Extracardiovascular injury complications in Kawasaki disease
ABSTRACT
Importance
Patients with Kawasaki disease (KD) experience various extracardiovascular injury complications, which may affect their outcomes.
Objective
To investigate the incidence and clinical characteristics of extracardiovascular complications in children with KD.
Methods
The clinical data of patients diagnosed with KD in the First Affiliated Hospital of Guangxi Medical University from January 2003 to January 2021 were reviewed. The clinical characteristics and extracardiovascular complications were compared among patients stratified by age, intravenous immunoglobulin (IVIG) therapy responsiveness, and coronary status.
Results
A total of 511 patients with KD were included, 357 (69.9%) were aged 1–5 years. Children aged <1 year (21.5%) and boys (70.8%) were more likely to have coronary artery lesions (CALs). The incidence of incomplete KD was lowest in 1–5-year-old patients (19.6%). Involvement of the hematological system gradually decreased with age (<1 year, 51.8%; 1–5 years, 36.7%; >5 years, 29.5%), whereas the involvement of the joints gradually increased with age (<1 year, 2.7%; 1–5 years, 6.2%; >5 years, 20.5%). Nervous system involvement was more common in IVIG non-responders (15.7% [13/83] vs. 5.4% [23/428], P = 0.001). However, there were no significant differences in extracardiovascular injury complications between patients with or without CALs.
Interpretation
KD can involve multiple organ injuries as well as cardiovascular complications, and nervous systerm involvement may be more common in patients unresponsive to IVIG.
INTRODUCTION
Coronary artery aneurysm (CAA) caused by Kawasaki disease (KD) has become the most common cause of acquired pediatric heart disease,1-3 and consequently, cardiovascular diseases resulting from KD have increasingly attracted attention from researchers. In clinical practice, the diagnosis of KD is primarily based on symptoms, including fever, bilateral bulbar conjunctival injection, lip, and oral cavity changes, rash, erythematous changes to the extremities, and cervical lymphadenopathy.4 However, the manifestations may vary greatly among children of different ages in terms of distribution, duration, and symptoms which in turn depend on the phase of the disease, thus creating a diagnostic dilemma. Delays in diagnosis and treatment can significantly increase the risk of cardiovascular complications, and additional diagnostic difficulties may occur when extracardiovascular complications are present. This leads in turn to missed best treatment windows, subsequently resulting in poor prognosis.5-8 To date, reports on extracardiovascular complications in different KD subgroups are scant. Therefore, this study aimed to explore the incidence and clinical characteristics of extracardiovascular complications in children with KD.
METHODS
Ethical approval
This research was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (code number: 2021[KY-E-240]). Written informed consent was waived as this was a retrospective study.
Study population
This retrospective study was performed at the Department of Pediatrics, First Affiliated Hospital of Guangxi Medical University, China. The medical records of all children who fulfilled the diagnostic criteria for KD at our hospital between January 2003 and January 2021, were reviewed. We excluded patients who did not receive intravenous immunoglobulin (IVIG), patients with other diseases known to mimic KD, patients with concomitant infection confirmed by positive pathogen examinations or based on clinical symptoms, and patients with recurrent KD because previous CAA may persist as a coronary complication.
The diagnostic criteria for KD were based on the American Heart Association (AHA) guidelines,2 and were re-evaluated in all cases according to the guidelines proposed by the Japan Kawasaki Disease Research Committee in 2020.4 All diagnoses were confirmed by more than two pediatric cardiologists. Complete KD was diagnosed when patients had a fever persisting for at least 5 days together with at least four of the following five clinical features: rash, bilateral bulbar conjunctival injection, non-suppurative cervical lymphadenopathy, changes of peripheral extremities, and oral mucosal changes. Patients with more than 5 days of fever accompanied by two or three of the above clinical features plus coronary artery abnormality by echocardiography were considered as having incomplete KD.2, 4 Coronary artery lesions (CALs) were defined by body surface area –adjusted Z scores for coronary arteries with an internal diameter ≥ 2.0, in either the proximal right coronary artery, the left main coronary artery or the left anterior descending artery. Patients with a maximal Z score of ≥ 2.5 for more than 1 month after disease onset were considered as CAA; those with a maximal Z score between 2.0 and 2.5 were considered as coronary artery dilation (CAD), as described in the AHA guidelines and guidelines on the diagnosis and management of cardiovascular sequelae in KD (JCS/JSCS 2020).2, 9 The Z scores were calculated using Dallaire equations.10
Once KD was established, treatment with any antiviral drug or other antibiotic was discontinued, and all patients were treated according to the AHA guidelines. Patients unresponsive to IVIG were defined as those with symptoms of persistent or recrudescent fever (temperature ≥ 38.0°C) for at least 36 h but not longer than 7 days after receiving the initial IVIG infusion (2 g/kg).2 Patients were grouped separately based on age, IVIG therapy responsiveness, and coronary status, and the baseline characteristics and extracardiovascular complications were analyzed among the groups.
The criteria for the diagnosis of extracardiovascular complications were as follows4, 11: (1) respiratory system injury in children with KD characterized by cough, rhinorrhea, retropharyngeal edema, pleural effusion, fixed small and medium blisters in the lungs, or an infiltrate on chest radiograph; (2) digestive system injury characterized by abdominal pain, vomiting, diarrhea, pericholecystic edema, bile duct dilation, jaundice, or abnormal liver function; (3) hematological system injury typically presenting as anemia, granulocytic deficiency, or symptoms compatible with macrophage activation syndrome; (4) nervous system injury characterized by symptoms including somnolence, extreme irritability, headache, seizures, facial nerve palsy, paralysis of the extremities, and cerebrospinal fluid pleocytosis (≥15 × 106/L); (5) joint injury presenting as joint swelling, heat, pain, and dysfunction; and (6) urinary system injury indicated by urethritis, proteinuria, or microscopic hematuria. As some patients may experience two or more simultaneous symptoms of the same system, we took these symptoms as one when estimating the incidence of system involvement.
Statistical analysis
The normality of distribution was verified using the Shapiro–Wilk and homogeneity tests. Normally distributed data are expressed as mean ± standard deviation. A two-independent sample t-test or one-way analysis of variance was performed to compare data between the groups. Data without a normal distribution are expressed as medians (interquartile range) and were compared using the Mann–Whitney U or Kruskal–Wallis H test. Enumeration data are expressed as percentages (%). The Chi-square or Pearson Chi-square test was used to perform intergroup comparisons. Two-tailed P-values < 0.05 indicated statistical significance, and Bonferroni correction was applied for multiple comparisons. All statistical analyses were performed using SPSS, version 26.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
During the study period, 716 children were admitted with a KD diagnosis, and 205 patients were excluded from this study, including 31 children without IVIG treatment, 114 with concomitant infection, 57 with other diseases known to mimic KD or with incomplete clinical data, and three with recurrent KD. Finally, we recruited 511 children with KD, of which 362 (70.8%) were boys and 149 (29.2%) were girls. The age at onset ranged from 2 months to 158 months, with a median age of 29 months and the peak age of 12–24 months. Of the patients, 110 (21.5%) were <1 year of age, 357 (69.9%) were 1–5 years of age, and 44 (8.6%) were >5 years of age. KD can occur year-round, but the highest incidence of onset was seen in the summer quarters (May to September) (Figure S1).
Among these patients with KD, the incidence of respiratory system involvement was highest (344, 67.3%), followed by the digestive system (236, 46.2%), the hematological system (201, 39.3%), the urinary system (57, 11.2%), the nervous system (36, 7.0%), and the joints (34, 6.7%).
Comparison of extracardiovascular complications in patients with different age
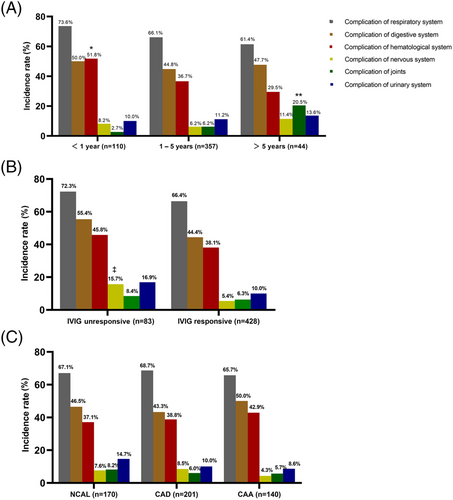
In our study population, most children with KD were 1–5 years of age (357/511, 69.9%) and more often presented with complete KD (287/357, 80.4%). Children aged ≤5 years had a significantly higher incidence of CALs (68.7% vs. 45.5%, P < 0.001), especially CAA, which were most frequent in those under 1 year of age (P = 0.002). Patients > 5 years of age were more likely to present joint swelling, heat, and pain than those <5 years of age (2.7% in <1 year vs. 5.3% in 1–5 years vs. 18.2% in > 5 years, P = 0.005). Patients aged 1–5 years were less likely to present with pleural effusion than patients >5 years of age (2.2% vs. 11.4%, P = 0.006), and less likely to present with granulocytic deficiency than patients <1 year of age (5.9% vs. 17.3%, P < 0.001) (Table 1). Involvement of the respiratory (73.6% in <1 year vs. 66.1% in 1–5 years vs. 61.4% in >5 years) and hematological (51.8% vs. 36.7% vs. 29.5%) systems gradually decreased with age, whereas the involvement of the joints (2.7% vs. 6.2% vs. 20.5%) and urinary (10.0% vs. 11.2% vs. 13.6%) system gradually increased with age (Figure 1A).
| Variables | < 1 year (n = 110) | 1–5 years (n = 357) | >5 years (n = 44) | P |
|---|---|---|---|---|
| Male | 86 (78.2) | 247 (69.2) | 29 (65.9) | 0.145 |
| Incomplete KD | 39 (35.5) | 70 (19.6)† | 15 (34.1) | 0.001 |
| IVIG unresponsive | 18 (16.4) | 57 (16.0) | 8 (18.2) | 0.931 |
| CALs | 89 (80.9) | 232 (65.0)† | 20 (45.5)†,‡ | <0.001 |
| CAA | 45 (40.9) | 85 (23.8)† | 10 (22.7) | 0.002 |
| Respiratory system | 81 (73.6) | 236 (66.1) | 27 (61.4) | 0.230 |
| Cough | 76 (69.1) | 223 (62.5) | 24 (50.0) | 0.208 |
| Rhinorrhea | 22 (20.0) | 80 (22.4) | 6 (13.6) | 0.383 |
| Retropharyngeal edema | 12 (10.9) | 36 (10.1) | 10 (22.7) | 0.076 |
| Fixed small and medium blisters in the lungs or an infiltrate on the chest radiograph | 34 (30.9) | 91 (25.5) | 14 (31.8) | 0.414 |
| Pleural effusion | 2 (1.8) | 8 (2.2) | 5 (11.4)‡ | 0.022 |
| Digestive system | 55 (50.0) | 160 (44.8) | 21 (47.7) | 0.620 |
| Abdominal pain | 11 (10.0) | 54 (15.1) | 10 (22.7) | 0.119 |
| Diarrhea | 26 (23.6) | 59 (16.5) | 7 (15.9) | 0.221 |
| Vomiting | 15 (13.6) | 50 (14.0) | 5 (11.4) | 0.891 |
| Pericholecystic edema or bile duct dilation | 11 (10.0) | 19 (5.3) | 6 (13.6) | 0.067 |
| Jaundice | 12 (10.9) | 40 (11.2) | 8 (18.2) | 0.380 |
| Abnormal liver function | 24 (21.8) | 68 (19.0) | 12 (27.3) | 0.402 |
| Hematological system | 57 (51.8) | 131 (36.7) | 13 (29.5) | 0.007 |
| Anemia | 46 (41.8) | 119 (33.3) | 13 (29.5) | 0.196 |
| Granulocytic deficiency | 19 (17.3) | 21 (5.9)† | 3 (6.8) | 0.002 |
| Symptoms compatible with macrophage activation syndrome | 0 | 1 (0.3) | 3 (6.8)‡ | 0.001 |
| Nervous system | 9 (8.2) | 22 (6.2) | 5 (11.4) | 0.423 |
| Somnolence | 4 (3.6) | 8 (2.2) | 3 (6.8) | 0.280 |
| Extreme irritability | 5 (4.5) | 7 (2.0) | 2 (4.5) | 0.164 |
| Headache | 1 (0.9) | 10 (2.8) | 3 (6.8) | 0.148 |
| Seizures | 4 (3.6) | 8 (2.2) | 1 (2.3) | 0.734 |
| Facial nerve palsy or paralysis of the extremities | 1 (0.9) | 3 (0.8) | 1 (2.3) | 0.417 |
| Cerebrospinal fluid pleocytosis | 5 (4.5) | 7 (2.0) | 2 (4.5) | 0.290 |
| Joints | 3 (2.7) | 22 (6.2) | 9 (20.5) | 0.002 |
| Joint swelling, heat, pain | 3 (2.7) | 19 (5.3) | 8 (18.2)†,‡ | 0.005 |
| Dysfunction | 1 (0.9) | 7 (2.0) | 1 (2.3) | 0.745 |
| Urinary system | 11 (10.0) | 40 (11.2) | 6 (13.6) | 0.814 |
| Urethritis | 11 (10.0) | 37 (10.4) | 6 (13.6) | 0.795 |
| Proteinuria | 2 (1.8) | 10 (2.8) | 3 (6.8) | 0.319 |
| Microscopic hematuria | 5 (4.5) | 9 (2.5) | 1 (2.3) | 0.560 |
- Data are expressed as n (%). †P < 0.016, compared with the < 1-year group; ‡ P < 0.016, compared with the 1–5 years group. KD, Kawasaki disease; IVIG, intravenous immunoglobulin; CALs, coronary artery lesions; CAA, coronary artery aneurysm.
Comparison of extracardiovascular complications between the subgroups based on IVIG therapy responsiveness
The distribution of extracardiovascular complications in patients with KD with and without response to IVIG is shown in Table 2. IVIG non-responders were more likely to present with respiratory diseases, including visible infiltrate on the chest radiograph (37.3% vs. 25.2%, P = 0.023), pleural effusion (8.4% vs. 1.9%, P = 0.004), and with digestive diseases, including pericholecystic edema or bile duct dilation (15.7% vs. 5.4%, P = 0.001), jaundice (27.7% vs. 8.6%, P < 0.001), abnormal liver function (33.7% vs. 17.8%, P = 0.001) than IVIG responders. The nervous system involvement was significantly higher in IVIG non-responders than in IVIG responders (15.7% vs. 5.4%, P = 0.001) (Figure 1B). Of those, IVIG non-responders were more likely to present somnolence (9.6% vs. 1.6%, P < 0.001), headache (9.6% vs. 1.4%, P < 0.001), and cerebrospinal fluid pleocytosis (8.4% vs. 1.6%, P = 0.002).
| Variables | IVIG unresponsive (n = 83) | IVIG responsive (n = 428) | P |
|---|---|---|---|
| Age (months) | 20.0 (13.0, 46.0) | 23.0 (13.0, 38.0) | 0.735 |
| Male | 60 (72.3) | 302 (70.6) | 0.751 |
| BMI (kg/m2) | 15.63 (14.07, 16.91) | 15.52 (14.49, 16.44) | 0.491 |
| Incomplete KD | 18 (21.7) | 106 (24.8) | 0.549 |
| CAA | 26 (31.3) | 114 (26.6) | 0.381 |
| Respiratory system | 60 (72.3) | 284 (66.4) | 0.292 |
| Cough | 56 (67.5) | 267 (62.4) | 0.379 |
| Rhinorrhea | 13 (15.7) | 95 (22.2) | 0.182 |
| Retropharyngeal edema | 9 (10.8) | 49 (11.4) | 0.874 |
| Fixed small and medium blisters in the lungs or an infiltrate on the chest radiograph | 31 (37.3) | 108 (25.2) | 0.023 |
| Pleural effusion | 7 (8.4) | 8 (1.9) | 0.004 |
| Digestive system | 46 (55.4) | 190 (44.4) | 0.065 |
| Abdominal pain | 16 (19.2) | 59 (13.8) | 0.196 |
| Diarrhea | 18 (21.7) | 74 (17.3) | 0.340 |
| Vomiting | 10 (12.0) | 60 (14.0) | 0.633 |
| Pericholecystic edema or bile duct dilation | 13 (15.7) | 23 (5.4) | 0.001 |
| Jaundice | 23 (27.7) | 37 (8.6) | <0.001 |
| Abnormal liver function | 28 (33.7) | 76 (17.8) | 0.001 |
| Hematological system | 38 (45.8) | 163 (38.1) | 0.189 |
| Anemia | 36 (43.4) | 142 (33.2) | 0.074 |
| Granulocytic deficiency | 9 (10.8) | 34 (7.9) | 0.384 |
| Symptoms compatible with macrophage activation syndrome | 4 (4.8) | 0 | <0.001 |
| Nervous system | 13 (15.7) | 23 (5.4) | 0.001 |
| Somnolence | 8 (9.6) | 7 (1.6) | <0.001 |
| Extreme irritability | 4 (4.8) | 10 (2.3) | 0.368 |
| Headache | 8 (9.6) | 6 (1.4) | <0.001 |
| Seizures | 2 (2.4) | 11 (2.6) | 1.000 |
| Facial nerve palsy or paralysis of the extremities | 2 (2.4) | 3 (0.7) | 0.187 |
| Cerebrospinal fluid pleocytosis | 7 (8.4) | 7 (1.6) | 0.002 |
| Joints | 7 (8.4) | 27 (6.3) | 0.477 |
| Joint swelling, heat, pain | 7 (8.4) | 23 (5.4) | 0.406 |
| Dysfunction | 2 (2.4) | 7 (1.6) | 0.972 |
| Urinary system | 14 (16.9) | 43 (10.0) | 0.071 |
| Urethritis | 13 (15.7) | 41 (9.6) | 0.099 |
| Proteinuria | 5 (6.0) | 10 (2.3) | 0.143 |
| Microscopic hematuria | 4 (4.8) | 11 (2.6) | 0.450 |
- Data are presented as median (interquartile range) or n (%). IVIG, intravenous immunoglobulin; BMI, body mass index; KD, Kawasaki disease; CAA, coronary artery aneurysm.
Comparison of extracardiovascular complications among the subgroups based on coronary status
We performed comparisons based on coronary status and did not observe significant differences in the distribution of extracardiovascular complications in patients with KD among any of the three subgroups (no CAL, CAD, and CAA) (Figure 1C). However, patients with CAA were more likely to present with pericholecystic edema or bile duct dilation than those without CAA (14.3% in the CAA group vs. 4.1% in no CAL group vs. 4.5% in the CAD group, P < 0.001), and more likely to present with pleural effusion than patients with CAD (6.4% vs. 0.0%, P = 0.001) (Table S1). Furthermore, patients with CALs were significantly younger, and incomplete KD was more frequent in patients without CALs than in those with CALs (Table 3).
| Variables | NCAL (n = 170) | CAD (n = 201) | CAA (n = 140) | P |
|---|---|---|---|---|
| Age (months) | 27.5 (15.8, 48.3) | 21.0 (12.0, 33.0)† | 18.0 (10.0, 33.8)† | <0.001 |
| Male | 105 (61.8) | 145 (72.1) | 112 (80.0)† | 0.002 |
| BMI (kg/m2) | 15.43 (14.22, 16.42) | 15.38 (14.23, 16.44) | 15.62 (14.81, 16.56) | 0.088 |
| Incomplete KD | 64 (37.6) | 38 (18.9)† | 22 (15.7)† | <0.001 |
| IVIG unresponsive | 25 (14.7) | 32 (15.9) | 26 (18.6) | 0.648 |
| Respiratory system | 114 (67.1) | 138 (68.7) | 92 (65.7) | 0.847 |
| Digestive system | 79 (46.5) | 87 (43.3) | 70 (50.0) | 0.471 |
| Hematological system | 63 (37.1) | 78 (38.8) | 60 (42.9) | 0.571 |
| Nervous system | 13 (7.6) | 17 (8.5) | 6 (4.3) | 0.311 |
| Joints | 14 (8.2) | 12 (6.0) | 8 (5.7) | 0.596 |
| Urinary system | 25 (14.7) | 20 (10.0) | 12 (8.6) | 0.183 |
- Data are presented as median (interquartile range) or n (%). †P < 0.016, compared with the NCAL group. NCAL, no coronary artery lesion; CAD, coronary artery dilation; CAA, coronary artery aneurysm; BMI, body mass index; KD, Kawasaki disease; IVIG, intravenous immunoglobulin.
DISCUSSION
This study aimed to evaluate the characteristics of extracardiovascular injury complications in children with KD. The incidence of KD was reported as 68.8 to 107.3 per 100 000 children aged <5 years from 2013 to 2017 in Shanghai, with a peak in May and baseline incidence on September,12 which is in line with our regional findings regarding the seasonal change in KD. Several studies showed that age <1 year and >5 years and males are risk factors for cardiovascular complications in patients with KD, which is partially consistent with our findings.3, 13-17 This study found that patients aged >5 years had the highest IVIG unresponsiveness incidence, but not CAA incidence. This result, however, was not statistically significant, probably because of the low number of patients in this group. IVIG unresponsiveness is a well-established risk factor for CAA formation in patients with KD,18-20 therefore, KD patients aged >5 years warrant special attention, as they frequently have an atypical, late presentation.
In Japan, approximately 0.08% of pediatric patients die of cardiac complications due to KD during the acute phase of the disease.21 The clinical diagnosis of KD is still challenging owing to the lack of a specific diagnostic modality to date, and the overlap between the clinical and laboratory manifestations of KD and other infectious diseases continues to confound the clinical diagnosis, particularly when combined with other complications. As such complications often prominently occur during the acute stage, these can lead to misdiagnosis and mistreatment of KD.11 Herein, the incidence of respiratory system involvement was highest, with bronchopneumonia and bronchitis as the main manifestations, and patients unresponsive to IVIG were more likely to present an infiltrate on chest radiograph and pleural effusion, which is consistent with previous findings.11 This could be due to increased vascular permeability and perivascular edematous changes in the lungs due to vessel inflammation resulting from KD. Moreover, we found that the involvement of the respiratory and hematological systems gradually decreased with age, whereas the involvement of the joints and urinary systems gradually increased with age. Anemia is the main hematological symptom of KD, particularly in patients aged < 1 year. Anemia may aggravate the amplification of the cytokine cascade caused by KD and cause further serious vascular endothelial injury and vascular dysfunction,22 which may explain why CAA is more frequent in children < 1-year-old. However, the pathophysiology underlying the association between hematological system injury and KD should be addressed in future studies.
Joint involvement in KD is more common in children >3 years of age and mainly manifests as joint pain and joint cavity effusion,11 in line with our findings. Previous studies have shown that large joints are more commonly involved, with knee joint involvement being the most common.23, 24 Joint involvement is transient and the prognosis is good. The most important aspect of treatment is to control the primary disease. However, in contrast to previous results, our study did not find that the incidence of coronary artery damage in children with joint involvement was higher than that in children without joint involvement.11 One reason for this discrepancy may be that different standards for group based on Z scores for coronary arteries may lead to different outcomes. Further and more comprehensive studies are required to confirm these observations.
The incidence of IVIG unresponsiveness was 16.2% (83/511) in our study, which was similar to findings in previous reports.25-29 It is worth noting that nervous system involvement was more common in patients unresponsive to IVIG. The incidence of neurological involvement (7.0%) in all patients with KD in this study was comparable to other studies (1%–30%),30 and was 15.7% in IVIG non-responders. Although the overall prognosis of patients with KD with neurological involvement is satisfactory,11 IVIG non-responders were more likely to present somnolence, headache, and cerebrospinal fluid pleocytosis, and similar findings were also evident in this study for pericholecystic edema or bile duct dilation, jaundice, abnormal liver function, and symptoms compatible with macrophage activation syndrome, which have been reported previously as risk factors of IVIG unresponsiveness.25-27, 31-33 Therefore, close ultrasound monitoring should be considered for these patients because unresponsiveness to IVIG is an important risk factor for CAA development. To the best of our knowledge, this is the first study to report an association between extracardiovascular injury complications and the response to IVIG therapy in patients with KD.
Coronary artery involvement is considered the most common complication associated with KD, and it may cause significant cardiovascular disease in children. The incidence of CAA in the current study (27.4%) was higher than that reported in the literature (2%–24.1%), which is probably due to the use of Z scores instead of the absolute values of the internal diameter of the coronary arteries. Alternatively, our hospital is a tertiary care center and received many referred patients with severe KD or treatment failure, which may also explain this result. However, no significant differences in extracardiovascular injury complications were observed among the subgroups stratified by coronary status, which suggests that there is no significant relationship between the occurrence of extracardiovascular complications and cardiovascular complications.11 Patients with CAA were more likely to present with pericholecystic edema or bile duct dilation than those without CAA, and presented more often with pleural effusion than patients with CAD, indicating that a strong inflammatory response stimulates the increased permeability of the endothelium, resulting in worse late coronary outcomes.
This study has several limitations. First, it must be viewed considering the potential limitations inherent to its retrospective nature and long observation period. Of note, we strictly applied the most up-to-date recommendations together with inclusion and exclusion criteria to further verify and select the target population and minimize selection bias. Second, our study was conducted in a tertiary pediatric hospital in southern China, and the results obtained may not extend to other ethnicities. Third, the quality of echocardiography may have varied among patients owing to differences in operator expertise, machine quality, and choice of sedation, thus leading to an overestimation of the true incidence of CAA. Hence, these findings should be interpreted with caution, and multicenter studies with large cohorts are needed.
In conclusion, KD can involve multiple organ injuries other than cardiovascular complications; particularly, nervous system involvement may be more common in IVIG non-responders. These complications should be promptly identified and patients should be followed more closely during their treatment.
ACKNOWLEDGMENTS
We thank Dr. Cheng Chen for his helpful advice and discussions. We also gratefully acknowledge Qiaoyu Yue, Kaizhi Liang, Baofeng Wang, and Changqing Wei from the Pediatrics Department of First Affiliated Hospital of Guangxi Medical University who instructed and assisted us during the manuscript preparation.
CONFLICT OF INTEREST
This article is included in the special issue Cardiovascular Disease of Pediatric Investigation and Dr. Yusheng Pang is the guest editor of this special issue.