Interaction between increasing body mass index and spinal cord injury to the probability of developing a diagnosis of nonalcoholic fatty liver disease
Abstract
Background
The prevalence of obesity and comorbidities is high in the population with spinal cord injury (SCI). We sought to determine the effect of SCI on the functional form of the relationship between body mass index (BMI) and risk of developing nonalcoholic fatty liver disease (NAFLD), and assess whether SCI-specific mapping of BMI to risk of developing NAFLD is needed.
Methods
Longitudinal cohort study comparing Veterans Health Administration patients with a diagnosis of SCI to a 1:2 matched control group without SCI. The relationship between BMI and development of NAFLD at any time was assessed with propensity score matched Cox regression models; NAFLD development at 10-year with a propensity score matched logistic model. The positive predictive value of developing NAFLD at 10 years was calculated for BMI 19–45 kg/m2.
Results
14,890 individuals with SCI met study inclusion criteria, and 29,780 Non-SCI individuals in matched control group. Overall, 9.2% in SCI group and 7.3% in Non-SCI group developed NAFLD during the study period. A logistic model assessing the relationship between BMI and the probability of developing a diagnosis of NAFLD demonstrated that the probability of developing disease increased as BMI increased in both cohorts. The probability was significantly higher in the SCI cohort at each BMI threshold (p < 0.01), and increased at a higher rate compared with the Non-SCI cohort as BMI increased 19–45 kg/m2. Positive predictive value for developing a diagnosis of NAFLD was higher in the SCI group for any given BMI threshold from 19 kg/m2 to BMI 45 kg/m2.
Conclusions
The probability of developing NAFLD is greater in individuals with SCI than without SCI, at every BMI level 19 kg/m2 to 45 kg/m2. Individuals with SCI may warrant a higher level of suspicion and closer screening for NAFLD. The association of SCI and BMI is not linear.
1 INTRODUCTION
There is a growing recognition of nonalcoholic fatty liver disease (NAFLD) as a global health concern, with increasing prevalence and potential implications for liver adverse outcomes.1, 2 Nonalcoholic fatty liver disease is now a leading cause of liver disease worldwide affecting up to 25% of the world adult population, is a growing cause of liver-related death, and is a significant burden on healthcare and health systems.3, 4 It is more common in males and demonstrates geographic variability, as well as ethnic and racial variation.5-7 Nonalcoholic fatty liver disease includes several progressive liver disorders. Its subtype, nonalcoholic steatohepatitis (NASH) demonstrates features of hepatocellular injury, which can be associated with inflammation and progress to liver fibrosis and cirrhosis.8 In fact, NASH is a growing cause of liver failure and indication for transplantation.5, 9, 10
While the mechanism of disease has not been fully elucidated, it is thought that fatty accumulation in the liver leads to a spectrum of pathological and clinical findings, resulting in a strong association with metabolic and endocrine disease. Thus, NAFLD is strongly associated with obesity, dyslipidemia, type 2 diabetes, and the metabolic syndrome generally, but may also be secondarily associated with polycystic ovary disease, hypothyroidism, and hypogonadism.11 In fact, endocrine disease may be associated with the development of NAFLD and its progression. A state of androgen excess seems to promote the pathogenesis of NAFLD, and estrogens may be protective of NAFLD,12 which may explain the male predilection of the diagnosis.
Nonalcoholic fatty liver disease is closely associated with the metabolic syndrome, and obesity is a known risk factor for NAFLD.13 The increase in prevalence of NAFLD mirrors that of global obesity, and the majority of individuals with NAFLD have a diagnosis of overweight or obesity.9, 14 However, the interaction of NAFLD and metabolic syndrome is complex. There is evidence to suggest that NAFLD is not a result of metabolic syndrome in all cases, but may actually be a risk factor for the development of other metabolic disease.15, 16 In fact, NAFLD is recognized in non-obese individuals. Interestingly, a study in an Asian population with a mean body mass index (BMI) of 23.7 + 1.1 kg/m2 quantified an elevated cardiovascular risk in subjects with NAFLD in the absence of obesity.17 Obesity with metabolic syndrome and obesity without metabolic syndrome may be different but related clinical entities, suggesting the importance of fat distribution and the cellular mechanisms at the level of the adipocyte. Important signaling pathways between hepatocytes, Kupffer cells, hepatic stellate cells, and liver sinusoidal endothelial cells have been implicated, along with multiple inflammatory mediators that are suggested in the mechanism of liver injury in obesity.18-20 Variants in specific genes that are involved in lipid metabolism alter the risk of development of NAFLD and its progression to NASH, fibrosis, and cirrhosis.21 Nonetheless, there is a strong pathophysiological link between NAFLD and obesity, and obesity increases the risk of progression of NAFLD/NASH to liver fibrosis.5, 18, 22, 23 While there is no specific treatment for NAFLD, histologic improvement can be seen after significant weight loss achieved by any method, and weight loss remains a mainstay of primary risk reduction and therapy in most cases.24-26
The prevalence of obesity and its related comorbidities is high in the population with spinal cord injury (SCI).27 Individuals with SCI are especially susceptible to obesity due to metabolic changes that occur after SCI, as well as decreased mobility and loss of muscle mass. These changes and differences relative to people without SCI mean that conventional BMI thresholds are suspected to underestimate clinical obesity and risk of developing obesity-related comorbidities in these individuals.28 Thus, based on anthropomorphic data and expert opinion, it is recommended that the appropriate obesity cutoff point for individuals with SCI is BMI ≥22 kg/m2.29 The implication is that individuals with SCI may have higher risk of developing NAFLD compared with someone with the same BMI without SCI. In this study we sought to determine the effect of SCI status on the functional form of the relationship between BMI and the risk of developing NAFLD, and whether SCI-specific mapping of BMI to risk of developing obesity-related comorbidities is needed. Our hypothesis is that the populations with SCI and the population without SCI (non-SCI) are different with respect to BMI-based risk for NAFLD, and that the population with SCI will demonstrate a greater risk for NAFLD at lower BMI compared with the non-SCI population. In addition, we suspect that conventional BMI zones will not serve as meaningful thresholds of risk.
2 MATERIALS AND METHODS
2.1 Data and design
We conducted a longitudinal cohort study of patients within the Veterans Health Administration (VHA) with chronic SCI, defined as a SCI diagnosis in fiscal years (FY) 2005–2007 and at least one additional diagnosis code documented at least two years prior to ensure that SCI was not a new diagnosis. The earliest date of SCI diagnosis documentation in FY 2005–2007 with available height (measured at any time prior) and weight (measured in 6 months prior) is the “index date”. Patients with a documented NAFLD diagnosis prior to index date, and patients without available height and weight measurements were excluded.
We constructed a cohort of matched controls for comparison from a random sample of 100,000 patients with at least one primary care visit in FY 2005–2007 and followed for a study period of 10 years. We identified patients with no documented SCI or central nervous system condition diagnosis. Matched controls had at least 1 year of health record data prior to primary care visit, and available weight measurement (within 6 months of primary care visit) and height measurement (at any point prior to primary care visit). Index date for controls was defined as the earliest primary care visit in FY 2005–2007 that met those criteria. We excluded patients with NAFLD diagnosis documented before index date. We matched 2:1 nearest neighbor with replacement on sex, race, ethnicity, Charlson Comorbidity Index, BMI, year of index visit, and geographic clinic location (Continental, Midwest, North Atlantic, Pacific, Southwest) to minimize bias. We assessed covariate balance using standardized mean difference (mean difference divided by pooled standard deviation), with <10% indicating sufficient balance.
2.2 Outcome
Our primary outcome was development of NAFLD, based on International Classification of Disease, Ninth Revision codes 571.5, 571.8, 571.9; or 10th Revision codes K74.0, K74.1, K74.60, K74.69, K76.0, K76.89, K76.9. Patients were followed from index date until last known date of care or death, within the Veterans Affairs health system.
2.3 Covariates
Our primary predictor was BMI, calculated with closest weight measurement in the 6 months prior to index date and mode of up to 10 closest height measurements prior to index date, or SCI status.
2.4 Statistical analysis
We assessed the relationship between BMI and development of NAFLD with a doubly-robust, propensity score matched Cox regression model, unadjusted for additional covariates. We assessed the proportional hazards assumption by including an interaction term with time and did not find evidence that it was violated. In this context, we think that positive predictive value (PPV) is the most relevant accuracy metric but we also calculated sensitivity, specificity, and negative predictive value for half-point increments of BMI stratified by SCI status, with the package TimeROC in R (R Foundation for Statistical Computing, Vienna, Austria).30 All other analyses were completed with Stata MP v.17 (StataCorp LLC, College Station, TX, USA).
The protocol for this study was approved by the Institutional review board of Stanford University School of Medicine.
3 RESULTS
3.1 Cohort matching
Between FY 2005–2007 there were 14,890 individuals with SCI within VHA that met inclusion criteria, and 29,780 Non-SCI individuals in the matched control group. Overall, 9.2% of the individuals in the SCI group and 7.3% in the Non-SCI group developed NAFLD during the study period. Both cohorts had a median BMI of 27 kg/m2 and the mean age of each cohort was similar (58 years, Non-SCI; 57 years, SCI). The SCI and Non-SCI groups were mostly male (96.9% and 97.8%, respectively), white (70.4% and 72.6%, respectively), and non-Hispanic (87.9% and 89.4%, respectively). The median Charlson Comorbidity Index was two in both groups, and the geographical distribution of the patients was similar throughout the 5 regions of VHA (Table 1).
| Non-SCI (n = 29,780) | SCI (n = 14,890) | Total (n = 44,670) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| Age at index, mean SD | 58 | 15 | 58 | 13 | 58 | 14 | 0.001 |
| Sex | <0.001 | ||||||
| Female | 652 | 2.2% | 463 | 3.1% | 1115 | 2.5% | |
| Male | 29,128 | 97.8% | 14,427 | 96.9% | 43,555 | 97.5% | |
| Race | <0.001 | ||||||
| American Indian or Alaska Native | 189 | 0.6% | 104 | 0.7% | 293 | 0.7% | |
| Asian | 89 | 0.3% | 58 | 0.4% | 147 | 0.3% | |
| Black or African American | 4607 | 15.5% | 2515 | 16.9% | 7122 | 15.9% | |
| Native Hawaiian or other Pacific Islander | 200 | 0.7% | 108 | 0.7% | 308 | 0.7% | |
| White | 21,618 | 72.6% | 10,485 | 70.4% | 32,103 | 71.9% | |
| Unknown | 3077 | 10.3% | 1620 | 10.9% | 4697 | 10.5% | |
| Ethnicity | <0.001 | ||||||
| Hispanic or Latino | 1194 | 4.0% | 759 | 5.1% | 1953 | 4.4% | |
| Not Hispanic or Latino | 26,625 | 89.4% | 13,084 | 87.9% | 39,709 | 88.9% | |
| Unknown | 1961 | 6.6% | 1047 | 7.0% | 3008 | 6.7% | |
| BMI | 27 | (24, 30) | 27 | (23.5, 30.5) | 27 | (23.5, 30.5) | 0.035 |
| Charlson index | 2 | (1, 4) | 2 | (1, 3) | 2 | (1, 3) | 0.819 |
| Index year | <0.001 | ||||||
| 2004 | 12,843 | 43.1% | 6016 | 40.4% | 18,859 | 42.2% | |
| 2005 | 10,428 | 35.0% | 5253 | 35.3% | 15,681 | 35.1% | |
| 2006 | 4326 | 14.5% | 2335 | 15.7% | 6661 | 14.9% | |
| 2007 | 2183 | 7.3% | 1286 | 8.6% | 3469 | 7.8% | |
| Clinic location | <0.001 | ||||||
| Continental | 5350 | 18.0% | 2488 | 16.7% | 7838 | 17.5% | |
| Midwest | 6528 | 21.9% | 2947 | 19.8% | 9475 | 21.2% | |
| North Atlantic | 6685 | 22.4% | 3106 | 20.9% | 9791 | 21.9% | |
| Pacific | 4736 | 15.9% | 2960 | 19.9% | 7696 | 17.2% | |
| Southeast | 6481 | 21.8% | 3389 | 22.8% | 9870 | 22.1% | |
3.2 Effect of spinal cord injury status on the functional form of the relationship between body mass index and the risk of developing nonalcoholic fatty liver disease
A model assessing the relationship between BMI and the probability of developing a diagnosis of NAFLD demonstrated that the probability of developing disease increased as BMI increased in both the SCI and Non-SCI cohorts (Figure 1). The distribution based on level of injury (tetraplegia (45.7%), paraplegia (47.5%)) demonstrated a significant difference between both levels of injury and the Non-SCI cohort in the lower BMI ranges, but the difference was much greater for the group with paraplegia in the upper BMI ranges (Figure 2). The probability was significantly higher in the SCI cohort at each BMI threshold, and increased at a higher rate compared to the Non-SCI cohort as BMI increased from 19 to 45 kg/m2 (p < 0.01 at each BMI increment of 0.5).
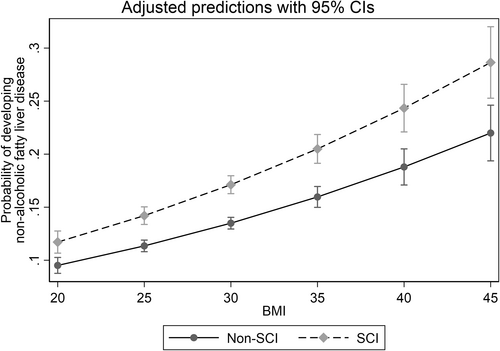
Relationship between body mass index (BMI) and the probability of developing a diagnosis of nonalcoholic fatty liver disease (NAFLD) in the cohort with spinal cord injury (SCI) and cohort without SCI (Non-SCI). 95% confidence intervals are indicated. BMI, body mass index, kg/m2; NAFLD, nonalcoholic fatty liver disease; SCI, spinal cord injury.
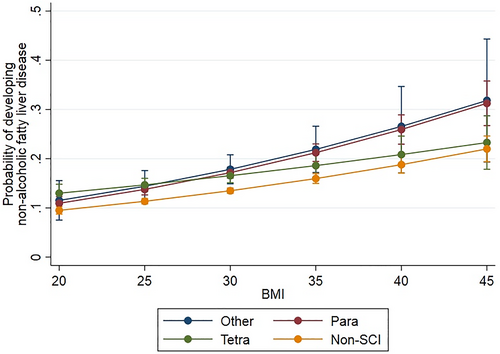
Relationship between body mass index (BMI) (in kg/m2) and the probability of developing a diagnosis of NAFLD in the cohort with spinal cord injury (SCI) and cohort without SCI (Non-SCI). Subset analysis based on level of SCI injury. Tetra (Tetraplegia), Para (Paraplegia), Other (conflicting documentation in dataset). 95% confidence intervals are indicated.
The positive predictive value for developing a diagnosis of NAFLD was higher in the SCI group versus Non-SCI group, for any given BMI threshold from 19 kg/m2 to BMI 45 kg/m2 (Table 2). For example, at BMI 35 kg/m2 PPV was 0.1177 versus 0.0881 for the SCI and Non-SCI groups respectively. Meanwhile, the negative predictive value was higher in the Non-SCI group for any BMI ≥21.5 kg/m2.
| BMI threshold | SCI | Non-SCI (comparison) | ||||
|---|---|---|---|---|---|---|
| PPV | NPV | # Above threshold | PPV | NPV | # Above threshold | |
| 19 | 0.0731 | 0.9587 | 14,668 | 0.0556 | 0.9324 | 29,489 |
| 19.5 | 0.0734 | 0.9568 | 14,453 | 0.0556 | 0.9383 | 29,167 |
| 20 | 0.0743 | 0.9648 | 14,168 | 0.0557 | 0.9441 | 28,758 |
| 20.5 | 0.0748 | 0.9608 | 13,850 | 0.0559 | 0.9504 | 28,233 |
| 21 | 0.0761 | 0.9653 | 13,506 | 0.0569 | 0.9631 | 27,615 |
| 21.5 | 0.0763 | 0.9585 | 13,112 | 0.0575 | 0.9653 | 26,865 |
| 22 | 0.0772 | 0.9567 | 12,698 | 0.0581 | 0.9647 | 26,079 |
| 22.5 | 0.0782 | 0.9552 | 12,222 | 0.0584 | 0.9626 | 25,280 |
| 23 | 0.0783 | 0.9503 | 11,713 | 0.0596 | 0.9646 | 24,293 |
| 23.5 | 0.0790 | 0.9483 | 11,148 | 0.0593 | 0.9590 | 23,144 |
| 24 | 0.0805 | 0.9488 | 10,587 | 0.0598 | 0.9577 | 21,956 |
| 24.5 | 0.0825 | 0.9495 | 9991 | 0.0599 | 0.9553 | 20,898 |
| 25 | 0.0849 | 0.9505 | 9415 | 0.0606 | 0.9550 | 19,668 |
| 25.5 | 0.0860 | 0.9487 | 8829 | 0.0616 | 0.9550 | 18,481 |
| 26 | 0.0874 | 0.9468 | 8182 | 0.0614 | 0.9526 | 17,040 |
| 26.5 | 0.0902 | 0.9475 | 7679 | 0.0623 | 0.9523 | 15,724 |
| 27 | 0.0925 | 0.9467 | 7052 | 0.0627 | 0.9513 | 14,477 |
| 27.5 | 0.0957 | 0.9465 | 6447 | 0.0635 | 0.9508 | 13,285 |
| 28 | 0.0971 | 0.9446 | 5894 | 0.0650 | 0.9508 | 12,007 |
| 28.5 | 0.0977 | 0.9426 | 5387 | 0.0653 | 0.9499 | 10,851 |
| 29 | 0.0982 | 0.9409 | 4917 | 0.0678 | 0.9503 | 9756 |
| 29.5 | 0.0966 | 0.9384 | 4507 | 0.0687 | 0.9498 | 8726 |
| 30 | 0.1019 | 0.9387 | 4036 | 0.0717 | 0.9500 | 7726 |
| 30.5 | 0.0983 | 0.9361 | 3661 | 0.0721 | 0.9493 | 6929 |
| 31 | 0.1000 | 0.9356 | 3321 | 0.0740 | 0.9491 | 6199 |
| 31.5 | 0.1005 | 0.9348 | 3007 | 0.0784 | 0.9493 | 5485 |
| 32 | 0.1047 | 0.9348 | 2703 | 0.0786 | 0.9486 | 4828 |
| 32.5 | 0.1043 | 0.9336 | 2402 | 0.0810 | 0.9484 | 4273 |
| 33 | 0.1093 | 0.9337 | 2167 | 0.0878 | 0.9486 | 3666 |
| 33.5 | 0.1145 | 0.9337 | 1955 | 0.0879 | 0.9480 | 3253 |
| 34 | 0.1165 | 0.9332 | 1761 | 0.0851 | 0.9472 | 2849 |
| 34.5 | 0.1220 | 0.9331 | 1563 | 0.0875 | 0.9470 | 2485 |
| 35 | 0.1177 | 0.9320 | 1402 | 0.0881 | 0.9467 | 2222 |
| 35.5 | 0.1201 | 0.9317 | 1271 | 0.0899 | 0.9465 | 1953 |
| 36 | 0.1268 | 0.9318 | 1149 | 0.0874 | 0.9460 | 1703 |
| 36.5 | 0.1320 | 0.9316 | 1019 | 0.0941 | 0.9461 | 1451 |
| 37 | 0.1353 | 0.9314 | 928 | 0.0873 | 0.9456 | 1286 |
| 37.5 | 0.1278 | 0.9305 | 823 | 0.0914 | 0.9456 | 1120 |
| 38 | 0.1258 | 0.9300 | 728 | 0.0796 | 0.9450 | 945 |
| 38.5 | 0.1290 | 0.9298 | 647 | 0.0803 | 0.9449 | 855 |
| 39 | 0.1266 | 0.9294 | 582 | 0.0875 | 0.9450 | 769 |
| 39.5 | 0.1258 | 0.9292 | 518 | 0.0936 | 0.9450 | 676 |
| 40 | 0.1286 | 0.9291 | 469 | 0.0852 | 0.9448 | 586 |
| 40.5 | 0.1290 | 0.9289 | 415 | 0.0876 | 0.9447 | 498 |
| 41 | 0.1248 | 0.9286 | 376 | 0.0822 | 0.9446 | 442 |
| 41.5 | 0.1260 | 0.9285 | 338 | 0.0783 | 0.9445 | 387 |
| 42 | 0.1240 | 0.9283 | 306 | 0.0871 | 0.9446 | 340 |
| 42.5 | 0.1281 | 0.9282 | 280 | 0.0849 | 0.9446 | 297 |
| 43 | 0.1137 | 0.9279 | 256 | 0.0766 | 0.9445 | 269 |
| 43.5 | 0.1092 | 0.9278 | 234 | 0.0467 | 0.9442 | 231 |
| 44 | 0.1184 | 0.9279 | 213 | 0.0516 | 0.9443 | 210 |
| 44.5 | 0.1187 | 0.9279 | 195 | 0.0627 | 0.9443 | 181 |
| 45 | 0.1044 | 0.9277 | 175 | 0.0576 | 0.9443 | 151 |
- Note: The number of individuals in each cohort with a BMI greater than the threshold is indicated.
4 DISCUSSION
In this study we demonstrate that the risk of obtaining a diagnosis of NAFLD over a 10-year period increases with increasing initial BMI. While this supports the current understanding of the deleterious metabolic effects of obesity in general and the increasing burden of fatty liver disease in the population with obesity in particular,31 our study findings are novel and important. First, our findings suggest that the metabolic impact observed with the increasing probability of developing a NAFLD diagnosis can be detected at BMI thresholds lower than those that define obesity for both SCI and non-SCI populations. We suspect that the risk of developing NAFLD is under-appreciated among healthcare providers and patients. We found this to be especially true in the population with SCI, which would specifically benefit from closer attention by clinical providers to the possibility of NAFLD in these patients. Other studies using health records have shown a linear relationship between risk of NAFLD diagnosis and BMI over a wide range of BMI thresholds.32 However, we found a progressive increase in the PPV for disease with increasing BMI, that did not persist at the highest BMI thresholds. We suspect that this is a result of the decreasing number of subjects at high BMI.
Second, we found that the risk for developing a diagnosis of NAFLD was higher for the population with SCI at any BMI level that we assessed, compared to matched controls without SCI. In fact, it appears that SCI confers an increased risk of NAFLD; particularly as BMI increases. This is in general agreement with Peterson et al. who used an insurance claims database to study the 4-year incidence of metabolic disease following SCI.33 They found that individuals with SCI have a higher risk for cardiometabolic disease compared to individuals without SCI, although obesity status (or BMI) was not examined in their study. Others have suggested an association between NAFLD and SCI in individuals with concurrent androgen deficiency.34 Animal studies, meanwhile, have demonstrated that acute SCI alone results in inflammation and lipid accumulation in the liver.35 The complex interaction between the metabolic changes after SCI, and the relationship between metabolic disease and NAFLD is yet to be fully elucidated. Nonetheless, our findings may argue for healthcare systems to employ screening protocols that are specific for patients with SCI to detect NAFLD. In addition, the population with SCI may benefit from aggressive management of metabolic disease, irrespective of BMI.
Third, the finding in this study of the functional form of the interaction between SCI, BMI, and NAFLD demonstrates that there are no clearly defined BMI thresholds that define zones of risk for metabolic disease. Thus, not only do BMI thresholds need to be adjusted for the population with SCI, as has been reported previously,29, 36 but these thresholds correlate poorly with risk for NAFLD. In fact, a clinical practice guideline established by a consortium focused on the care of veterans with SCI recommended that all adults with SCI be evaluated for cardiometabolic disease at the time of discharge from rehabilitation. This aligns with our findings of NAFLD risk in this population.
Limitations of this study include that NAFLD is likely under-diagnosed by our study methods, due to lack of routine screening in VHA; while at the same time it is unknown whether individuals were diagnosed with NAFLD by a standard diagnostic method. However, this should apply to both SCI and non-SCI populations, and we suspect that correcting for this limitation would further amplify our findings. In addition, the sample size decreased significantly at the highest BMI levels, which may account for our finding that the PPV for NAFLD did not continue to increase in these individuals in both cohorts. Our sample size was also too small to perform a meaningful subset analysis in the female and non-white populations; a reflection of the demographics of the U.S. veteran population in general. Furthermore, the care of individuals with SCI in VHA is likely different, and perhaps better, than individuals with SCI from the general population, due to its integrated system of care. Thus, our findings may not be generalizable. Further study is necessary to determine the risk of other metabolic and endocrine disease in the population with SCI. The strength of this study is that the Department of Veterans Affairs has one of the largest databases of individuals with SCI and allows access to data from thousands of patients that can be followed longitudinally. Future study is necessary to investigate the quality of accepted BMI risk zones in assessing risk for metabolic disease in general, and NAFLD in particular, especially in the population with SCI.
In conclusion, we found an interaction between SCI and increasing BMI to the probability of developing a diagnosis of NAFLD. This has important implications for the population with SCI in particular. Our findings support that standard mapping of BMI to risk of NAFLD should be adjusted to account for SCI status. Because the associations are not additive, findings such as those produced in our study can be used to provide patient-specific estimates of NAFLD risk based on BMI and SCI status. Long-term screening for NAFLD may be indicated in all adults with chronic SCI, and specifically in adults who have SCI and overweight/obesity.
AUTHOR CONTRIBUTIONS
Eisenberg: Conceptualization, Methodology, Investigation, Data curation, Validation, Writing original draft, Writing review & editing, Supervision, Funding acquisition. Arnow: Methodology, Formal analysis, Investigation, Data curation, Writing original draft, Writing review & editing. Barreto: Methodology, Formal analysis, Investigation, Data curation, Writing review & editing. Davis: Conceptualization, Project administration, Writing original draft, Writing review & editing. Frayne: Conceptualization, Writing review & editing. Lavela: Conceptualization, Writing review & editing. Nevedal: Conceptualization, Writing review & editing. Wu: Conceptualization, Writing review & editing. Harris: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing review & editing, Funding acquisition.
ACKNOWLEDGMENTS
This study was supported by Merit Review Award number IIR 17–047 (Eisenberg) and Research Career Scientist Award number RCS 14–232 (Harris) from the United States (U.S.) Department of VA, HSR&D.
CONFLICTS OF INTEREST
The contents do not represent the views of the VA or the United States Government. The authors have no conflicts of interest to disclose.