Cold exposure modulates potential brown adipokines in humans, but only FGF21 is associated with brown adipose tissue volume
Jonatan R. Ruiz, Guillermo Sanchez-Delgado, Andrea Mendez-Gutierrez, and Concepcion M. Aguilera contributed equally to this work.
Abstract
Objective
The study objective was to investigate the effect of cold exposure on the plasma levels of five potential human brown adipokines (chemokine ligand 14 [CXCL14], growth differentiation factor 15 [GDF15], fibroblast growth factor 21 [FGF21], interleukin 6 [IL6], and bone morphogenic protein 8b [BMP8b]) and to study whether such cold-induced effects are related to brown adipose tissue (BAT) volume, activity, or radiodensity in young humans.
Methods
Plasma levels of brown adipokines were measured before and 1 h and 2 h after starting an individualized cold exposure in 30 young adults (60% women, 21.9 ± 2.3 y; 24.9 ± 5.1 kg/m2). BAT volume, 18F-fluorodeoxyglucose uptake, and radiodensity were assessed by a static positron emission tomography-computerized tomography scan after cold exposure.
Results
Cold exposure increased the concentration of CXCL14 (Δ2h = 0.58 ± 0.98 ng/mL; p = 0.007), GDF15 (Δ2h = 19.63 ± 46.2 pg/mL; p = 0.013), FGF21 (Δ2h = 33.72 ± 55.13 pg/mL; p = 0.003), and IL6 (Δ1h = 1.98 ± 3.56 pg/mL; p = 0.048) and reduced BMP8b (Δ2h = −37.12 ± 83.53 pg/mL; p = 0.022). The cold-induced increase in plasma FGF21 was positively associated with BAT volume (Δ2h: β = 0.456; R2 = 0.307; p = 0.001), but not with 18F-fluorodeoxyglucose uptake or radiodensity. None of the changes in the other studied brown adipokines was related to BAT volume, activity, or radiodensity.
Conclusions
Cold exposure modulates plasma levels of several potential brown adipokines in humans, whereas only cold-induced changes in FGF21 levels are associated with BAT volume. These findings suggest that human BAT might contribute to the circulatory pool of FGF21.
Study Importance
What is already known?
- Brown adipose tissue can secrete molecules to the bloodstream (i.e., brown adipokines) that can impact the function of other tissues and organs.
- Most human adults present with metabolically active brown adipose tissue, although the endocrine function of this tissue is largely unknown.
What does this study add?
- A 2-h personalized mild cold exposure activating brown adipose tissue elevated the circulating concentrations of FGF21, IL6, CXCL14, and GDF15, while decreasing BMP8b, in young adults.
- The cold-induced changes in FGF21 concentration, but not the changes in the other analyzed brown adipokines, were associated with brown adipose tissue volume.
How might these results change the direction of research or the focus of clinical practice?
- The results of this study shed some light on the endocrine function of human brown adipose tissue in humans. Whereas we observed that the cold-induced changes in the concentration of FGF21 are associated with brown adipose tissue volume, the changes in IL6, CXCL14, GDF15, and BMP8b concentration seem not to be related to the amount of brown adipose tissue, suggesting that other tissues might be responsible for secreting these molecules upon cold exposure.
INTRODUCTION
In mammals, adipose tissue can mainly be found in two different forms: white adipose tissue (WAT) and brown adipose tissue (BAT), with somehow opposite roles in whole-body energy metabolism [(1)]. Whereas the main role of WAT is to store and release energy, the main function of BAT is to produce heat to maintain body temperature [(1)]. Brown adipocytes are composed of multiple small lipid droplets and are rich in mitochondria expressing uncoupling protein-1 (UCP-1) [(2)]. UCP-1 offers an alternative pathway for protons to return to the mitochondrial matrix, generating heat instead of synthesizing ATP [(2)]. Although BAT has been long recognized as a thermogenic organ responsible for non-shivering thermogenesis, it was in 2007 when, thanks to the use of positron emission tomography-computerized tomography (PET-CT) technology, the presence of metabolically active BAT in adult humans was proposed and then finally confirmed in 2009 [(3)]. Some findings have suggested that BAT presence/activity is protective against obesity and related metabolic and cardiovascular diseases [(4)]. Thus, BAT is being investigated as a promising therapeutic target to treat obesity and related comorbidities.
BAT secretes multiple molecules, known as brown adipokines or batokines (hereafter referred to as batokines), which exert autocrine, paracrine, and endocrine functions [(5)]. The relevance of its endocrine function has been manifested by BAT transplantation in preclinical models. These BAT transplants have consistently resulted in weight loss, improvement of glucose homeostasis, and cardioprotective effects, among other benefits [(5)]. These beneficial effects can hardly be attributed to the thermogenic activity of BAT explants and are therefore hypothesized to be mediated by the BAT secretome. Thus, the identification of batokines in humans could represent a potential tool for the development of strategies to treat metabolic diseases such as obesity, diabetes, and cardiovascular diseases. Multiple preclinical studies have identified batokines such as chemokine ligand 14 (CXCL14) [(6)], growth differentiation factor 15 (GDF15) [(7)], fibroblast growth factor 21 (FGF21) [(8)], interleukin 6 (IL6) [(9)], and bone morphogenic protein 8b (BMP8b) [(10)]. However, translating findings from rodents to humans is particularly challenging when it comes to BAT physiology [(11)]. Therefore, whether the secretion of these batokines by human BAT significantly contributes to the systemic pool needs to be elucidated. The present study is grounded on the hypothesis that, upon cold exposure (the best stimuli to activate BAT in humans), BAT should release a certain amount of these batokines to the circulation that may be detectable in plasma. If this assumption is true, the plasma levels of cold-induced batokines should be associated with BAT volume, 18F-fluorodeoxyglucose (18F-FDG) uptake, and/or radiodensity.
Therefore, the present study aimed to determine the effect of a 2-h personalized cold exposure on the plasma levels of CXCL14, GDF15, FGF21, IL6, and BMP8b and their association with BAT volume, 18F-FDG uptake, and/or radiodensity in young humans.
METHODS
Study design
This study was conducted under the framework of the ACTIBATE (Activating brown adipose tissue through exercise) randomized controlled trial [(12, 13)], which was designed to study the effect of an exercise program on BAT volume and activity (ClinicalTrials.gov NCT02365129) [(13)]. All the measurements included in this manuscript were collected between September and November of 2016 and 2017 in Granada (Spain). The outdoor temperature during this period has been reported elsewhere [(13)]. Eligibility criteria were as follows: being 18 to 25 years old, reporting no more than 20 min of moderate-vigorous physical activity on a maximum of 3 days per week, absence of cardiometabolic disease, being a nonsmoker, not taking any medication affecting energy metabolism, and having stable body weight during the previous 3 months. This study was conducted following the last version of the Declaration of Helsinki, and the protocol and written informed consent were approved by the Ethics Committee on Human Research of the University of Granada (n° 924) and “Servicio Andaluz de Salud.”
The study design is summarized in Figure 1. A total of 30 participants (18 women and 12 men) were included in this study. Participants were 21.92 ± 2.1 years old, had a mean body mass index (BMI) of 24.9 ± 5.1 kg/m2, and had a mean fat mass percentage of 24.9% ± 8.3%. Additional characteristics are shown in Table 1. At 48 to 72 h before collecting blood samples and assessing BAT by a static 18F-FDG PET-CT, the participants' shivering threshold was determined. Then, a 2-h personalized cooling protocol was used to stimulate BAT activity immediately before the PET-CT scan. Blood samples were collected before and 1 h and 2 h after starting the cold exposure preceding the PET-CT to determine the plasma concentration of batokines.
| All (N = 30) | Men (n = 12) | Women (n = 18) | |
| Demographics | |||
| Age (y) | 21.92 (2.3) | 22.2 (2.5) | 21.8 (2.3) |
| Body composition | |||
| Weight (kg) | 72.9 (18.2) | 84.2 (21.1) | 65.4 (11.3) |
| Height (cm) | 171.1 (8.1) | 177.2 (5.4) | 166.2 (6.5) |
| BMI (kg/m2) | 24.9 (5.1) | 26.7 (6.4) | 23.7 (3.8) |
| Waist circumference (cm) | 82.3 (14.8) | 88.2 (18.9) | 78.3 (9.9) |
| Lean mass (kg) | 43.5 (10.4) | 53.6 (8.3) | 36.8 (4.4) |
| Fat mass (kg) | 25.4 (98.8) | 26.1 (13.1) | 24.9 (7.5) |
| Fat mass (%) | 34.9 (8.3) | 29.9 (9.1) | 38.4 (5.9) |
| VAT mass (g) | 364 (215) | 442 (244) | 313 (182) |
| Cardiometabolic risk factors | |||
| Glucose (mg/dL) | 89.6 (8.4) | 91.8 (10.7) | 88.2 (6.4) |
| Insulin (μU/mL) | 10.1 (9.3) | 13.8 (13.1) | 7.7 (4.4) |
| HOMA-IR | 2.4 (2.6) | 3.4 (3.7) | 1.7 (1.1) |
| Triglycerides (mg/dL) | 98.5 (69.3) | 128.1 (92.3) | 78.7 (40.6) |
| Cholesterol (mg/dL) | 171.0 (36.9) | 173.3 (46.7) | 169.4 (29.9) |
| HDL cholesterol (mg/dL) | 53.0 (12.9) | 44.2 (6.9) | 58.9 (12.6) |
| LDL cholesterol (mg/dL) | 98.7 (27.8) | 104.8 (34.2) | 94.7 (22.8) |
| Systolic BP (mmHg) | 117.7 (12.4) | 129.3 (9.6) | 110.7 (7.8) |
| Diastolic BP (mmHg) | 72.0 (7.3) | 76.4 (7.5) | 69.3 (5.8) |
| Fatty liver index | 24.3 (29.7) | 39.1 (37.7) | 14.48 (18.0) |
| Brown adipose tissue | |||
| BAT volume (mL) | 72.1 (67.0) | 67.8 (74.1) | 75.1 (63.9) |
| BAT SUV peak | 10.5 (7.3) | 8.2 (6.1) | 12.1 (7.8) |
| BAT mean radiodensity (HU)a | −59.9 (13.1) | −54.4 (11.5) | −57.3 (11.5) |
- Note: Results are shown as mean (standard deviation).
- Abbreviations: BAT, brown adipose tissue; BP, blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HU, Hounsfield units; LDL, low-density lipoprotein; SUV, standardized uptake value; VAT, visceral adipose tissue.
- a Sample size for this variable = 24 (9 men, 15 women).
Shivering threshold test
The participants' shivering threshold was assessed to then personalize the cold exposure used to activate BAT before the PET-CT scan. To do so, participants first entered a warm room (22.1°C ± 1.6°C) for 30 min. After that, participants were transferred to and remained seated in a room at 19.8°C ± 0.5°C, wearing a water-perfused cooling vest connected to a temperature-controlled chiller unit (Polar Products Inc.). The vest covered the clavicular, chest, abdominal, and back regions. The water temperature was initially set at 16.6°C and was progressively reduced every 10 to 15 min until shivering onset (determined visually and/or self-reported) or a water temperature of 3.8°C was reached. If participants did not shiver, they continued in the cold room, with the water temperature set at 3.8°C, additionally for 45 min. The individual shivering threshold was established as the temperature at which participants started to shiver [(14)].
Personalized cooling protocol
Two or three days after determining the shivering threshold, at the same time of the day, the personalized cooling protocol started with participants staying in a warm room (22.2°C ± 0.5°C) for 30 min. A peripheral catheter was inserted into the antecubital vein, and blood was collected before starting the personalized cooling protocol. Immediately after, they were moved into a cold room (20.2°C ± 0.3°C), where they stayed seated wearing the cooling vest with the water temperature set 4°C above the individual's shivering threshold. For those participants who did not shiver in the shivering threshold test, the water temperature was set at 3.8°C. After 1 h of cold exposure, another blood sample was extracted, a bolus of 18F-FDG (185 megabecquerel, MBq; ~2.8 MBq/kg) was injected, and the water temperature was increased by 1°C. If the participants started to shiver at any time, the water temperature was immediately increased by 1°C, and the participants were covered with a bathrobe until shivering disappeared. After 2 h of cold exposure, one last blood sample was obtained, and participants were transferred to another room where the PET/CT scan (Siemens Biograph 16 PET-CT system) was performed. Blood samples were collected in an EDTA-containing tube and were immediately centrifuged (10 min, 3000 rpm, 4°C). Plasma aliquots were then stored at −80°C until analyses.
PET-CT analysis
The CT image was obtained by applying 120 kV and 61 ± 11.2 mAs, for a matrix size of 512 × 512 × 198 × 1 and a pixel size of 0.98 × 0.98 × 1 mm. The PET image was obtained immediately after the CT using two 16-cm bed positions (6 min acquisition per bed position), from atlas vertebrae to mid-chest, with a matrix size of 168 × 168 × 65 × 1 and a pixel size of 4 × 4 × 5 mm. Both images were acquired using a Siemens Biograph 16 PET/CT. PET-CT scans were analyzed using the FIJI software as previously described [(14)], establishing the regions of interest (ROIs) from cervical vertebra 1 to thoracic vertebra 4 on both sides of the body (laterocervical, supraclavicular, mediastinum, and paravertebral). These ROIs were semiautomatically outlined and were computed as a single ROI, as extensively described elsewhere [(14)]. The standardized uptake value (SUV) was calculated as 18F-FDG uptake (kBq/mL)/(injected dose [kBq]/patient weight [g]). Then, an SUV threshold of 1.2/(lean body mass/body mass) and a radiodensity range of −190 to −10 were used to delineate BAT, following the BARCIST 1.0 recommendations [(15)]. Finally, BAT volume, SUV peak, and mean radiodensity were calculated by the software.
Plasma levels of batokines
The plasma levels of CXCL14, GDF15, FGF21, and BMP8b were determined using enzyme-linked immune-absorbent assay (ELISA) kits, according to manufacturer instructions. CXCL14 was determined by ELH-CXCL14-1 (RayBiotech; coefficient of variation [CV] = 9.2%), GDF15 by RD191135200R (Biovendor; CV = 6.8%), FGF21 by RD191108200R (Biovendor; CV = 7.11%), and BMP8b by MBS944757 (MyBioSource; CV = 8%). IL6 plasma levels were determined using XMAP technology by HSTCMAG-28SK (Luminex Corp.; CV = 11.5%).
Body composition
On a different day, body weight and height were measured using a SECA scale and stadiometer (model 799, Electronic Column Scale) while participants wore light clothing and were barefoot. Lean mass, fat mass, and visceral adipose tissue mass were assessed by dual-energy x-ray absorptiometry (Discovery Wi, Hologic). Waist circumference (WC) was measured twice with a non-elastic–plastic tape, and the mean value was obtained.
Cardiometabolic risk factors
Systolic and diastolic blood pressure were measured twice on three different days using an automatic sphygmomanometer Omron M2, and the average of these values was used for the analyses.
Statistical analyses
Descriptive data are presented as mean ± standard deviation unless otherwise stated. The effect of the personalized cooling protocol on the batokines' concentration was analyzed by repeated measures ANOVA. The samples in which the batokine concentration was below the range of detection (one individual for CXCL14, four for GDF15, six for IL6, and three for BMP8b) were given a value equal to half the magnitude of the lower end of the detection range and included in the analyses (although all the results remained unaltered when these participants were excluded from the analyses). FGF21 and IL6 did not follow a normal distribution. However, we observed similar results when performing the Friedman nonparametric test (data not shown). There were no Sex × Time interaction effects; therefore, the analyses were conducted combining men and women. However, exploratory analyses were conducted separately in men and women, and similar trends were observed (Figures S1 and S2).
To analyze the association between the cold-induced changes in batokine concentration and BAT-related variables, we calculated the change (Δ) in circulating levels of the batokines by subtracting the warm period value from the 1-h and 2-h cold exposure value. Then we analyzed, by simple linear regression (Model 1), the association between both the Δ and the warm period batokine concentrations, and BAT volume, SUV peak, and mean radiodensity. These associations were also tested in multiple linear regression adjusting for the PET-CT scan date (Model 2) as a proxy of the outdoor ambient temperature, sex (Model 3), BMI (Model 4), and sex and BMI together (Model 5). Similarly, we analyzed the association between the plasma levels of the batokines and body composition and cardiometabolic risk factors.
RESULTS
Cold exposure modulates plasma levels of batokines in young human adults
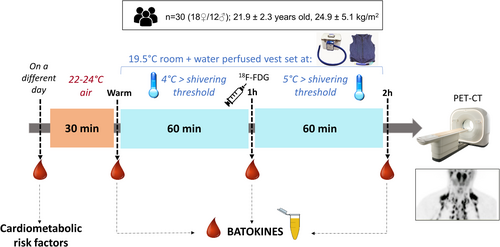
After 2 h of cold exposure, an increase in the concentration of CXCL14 (6.26 ± 2.18 vs. 6.84 ± 2.45 ng/mL, Δ = 9.3%, p = 0.007), GDF15 (290.40 ± 96.20 vs. 310.02 ± 102.82 pg/mL, Δ = 6.8%, p = 0.013), and FGF21 (146.42 ± 84.07 vs. 180.15 ± 122.28 pg/mL, Δ = 23%, p = 0.003) was observed. Moreover, plasma levels of IL6 were also upregulated after 1 h of cold exposure (6.40 ± 10.49 vs. 8.38 ± 12.86 pg/mL, Δ = 31%, p = 0.048). In contrast, circulating BMP8b levels were reduced after 2 h of cold exposure (367.23 ± 224.83 vs. 314.87 ± 216.73 pg/mL, Δ = −14.3%, p = 0.022) (Figure 2).
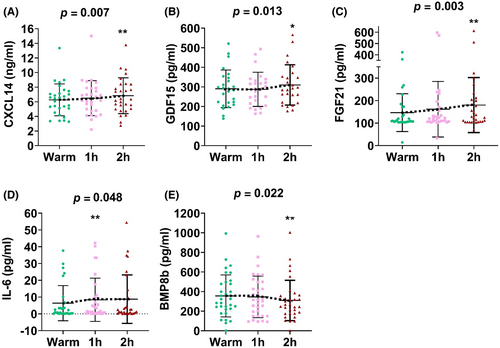
The cold-induced change in FGF21 levels, but not the other batokines, is associated with BAT volume
The cold-induced increase in FGF21 plasma levels after 1 h (β = 0.389, R2 = 0.426, p = 0.005) and 2 h (β = 0.456, R2 = 0.307, p = 0.001) of cold exposure was positively associated with BAT volume (Figure 3), and these results remained after adjusting for the PET-CT scan date, sex, and BMI (Tables S2 and S3). To confirm that these results were independent of warm period plasma levels, we repeated the analyses using fold change instead of the Δ, and the pattern remained, although the strength of association was attenuated (data not shown). In contrast, neither the FGF21 levels during the warm period nor the cold-induced change in its concentration was associated with BAT SUV peak or radiodensity (Figure 3).
On the other hand, plasma levels of GDF15 during the warm period were negatively associated with BAT mean radiodensity (β = −3.853, R2 = 0.223, p = 0.026; Table 2) but not BAT volume or 18F-FDG uptake, which persisted after adjusting for the PET-CT scan date, PET-CT scan date + sex, PET-CT scan + BMI, or sex + BMI together (Table S1). This contrasts with the lack of association between the cold-induced changes in GDF15 levels and all BAT-related variables (Table 2). The changes in plasma levels of IL6 and BMP8b after 1 h of cold exposure were associated with BAT mean radiodensity only when the analyses were adjusted for the PET-CT scan date, sex, or BMI (Table S2). In contrast, no associations were observed with the warm and 2-h change levels or any other BAT variable (Table 2). None of the other batokines was associated with any BAT-related parameter.
| CXCL14 (ng/mL) | GDF15 (pg/mL) | IL6 (pg/mL) | BMP8b (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | p | β | R2 | p | β | R2 | p | β | R2 | p | |
| Warm period | ||||||||||||
| BAT volume (mL) | 0.004 | 0.016 | 0.511 | 0.151 | 0.009 | 0.643 | 0.004 | 0.001 | 0.900 | 0.755 | 0.052 | 0.243 |
| BAT SUV peak | 0.043 | 0.021 | 0.453 | −1.688 | 0.012 | 0.589 | 0.099 | 0.005 | 0.717 | 3.028 | 0.010 | 0.612 |
| BAT radiodensity (HU) | 0.073 | 0.127 | 0.088 | −3.853 | 0.223 | 0.026 | 0.378 | 0.153 | 0.059 | −2.563 | 0.011 | 0.649 |
| Δ 1 h of cold exposure | ||||||||||||
| BAT volume (mL) | 0.001 | 0.004 | 0.742 | 0.014 | 0.001 | 0.910 | −0.007 | 0.018 | 0.480 | −0.055 | 0.002 | 0.814 |
| BAT SUV peak | 0.031 | 0.052 | 0.236 | 0.860 | 0.021 | 0.478 | −0.049 | 0.010 | 0.599 | 1.481 | 0.020 | 0.486 |
| BAT radiodensity (HU) | 0.025 | 0.069 | 0.215 | 1.214 | 0.118 | 0.118 | 0.132 | 0.152 | 0.059 | 3.003 | 0.154 | 0.079 |
| Δ 2 h of cold exposure | ||||||||||||
| BAT volume (mL) | 0.001 | 0.001 | 0.844 | 0.108 | 0.020 | 0.487 | −0.001 | <0.001 | 0.946 | 0.194 | 0.025 | 0.424 |
| BAT SUV peak | 0.002 | <0.001 | 0.953 | 2.482 | 0.116 | 0.089 | 0.043 | 0.002 | 0.803 | 1.030 | 0.008 | 0.643 |
| BAT radiodensity (HU) | 0.029 | 0.105 | 0.123 | 0.440 | 0.010 | 0.655 | 0.089 | 0.020 | 0.515 | 1.134 | 0.017 | 0.568 |
- Note: Unstandardized coefficient of regression (β), coefficient of determination (R2), and p value from simple regressions.
- Abbreviations: BAT, brown adipose tissue; BMP8b, bone morphogenic protein 8b; CXCL14, chemokine ligand 14; GDF15, growth differentiation factor 15; HU, Hounsfield units; SUV, standardized uptake.
Associations of plasma levels of batokines with body composition and cardiometabolic risk factors
The associations of the batokines concentration with body composition and cardiometabolic risk factors are shown in Tables S4 and S6. Levels of FGF21 during the warm period was positively associated with BMI, WC, lean mass, fat mass, visceral adipose tissue mass, circulating glucose and insulin levels, HOMA-IR, triacylglycerols, total cholesterol, LDL-C, systolic and diastolic blood pressure, and fatty liver index (Table S4). Despite these associations not being replicated with the cold-induced change in FGF21 levels 1 h after cold exposure (Table S5), we observed a positive association between the cold-induced change in FGF21 concentration after 2 h of cold exposure and insulin, HOMA-IR, triacylglycerols, total cholesterol, and LDL-C (Table S6). However, these associations disappeared after adjusting for FGF21 concentration during the warm period. On the other hand, levels of CXCL14 during the warm period were positively associated with fasting insulinemia (β = 1.606, R2 = 0.138, p = 0.048), LDL-C (β = 4.805, R2 = 0.139, p = 0.047), and systolic blood pressure (β = 2.467, R2 = 0.198, p = 0.018) (Table S4). Plasma levels of GDF15 during the warm period were also positively associated with insulin levels (β = 0.048, R2 = 0.272, p = 0.006) and HOMA-IR (β = 0.013, R2 = 0.248, p = 0.010) (Table S4).
DISCUSSION
The results of this study show that the levels of the five potential human batokines (CXCL14, GDF15, FGF21, IL6, and BMP8b) are modified by a 2-h cold exposure in young human adults. Moreover, the cold-induced change in FGF21 levels was positively associated with BAT volume. Overall, these results suggest that these five batokines may be part of the endocrine response to cold exposure in humans, with FGF21 possibly being secreted by BAT. However, more human studies are needed to clarify the role of BAT in the release of FGF21 and the rest of the batokines.
We first investigated whether the levels of the five potential batokines (CXCL14, GDF15, FGF21, IL6, and BMP8b) were modulated by a cooling protocol able to activate BAT in humans. We observed a cold-induced increase in the concentration of CXCL14, GDF15, IL6, and FGF21. We have previously shown that circulating levels of CXCL14 are increased in response to cold exposure in mice, and that BAT secretion is partially responsible for such an increase [(18)]. Importantly, this increase in CXCL14 was associated with improved glucose homeostasis, greater BAT activity, and WAT browning through the recruitment of alternatively activated, noninflammatory macrophages [(18)]. In the present human study, the cold-induced increase in plasma levels of CXCL14 was not associated with BAT volume, 18F-FDG uptake, or radiodensity. In contrast, García-Beltran et al. [(19)] reported that CXCL14 levels correlated positively with the area of active cervical BAT, although only in 1-year-old girls [(19)], which concurred with high CXCL14 gene expression levels in neonatal BAT [(19)]. Besides the age of the participants being an obvious key difference in our study, it should also be noted that García-Beltran et al. [(19)] used infrared thermography instead of PET-CT to evaluate BAT activity. While the extant literature suggests correlations between the two BAT imaging techniques to varying extents, differences in sensitivity and accuracy might impair the ability to replicate some findings with these two methods [(20-23)]. On the other hand, GDF15, a hormone that is currently being intensively investigated due to its potential role in appetite regulation, glucose homeostasis and lipolysis [(24)], seems to be secreted by murine brown adipocytes in response to cold exposure [(7)]. Here we observed a cold-induced increase in GDF15 levels, which would be compatible with a BAT secretion in humans. However, as for CXCL14, we did not observe consistent associations between the cold-induced increase in GDF15 concentration and any BAT-related variable. Finally, our analyses also showed an increase in IL6 plasma levels after 1 h of cold exposure. It is well known that brown adipocytes secrete IL6 [(9)], orchestrating a BAT-to-liver communication [(25)]. IL6 can promote WAT browning and BAT recruitment by activation of M2-type macrophage [(26)], and it seems to be a key mediator of the benefits associated with BAT transplantation in murine models [(27)]. However, we did not find consistent associations between the cold-induced changes in IL6 levels and BAT volume, 18F-FDG uptake, or radiodensity. It should be noted that skeletal muscle is a well-known secretor of IL6, and therefore cold-induced muscle activity (and IL6 release) might be confounding our results, even if our cooling protocol precluded shivering [(28)]. Thus, future studies are necessary to ascertain whether the cold-induced increases observed in this study are indeed due to the increased BAT secretion of these molecules.
FGF21 was one of the first described batokines [(8)] and has been shown to target several tissues and organs such as adipose tissue, the heart, and the liver [(29)]. In our study, we observed an increase in FGF21 levels in response to cold exposure with this increase being robustly associated with BAT volume. This finding concurs with a previous study showing an increase in plasma levels of FGF21 after 12 h of exposure to mild cold (19°C) air [(30)]. The same research group [(31)], and others [(32)], observed that this increase was more pronounced in BAT-positive than in BAT-negative participants. Moreover, another study found a positive association between the levels of FGF21 and BAT glucose uptake [(33)]. Overall, our results and previous studies suggest that FGF21 may be secreted by human BAT to an extent that may affect systemic circulation. However, it is known that BAT is not the only source of FGF21 during cold exposition. Reports using various cold-induced experimental setting in rodent models claimed distinct roles of liver and BAT as contributing to systemic FGF21 levels after cold exposure [(34)]. Therefore, further studies in humans are required to analyze the tissue(s) responsible for the increase of FGF21 in the circulation and its metabolic role. FGF21 modulates insulin sensitivity [(29)], protects the liver against steatosis [(35)], and exerts cardioprotective actions in mouse models of hypertension [(36)], among others. Therefore, it might seem paradoxical that we observed a positive association between the plasma levels of FGF21 during the warm period and several markers of adiposity and cardiometabolic risk, including BMI, WC, visceral adipose tissue mass, insulin, HOMA-IR, and cholesterol. Consistent with the results observed in our study, others have also reported increased FGF21 levels in individuals with obesity and a positive correlation with adiposity, insulin, and triglycerides [(37)]. This paradox between the beneficial effects of FGF21 on metabolism and the observed augmented circulating levels in human adults with overweight and obesity might be explained by an “FGF21-resistant” state in these individuals [(38)]. Further studies with a larger sample are needed to clarify not only the role of BAT in FGF21 secretion but also its impact on human cardiometabolic health.
In contrast with the changes observed in the rest of the batokines, we observed a cold-induced decrease in BMP8b. BMP8b seems to be secreted by brown adipocytes according to mice and in vitro studies [(10)]. However, its role in BAT activity is controversial [(39)]. Whereas Urisarri et al. [(40)] observed a positive association between BMP8b circulating levels and BAT thermogenic response, measured with infrared thermography after cold exposure in neonates, Garcia-Beltran et al. [(39)] did not observe any correlation in 1-year-old infants, despite reporting a high BMP8b expression in BAT compared to WAT in neonates. The apparent discordance between previous studies and our results might be due to several reasons. Firstly, most BAT-derived BMP8b may exert autocrine and paracrine roles [(41)], with negligible contributions to the circulatory pool, at least in human adults. Second, a longer cooling protocol may yield different results, taking into account that a 2-h cooling period is likely too short to reflect changes in protein expression [(42)]. Third, changes in the levels of BMP8b cannot be interpreted exclusively as a function of BAT secretion, as other tissues also express this protein (https://www.proteinatlas.org/ENSG00000116985-BMP8B/tissue). Either a reduction of BMP8b secretion by other organs or an increase in degradation would also explain why its blood levels are reduced despite a presumable increase in BAT secretion. Finally, the distinct methods used for assessing BAT (PET-CT vs. infrared thermography) are not comparable and may be responsible for the observed discrepancies among studies.
In this study, we aimed to investigate the modulation of selected batokines after BAT thermogenic stimulation in humans. We hypothesized that if BAT secretes a molecule to a sufficient extent to impact the systemic circulating pool, the concentration of this molecule should increase after BAT activation by cold exposure. Likewise, such an increase in its concentration should correlate with BAT volume and/or activity. Although the confirmation of these hypotheses would be highly suggestive of a physiologically relevant secretion of this molecule by BAT, the refutation of the hypotheses does not disprove this possibility. Firstly, it should be considered that despite BAT being unequivocally able to secrete these molecules [(5)], no batokine identified to date is exclusively secreted by BAT [(34)]. Secondly, it should be considered that despite that the static 18F-FDG PET-CT scan is the most widely used technique for assessing BAT volume and function [(43)], it presents several limitations for assessing BAT metabolic activity [(4)]. Moreover, even if the 18F-FDG PET-CT provided an accurate assessment of BAT thermogenic activity, it should not be assumed that the tissue endocrine activity is necessarily proportional to its thermogenic activity. Finally, as mentioned earlier, the 2-h cooling protocol may not be long enough to elicit a significant secretion of some batokines, if such secretion is largely dependent on protein expression (as opposed to secretion of previously synthesized and stored proteins). Nonetheless, even if testing the two proposed hypotheses cannot rule out that human BAT significantly secretes these molecules, we found that the analyses of FGF21 support both hypotheses, thus suggesting that BAT might significantly contribute to the circulatory pool in adult humans.
Besides the study limitations mentioned before, it should be considered that this study investigated an arbitrarily selected group of potential batokines. Several other molecules have been suggested to be secreted by human BAT and were not investigated in this study [(5)]. On the other hand, althought the largest BAT depots are contained within the region covered by the PET-CT in our study, other depots are not (e.g., suprarenal BAT depots) [(43)]. Moreover, the use of other radiotracers (e.g., [15O]-O2 or 11C-acetate) or imaging methods (magnetic resonance, dynamic PET acquisition) might be more adequate to assess BAT function than the static 18F-FDG PET-CT performed in this study. That might be of special relevance when considering individuals that do not exhibit 18F-FDG uptake by BAT, since it is known that some of these individuals have BAT that is detectable by other methods [(44, 45)]. Lastly, it should be noted that the absence of a control condition limits our ability to analyze the cold-induced effect on circulating batokines.
In summary, we found that a 2-h personalized cold exposure protocol increases plasma levels of CXCL14, GDF15, FGF21, and IL6 in young adults. The observed association between the cold-induced increase in FGF21 and BAT volume suggests that human BAT may secrete this molecule to an extent able to impact the circulating pool, although further studies are needed to confirm this hypothesis and the potential applications of personalized cold exposure protocols in obesity and diabetes management.
ACKNOWLEDGMENTS
This study was performed as part of a Doctoral Thesis conducted within the Official Doctoral Program in Biomedicine of the University of Granada, Spain. Data can be made available upon reasonable request to the corresponding author(s), subject to a data transfer agreement.
FUNDING INFORMATION
The study was supported by the Spanish Ministry of Economy and Competitiveness via Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393) and PTA 12264-I, Retos de la Sociedad (DEP2016-79512-R), and European Regional Development Funds (ERDF), by the Spanish Ministry of Education (FPU13/04365, FPU16/02828, FPU16/03653), the Fundación Iberoamericana de Nutrición (FINUT), the Redes Temáticas De Investigación Cooperativa RETIC (Red SAMID RD16/0022), Fundación Alfonso Martin Escudero, AstraZeneca HealthCare Foundation, the University of Granada Plan Propio de Investigación 2016-Excellence actions: Unit of Excellence on Exercise and Health (UCEES), the University of Granada Plan Propio de Investigación 2018-Contrato Perfeccionamiento de Doctores, Plan de Resilencia UGR: Ayudas Margarita Salas, and by the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF, SOMM17/6107/UGR), and Consejería de Economía, Conocimiento, Empresas y Universidad (P18-RT-4455). Funding for the open access charges provided by the Universidad de Granada / CBUA.
CONFLICT OF INTEREST STATEMENT
Guillermo Sanchez-Delgado participates in studies funded by Eli Lilly and Co and Novo Nordisk A/S, has served as a speaker for Eli Lilly and Co, and has received equipment support from Maastritch Instrument Inc., Cosmed, ParvoMedics, and Vyaire. The other authors declared no conflict of interest related to this work.





