Disease characteristics and neuropathological changes associated with cognitive dysfunction in obstructive sleep apnea
ABSTRACT
Obstructive sleep apnea (OSA) is a common sleep-disordered breathing disease that often leads to many comorbidities (e.g., cognitive dysfunction), which adversely affect the quality of life for patients with OSA. Thus far, the underlying mechanisms of this dysfunction remain unclear. Many studies have focused on OSA-related characteristics, including intermittent hypoxemia and sleep fragmentation. There is increasing emphasis on neuroimaging studies to explore underlying relationships between neuropathological changes and cognitive dysfunction. This article reviews recent research progress concerning cognitive dysfunction associated with OSA to reveal potential mechanisms that contribute to this dysfunction.
1 Introduction
Obstructive sleep apnea (OSA) is one of the most common sleep-disordered breathing diseases that is characterized by prolonged partial upper airway obstruction (hypopnea) and/or complete obstruction (apnea), both of which disrupt ventilation and normal sleep patterns.1 Its prevalence is 1.2%–5.7% in children2, 3 and 24% in adult men aged 30–60 years along with 9% in adult women.4, 5 Chronic OSA is typically accompanied by comorbidities such as cardiovascular diseases and cognitive dysfunction; common adverse effects of cognitive dysfunction include irritability, attention deficit, and memory and execution disorders.6-10 These learning and behavioral disorders presumably have severe adverse effects on physical and mental health. Furthermore, irreversible cognitive Impairments may occur in some patients with OSA.11, 12 Notably, children are in an important period of continuous development and maturation of the nervous system; thus, neuropathological changes in the nervous system may adversely affect children’s long-term growth and development.8, 13 The American Academy of Pediatrics has emphasized the necessity of assessing cognitive function in children with OSA, before and after treatment.2 Therefore, cognitive dysfunction is an important consideration in patients with OSA.
However, the mechanisms underlying cognitive dysfunction associated with OSA remain unknown. In recent years, there has been an increasing number of studies involving cognitive dysfunction in patients with OSA. The findings have confirmed that this dysfunction is associated with OSA characteristics and specific neuropathological changes. This review discusses the relevant studies regarding these characteristics and the neuropathological changes in patients with OSA, with the aim of providing a reference to explore the underlying mechanisms.
The existing literature mainly suggests that cognitive dysfunction might be associated with OSA-related characteristics, including intermittent hypoxemia (IH) and sleep fragmentation. Moreover, with the development of neuroimaging technologies, there has been increasing use of tools based on magnetic resonance imaging in clinical studies of patients with OSA. Substantial neuropathological changes have been observed in brain metabolites, brain morphology structure, and brain functions in patients (both children and adults) with OSA; these changes have been observed using tools such as magnetic resonance spectroscopy, high-resolution T1-weighted images, diffusion tensor imaging, and resting-state functional magnetic resonance imaging. The findings may aid in understanding of the underlying mechanisms of cognitive dysfunction.
2 OSA-related characteristics
2.1 Intermittent hypoxemia
IH refers to intermittent collapse of the upper airway during sleep and recurrent hypoxic episodes. It is currently considered a potential major pathogenic factor for OSA-related comorbidities,14 especially cognitive dysfunction.15, 16 Many studies have shown that OSA might cause cognitive dysfunction due to IH during sleep.14, 17 The underlying mechanisms may be related to the oxidative stress response promoted by IH, such as increased presence of reactive oxygen species and angiogenesis, stimulation of sympathetic activation, and onset of systemic and vascular inflammation.18-20
Furthermore, the high-frequency IH associated with OSA is characterized by adverse cycles of hypoxemia with re-oxygenation, and considerably differs from sustained low-frequency hypoxia14; high-frequency IH is presumably associated with greater risk for ischemia-reperfusion injury in the brain, which may result in important pathological changes. For example, using an ischemia-reperfusion injury rat model, Ravindran and Kurian17 compared cognitive performance after short-term (15 min) and long-term (30 min) exposure to ischemia. They found that the long-term exposure caused significantly more cognitive dysfunction in terms of learning and memory. Zhu et al21 studied dynamic changes in mice subjected to ischemia-reperfusion injury; they confirmed that perinuclear and dendritic cavitations of neurons were induced, while the response of multiple wake-active neurons to c-Fos stimulation was markedly impaired, following 8 weeks of exposure to a hypoxia/re-oxidation environment. Moreover, the number of catecholaminergic wake-active neurons associated with executive and emotional function was reduced by 40 percent after 6 months of exposure. These results suggested that high-frequency IH and cerebral ischemia-reperfusion injury associated with OSA may cause cognitive dysfunction, which might also explain the mechanisms underlying neuronal injury in patients with OSA.
2.2 Sleep fragmentation and subsequent cytokine regulation
Sleep fragmentation is a sleep deprivation behavior caused by repeated nocturnal awakening during sleep in patients with OSA. Some studies have also confirmed that sleep fragmentation is associated with sustained attention deficit and excessive daytime somnolence in patients with OSA.22, 23 These impairments may be caused by enhancement of sleep regulatory substances including interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), nerve growth factor, epidermal growth factor, IL-4, and IL-10.24-27 Notably, these cytokines are involved in inflammatory reactions, as well as regulation of the sleep biochemical network.
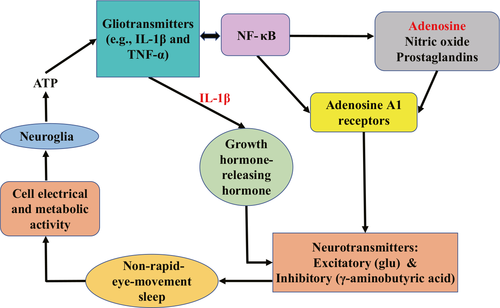
For example, in the brain, adenosine triphosphate released from neuroglia induces the release of gliotransmitters (e.g., IL-1β and TNF-α). IL-1β then induces the release of growth hormone-releasing hormone and the production of an inhibitory neurotransmitter (γ-aminobutyric acid) to further activate sleep-active neurons and inhibit wake-active neurons; these substances promote entry into non-rapid-eye-movement sleep.27-30 Additionally, these cytokines promote their own proliferation and interact with various other substances by means of nuclear factor-κB (NF-κB).31, 32 Multiple molecules (e.g., adenosine, nitric oxide, and prostaglandins) in downstream mechanisms activated by NF-κB have been recruited into the biochemical cascade of sleep regulation.33, 34 Among these molecules, adenosine acts on adenosine A1 receptors on postsynaptic neurons to promote neurotransmitter release and regulate the sleep state (see Figure 1 for details).24, 35 Although the underlying biochemical mechanisms have not been fully elucidated, patients with OSA might exhibit sleep fragmentation and subsequent cytokine regulation by means of a complex network of neuronal factors.
3 Specific neuropathological changes in the brain
3.1 Brain metabolites changes
Magnetic resonance spectroscopy has been applied to noninvasively detect brain metabolic changes and assess neuronal damage before the occurrence of morphological changes.36 For example, Alkan et al36 found that the metabolites ratios (i.e., N-acetyl aspartate/creatine, choline/creatine, and N-acetyl aspartate/choline) significantly differed in multiple brain regions, including hippocampus and putamen, between mild and severe OSA groups; these abnormalities were more pronounced as OSA severity increased. O’Donoghue et al37 showed that patients with OSA had significant differences in hippocampal choline/creatine ratios before morphological changes; these changes were no longer statistically significant after 6 months of continuous positive airway pressure (CPAP), highlighting the importance of early diagnosis and treatment of OSA. Furthermore, Macey et al38 confirmed that patients with OSA showed reduced levels of N-acetyl aspartate, which indicated neuronal injury or dysfunction. These studies revealed microstructural changes in the brains of patients with OSA and provided insights into exploring neuropathological changes.
3.2 Brain morphology structural changes
Many studies have reported that the morphological structures of different brain areas substantially changed in children with OSA; moreover, these changes may be associated with cognitive dysfunction.39-44 Chan et al39 observed that the gray matter volumes were reduced in prefrontal and temporal regions in children with moderateto-severe OSA who exhibited impairments involving attention and visual-fine motor coordination. Another study40 revealed statistically significant changes in cortical thickness among multiple regions including the frontal, insular, and parietal cortices. Based on the findings in that study,40 both thinning and thickening of the brain cortex were presumed to contribute to cognitive and behavioral dysfunction in pediatric patients with OSA. Kumar et al41 found that the gray matter volume in the putamen and its subregions changed significantly in children and adults with OSA; these regional changes were associated with specific OSA-related deficits in motor, autonomic, and neuropsychological functions. Similarly, a reduced mammillary body volume was associated with emotional and impaired cognitive function in patients with OSA.43 Canessa et al44 also found that cognitive function of memory, attention, and execution impaired in untreated patients with OSA and these impairments were associated with reduced gray matter volumes in multiple brain areas including the left hippocampus, left posterior parietal cortex, and right superior frontal gyrus.
Importantly, Chen et al45 analyzed diffusion tensor imaging-related indicators (e.g., fractional anisotropy, axial diffusivity, radial diffusivity, and mean diffusivity) in patients with severe OSA, compared with healthy participants. They found that white matter integrity was impaired in various regions; moreover, this impairment was associated with enhanced OSA severity. Another study46 confirmed that white matter integrity and structural connectivity were substantially altered in patients with OSA; notably, white matter integrity was associated with brain network properties (i.e., strength, global efficiency, and local efficiency), which helped to explain neurocognitive impairments in affected patients. Cha et al42 compared the mean diffusivity and fractional anisotropy between children with OSA and health controls they found that the volume of the left dentate gyrus was significantly reduced in the OSA group, while a reduced mean diffusivity in the dentate gyrus was positively correlated with a lower verbal learning score. Moreover, Castronovo et al47 observed that the white matter integrity was reduced in multiple brain areas in patients with OSA; these abnormalities were completely reversed after 12 months of CPAP. Based on these findings, early diagnosis and treatment are essential for patients with OSA. These changes in brain structure may reflect the progression from onset of OSA to chronic disease, perhaps associated with hypoxia or changes of glial perfusion and disruption of neural development.41, 44
3.3 Brain functional changes
Several studies have been published in the past 10 years regarding brain functional changes in adult patients with OSA. For example, Li et al48 analyzed the intensity of spontaneous brain activity in men with severe OSA, measured by amplitude of low-frequency fluctuation (ALFF). They observed significantly enhanced ALFF in the left inferior frontal gyrus, along with reduced ALFF in the right precuneus and bilateral posterior cingulate gyrus; the reduced ALFF in this cluster was positively correlated with Montreal Cognitive Assessment (MoCA) score. Additionally, Peng et al49 compared the local synchronicity of spontaneous neural activity between men with severe OSA and controls, measured by regional homogeneity (ReHo). They found that patients with OSA showed lower ReHo in the right medial frontal gyrus, right superior frontal gyrus, right precuneus and angular gyrus, and left superior parietal lobule; they also showed higher ReHo in the right posterior lobe of the cerebellum, right cingulate gyrus, and a bilateral cluster comprising the lentiform nucleus, putamen, and insula. In a study including 14 patients with OSA and 16 healthy participants (all Tibetan men), Kang et al50 reported that patients with OSA showed higher ALFF in multiple brain areas including the frontal and insular lobes and the cingulate and paracingulate gyri, as well as enhanced ReHo in the superior frontal dorsolateral gyrus, left middle frontal gyrus, and superior frontal medial gyrus; these patients also exhibited reduced ReHo in the left fusiform gyrus and cerebellum lobule 6. These results in adult men with OSA may have been influenced by factors such as disease severity and living environment (e.g., high altitude and hypoxia associated with residence on the Qinghai-Tibet Plateau).50
Notably, functional connectivity is reportedly impaired between the hippocampus and other brain areas (e.g., thalamus, parahippocampal gyrus, medial and superior temporal gyri, insula, and posterior cingulate cortex) in adult patients with OSA51; these changes have been associated with behavioral and neuropsychological performance. Chen et al52 confirmed that patients with OSA showed abnormal functional connectivity in several network regions including the default mode network, salience network, and central executive network; these topological properties could contribute to altered Epworth Sleepiness Scale and MoCA score. Another study53 revealed that the functional connectivity patterns in amygdala subregions showed substantial and complex changes in men with severe with OSA, compared with healthy controls. The changes included both enhanced and reduced functional connectivity; moreover, these abnormalities might reveal mechanisms of OSA-related emotional and executive disorders. These findings might aid in exploration of the underlying neuropathological mechanisms of cognitive dysfunction in adults with OSA. However, there are limited data regarding brain functional changes in children with OSA,54 indicating a need for further research in these patients.
4 Conclusion
OSA is an independent sleep-disordered breathing disease with high prevalence, which leads to cognitive dysfunction. Recent studies have confirmed that this dysfunction is not limited to characteristics of OSA; it is also related to neuropathological changes in brain metabolism, neuronal morphology, and neuronal function. These changes may be regarded as biomarkers for detection and evaluation of cognitive dysfunction. Although these findings provide some insights that may aid in the investigation of neuropathological mechanisms of cognitive dysfunction related to OSA, more prospective clinical studies are needed to clarify the specific mechanisms of cognitive dysfunction in patients with OSA.
5 Funding source
Capital Funds for Health Improvement and Research (2018-1-2091); National Natural Science Foundation of China (81970900); Natural Science Foundation of Beijing Municipality (7194262); Pediatric Medical Coordinated Development Center of Beijing Municipal Administration (XTZD20180101)
CONFLICT OF INTEREST
All the authors listed have read through the manuscript, approved for publication and declared no conflict of interest.




