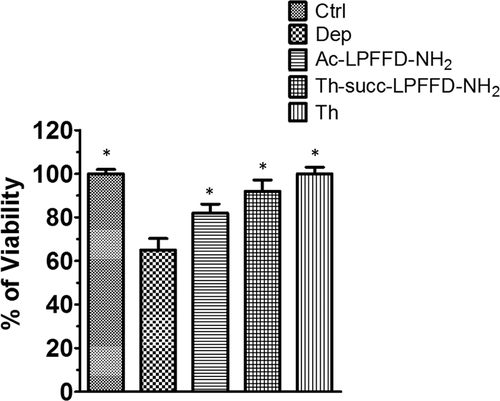
Cell viability was measured by MTT assay. Pure neuronal cultures were exposed for 48 hr to 100 nM of Ac-LPFFD-NH2 or Ac-LPFFD-Trehalose (Ac-LPFFD-Th) or Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2) after insulin withdrawal. Bars are Means ± SEM of three experiments (n = 3). Significant at P < 0.05 versus Deprivated (Dep.) (*)(One Way ANOVA + Turkey's multiple comparison Test
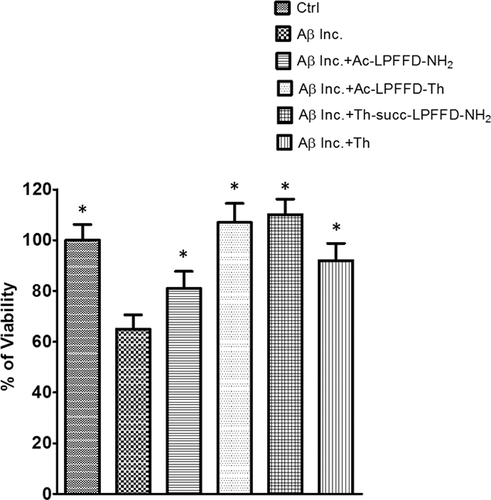
Cell viability was measured by MTT assay. Pure neuronal cultures were treated with pre-incubated samples of Aβ42 alone (Aβ inc.) or in the presence of Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2). Aβ42 and Th-Succ-LPFFD-NH2 were added in the 1:5 molar ratio. Values are the mean ± SD of three independent experiments. Significant at P < 0.05 versus Aβ42 incubated (inc.) (*) (One-Way ANOVA + Turkey's multiple comparison Test)
3.4 Quantitation of the Ac-LPFFD-NH2, Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th upon cell interaction
To quantify the amount of Ac-LPFFD-NH2 and its Th derivatives (Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th) that interact with the cells, the same cell lysates produced for western blot processing were analyzed by UHPLC-HRMS, after the removal of the protein content. We also checked (data not shown) that the treatment of the cell lysates with the organic solvent (methanol and chloroform) do not affect the concentration of Ac-LPFFD-NH2 and its trehalose derivatives of the aqueous solution.
Our optimized chromatographic process linked to the high resolution of the mass analyzer was a powerful approach to detect and effectively quantify both the LPFFD peptide and its N- and C-terminal Th derivatives. To maximize the detection of the peptides, chromatograms were acquired by monitoring a narrow range of m/z values that included those related to the most abundant ions of the analytes. As reported in the targeted SIM (tSIM) acquisitions (Figure 9), the N-terminal and C-terminal derivatives of LPFFD as well as the underivatized peptide, were monitored within the m/z range 1060.20–1063.60, 1003.20–1006.60, and 679.20–681.50, respectively.
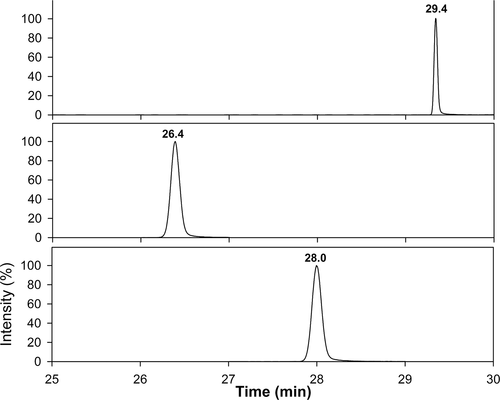
Chromatographic profiles in Targeted SIM (tSIM) mode of the SHSY5Y lysates treated with Ac- LPFFD-NH2A, Trehalose-Succinyl-LPFFD-NH2 (Th-Succ-LPFFD-NH2)B, and Ac-LPFFD-Trehalose (Ac-LPFFD-Th)C
The isotopic distribution of the most abundant ions of the analytes (Supporting Information Figure S1) clearly rationalizes the chosen m/z ranges for the MS acquisition.
By using an external standard calibration approach (Supporting Information Figure S2), the amounts of Ac-LPFFD-NH2 and its trehalose derivatives interacting with cells were quantified. As reported in Supporting Information Table S1, both Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th were found in the cell lysates, thus confirming a cell penetration and/or a membrane interaction of these derivatives. The peptide Ac-LPFFD-NH2 also interacts with the cell system and its concentration is significantly higher than that reported for both the trehalose derivatives. The higher hydrophobicity of the un-conjugated peptide, compared to the N-terminal and C-terminal trehalose derivatives, could reasonably account for this important difference. In spite of this, all the tested compounds showed similar effects on the activation of the cell signals, thus demonstrating greater efficiency of Th-Succ-LPFFD-NH2 and Ac-LPFFD-Th with respect to the Ac-LPFFD-NH2 parent peptide. The stability of the N-terminal and C-terminal trehalose derivatives towards the protease action, higher than that of the Ac-LPFFD-NH2 peptide,26 is a further confirmation of the promising role of these derivatives as therapeutic agents.
4 CONCLUDING REMARKS
Current available treatments for Alzheimer's disease is presently only symptomatic and do not really affect the underlying disease nor delay its progression. Thus, extensive scientific efforts are currently ongoing to find out new ways to halt Alzheimer's progression. An innovative Alzheimer's drug, is expected to treat the underlying molecular pathogenic mechanisms also preventing the degeneration of neurons, a major event responsible of the worsening of the disease's clinical conditions. A large number of peptides that specifically target Aβ have been reported in the literature, among these, inhibitors based on the hydrophobic core sequence of Aβ have been modified using a variety of different ways including peptide chain cyclization, N-Alkylation, introduction of non-natural aminoacid, addition of basic or acid homopeptides at N-or C-termini, conjugation with a number of natural and synthetic small molecules.69, 70 To our knowledge, peptide based inhibitors bearing glyosidic moieties are less represented.30, 71, 72 In our previous work, we described some chief advantages of the trehalose conjugated peptide inhibitors in terms of water solubility, selectivity toward Aβ peptides with dose response antifibrillogenic activity and enhanced resistance to proteases in biological fluids.5, 26 It is expected that trehalose conjugation to the peptide sequence offer better chance to cross the Blood Brain Barrier. To this regard, our derivatives represent a step further toward the design of potential therapeutics for AD. Moreover, in the present work we explored other neuroprotective capabilities of the new derivatives. For this reason, we used the NMDA neurotoxic insult as an additional model of neuronal death. We have shown that the β-sheet breaker LPFFD peptide and its trehalose derivatives protect neuronal cells from NMDA-mediated excitotoxic insult and evoke prosurvival signal pathways as an additional cell protective action. As a main difference from other glicopeptidomimetics appeared in the literature, we choose the natural occurring disaccharide trehalose. Trehalose displays many remarkable qualities including protein homeostasis and anti-amyloidogenic properties. Compared to other studies that used molecular trehalose beyond millimolar concentrations, the results reported in this work revealed in vitro performances of the disaccharide, as well as the derived peptide conjugates, in the nano- sub-micro molar range of concentrations. Considering that even at high doses trehalose does not cause toxicity to primary neurons, it can be expected that under in vivo conditions our compounds may achieve optimal concentration levels to perform neuroprotection. Our preliminary results may pave the way for further, yet more comprehensive, biological experiments to disclose promising drugs that combine targeting and scavenging of toxic forms of aggregated Aβ and inherent pro-survival activity toward neuronal cells. However, more data have to be collected before these new derivatives might be included in a drug development and testing program to fill the pipeline of the clinical trials. To this regard, future work should focus on in vivo experimentation.
ACKNOWLEDGMENTS
CNR is acknowledged for partial financial support.