Effectiveness of stenting and subsequent re-implantation of disconnected pulmonary artery to restore the pulmonary hemodynamics
ABSTRACT
Introduction
Early intervention in patients with congenitally disconnected pulmonary artery improves long-term outcome.
Case presentation
We present 3 cases of isolated disconnected pulmonary artery in the absence of associated structural heart disease during a period of 3 years.
Conclusion
Transcatheter stenting of the feeding ductus arteriosus re-established pulmonary artery flow and growth. Successful surgical repair was achieved, and normal perfusion and complete function of the ipsilateral lung were regained.
1 INTRODUCTION
Isolated disconnected pulmonary artery (PA), also known as ductal origin of PA, is a rare condition with an estimated incidence of approximately 1:200 000.1 Flow to a disconnected PA usually originates from a systemic artery through the patent ductus arteriosus (PDA). Although early surgical treatment is mandatory to support disconnected PA growth, this branch is small with insufficient blood flow, which makes it unsuitable for surgical correction.2
During a period of 3 years, 3 children have presented to our cardiac center with isolated disconnected PA (with no other associated intracardiac anomalies). We report our experience in the diagnosis of this anomaly, and describe the transcatheter approach and subsequent surgical interventions.
2 CASE REPORT
2.1 Patient 1
A male patient (5 days old) presented with dysmorphic features and central cyanosis. Chest X-ray showed a normal cardiac shadow with exaggerated peri-hilar markings in the right lung field. Echocardiography showed a right-sided aortic arch, with the main PA (MPA) giving rise to the right PA (RPA) branch, while the left PA (LPA) branch was not visible. A diagnosis of disconnected LPA was suspected.
At the cardiac catheterization laboratory, access was achieved via the femoral artery and femoral vein. A 100 U/kg dose of heparin and intravenous antibiotics were administered prior to the procedure. A preliminary hemodynamic study was not performed to avoid prolonging the procedure time. Angiography of the right ventricle (RV) confirmed the suspected diagnosis (Figure 1A). Angiography of the ascending aorta showed the disconnected LPA arising from the origin of the left brachiocephalic artery via a 2 mm ductus arteriosus (i.e. aorto-pulmonary collateral artery). The Driver, bare-metal stent (BMS) (4 mm × 16 mm. Medtronic, Inc., Minneapolis, MN, USA) was deployed successfully across the collateral artery to the LPA branch to augment flow and growth via the disconnected LPA branch (Figure 1B). Heparin was not reversed with protamine. The baby was discharged from hospital the following day after being prescribed antiplatelet agents (aspirin 5–10 mg·kg−1·d−1 PO q8hr; clopidogrel 0.2 mg·kg−1·d−1 PO q12hr) to maintain stent patency until the date of subsequent surgical intervention. This post-stent management protocol was implemented for all subsequent patients.
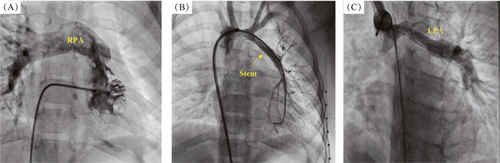
The patient was followed up on a monthly basis (clinically and by echocardiography) for a period of 18 months. A second diagnostic cardiac catheterization showed MPA, RPA, and LPA branches (20 mm, 13 mm, and 8 mm, respectively); and a mean pulmonary pressure of 13 mmHg (Figure 1C). Total surgical correction was performed after 2 weeks. The stent was removed, and the LPA was mobilized and implanted directly into the MPA posteriorly using continuous proline sutures. Postoperative follow-up echocardiography was performed to visualize the non-turbulent flow via the MPA and its two branches.
2.2 Patient 2
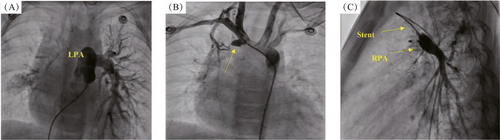
A 10-days-old child presented for evaluation of an accidentally discovered murmur during routine screening for congenital heart diseases. Echocardiography showed a left-sided aortic arch with normal intracardiac structures and an absent RPA branch. A tiny right ductus measuring 2 mm arose from the ascending aorta. Continuous infusion of prostaglandin-E1 (0.1 mcg·kg−1·min−1) was administered, and the baby was referred to the cardiac catheterization laboratory. Access was achieved via the femoral artery and femoral vein. Right ventricular outflow tract angiography revealed that the MPA continued as an LPA branch (Figure 2A). Angiography of the ascending aorta revealed neck branches with a bovine arch distribution (only 2 major branches arising from the arch). A small PDA originating from the ascending aorta opposite to the second rib and before the origin of neck branches gave rise to the disconnected hypoplastic RPA (Figure 2B). Successful PDA stenting using a 4 mm × 18 mm BMS was performed for the disconnected RPA branch (Figure 2C). Follow-up echocardiography showed a PDA stent with an augmented flow to the disconnected RPA branch. Six months later, the baby was referred for complete surgical repair, and re-implantation of the RPA branch was performed using a pericardial patch and MPA flap. Follow-up echocardiography showed that the implanted RPA branch was well connected to the MPA with augmented flow. Distally, the RPA branch was small with mild turbulence (maximum pressure gradient, 30 mmHg). A mildly dilated right atrium and MPA and mild RV hypertrophy were observed. Sildenafil (3 mg orally) was advised for 12 months, which was later discontinued after an improvement was observed echocardiographic findings.
2.3 Patient 3
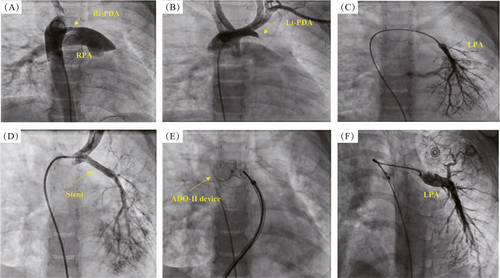
A female infant presented at 18 months of age with cyanosis during crying. The patient underwent echocardiography, which revealed a 3.5-mm right-sided PDA, and she was administered oral frusemide (1 mg∙kg−1∙d−1). At the age of 4 years, the patient was re-evaluated by echocardiography, which showed a right-sided aortic arch. The MPA continued only to the RPA branch, with a 3.5-mm patent ductus arteriosus connected to it. The RV was mildly dilated, and trivial tricuspid valve insufficiency was present. A diagnosis of a disconnected LPA branch was suggested. The patient was transferred to the catheterization laboratory. Access was achieved via the femoral artery and femoral vein. MPA angiography showed branching to the RPA only. Descending aortic angiography confirmed a diagnosis of disconnected LPA branch and showed a 3.5-mm patent ductus arteriosus with a left-to-right shunt connected to the RPA branch (Figure 3A). Selective left innominate artery angiography showed a 2.5-mm left-sided PDA supplying the disconnected LPA branch (the stump measured 3 mm before its bifurcation) (Figures 3B and 3C). A 3.5 mm × 24 mm coronary stent (OMEGA™ Platinum Chromium Bare-Metal Coronary Stent, Boston Scientific Corporation, NY, USA) was inserted via the left-sided PDA crossing to the disconnected LPA branch to maintain adequate flow (Figure 3D). The right-sided PDA was successfully closed using the Amplatzer Duct Occluder type-II (10 mm × 12 mm) (Figure 3E). The patient was followed up by echocardiography for approximately 12-months. The right-sided PDA closing device remained in place with no residual leak, and the left-sided patent PDA stent facilitated augmented flow to the LPA branch. Thereafter, diagnostic catheterization was performed to evaluate the patient before surgery, which revealed a left-sided PDA stent with an improvement in the size of the LPA branch (10 mm) and a mean LPA pressure of 4 mmHg (Figure 3F). The patient was referred for surgery in the same week. The PDA stent was removed, and re-implantation of the disconnected LPA branch to the MPA trunk was performed using a pericardial patch and MPA flap, ensuring normal flow. Follow-up echocardiography showed an improvement in the size of both PA branches with patent flow.
3 DISCUSSION
Adequate pulmonary blood flow is vital for optimal development of the lung segments. Any delay in corrective surgery can lead to hypoplasia of the affected lung and ventilation–perfusion mismatch, which cannot be corrected.3
We report three children with congenital isolated disconnected PA who presented to our center during a 3-years period. This series demonstrates the importance of echocardiography in suspecting this rare condition and the vital role of angiography in reaching an accurate diagnosis. Catheter recanalization should be attempted to promote perfusion and growth of the disconnected PA.4 Understanding the embryological origin of these types of lesion, as well as achieving an accurate angiographic diagnosis, is important for choosing between direct anastomosis and pericardial patch.
Trivedi et al5 reported the largest series of patients with isolated disconnected PA (16 children with isolated disconnected PA during a 31-year period). Surgical intervention was performed in only 7 patients (44%), in which interposition graft connection to the MPA and shunt placement were used, while direct anastomosis was not used. During follow up, 4 deaths were reported over a 5-year period. Although the remaining 9 patients (who did not undergo any intervention) suffered from complications, such as lung hypoplasia, pulmonary hypertension, hemoptysis, and chronic lung infection, they survived during the 5-year follow-up period.5 Few studies have compared treatment interventions in children with isolated disconnected PA (Table 1).
| Study | No. of patients who underwent intervention for disconnected PA (stent + surgical repair) & lesion types | Types of surgical interventions and their outcome over a 5-year follow-up period |
|---|---|---|
| Trivedi KR et al5 | 32 patients (7 isolated lesions; 25 complex lesions [e.g. TOF, pulmonary atresia, VSD, heterotaxy]) |
3 patients with isolated lesions: interposition graft connection to the MPA (1 death) 10 patients (4 with isolated lesions and 6 with complex lesions): shunt placement (4 deaths) 19 patients with complex lesions: direct anastomosis (4 deaths) |
| Cox D et al6 | 3 patients (isolated lesions) | 3 patients with isolated lesions: interposition graft connection to the MPA (0 deaths) |
| Kim GB et al7 | 11 patients (7 isolated lesions, 4 patients with TOF) |
5 patients: main pulmonary artery flap angioplasty (0 deaths) 6 patients: tube graft interposition (0 deaths) |
| Al-Khaldi A et al8 | 20 patients (8 isolated lesions, 12 complex lesions [e.g. TOF, AVSD, DORV]) |
18 patients: direct tissue-to-tissue connection (0 deaths) 2 patients: interposition tube grafts (0 deaths) |
- PA, pulmonary artery; TOF, tetralogy of Fallot; VSD, ventricular septal defect; AVSD, atrioventricular septal defect; DORV, double-outlet right ventricle; MPA, main pulmonary artery.
In our series, we used the direct anastomosis technique in all 3 cases. We did not observe any morbidity or mortality, and adequate blood flow to the previously isolated PA was achieved during the 3-years follow-up period.
In conclusion, although isolated disconnected PA is rare, we reported 3 cases that presented to our center over a 3-years period. In all 3 cases, PDA stenting was performed to promote growth of the disconnected PA for a period of 6–18 months. Total surgical correction using the direct anastomosis technique, which aimed to re-implant the disconnected PA branch into the MPA, was achieved successfully with establishment of normal antegrade flow.
4 CONSENT FOR PUBLICATION
Informed consent was taken from all individual participants/parents included in the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.




