A ketogenic diet lowers myocardial fatty acid oxidation but does not affect oxygen consumption: a study in overweight humans
Abstract
Objective
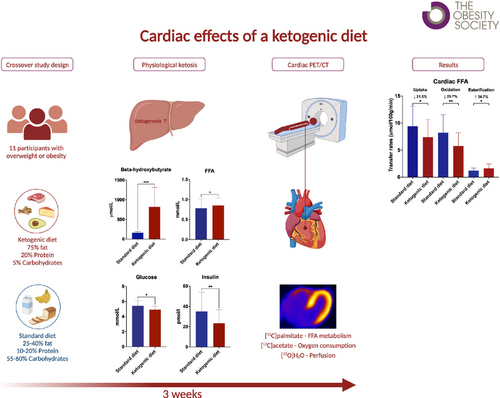
A ketogenic diet (KD) characterized by very low carbohydrate intake and high fat consumption may simultaneously induce weight loss and be cardioprotective. The “thrifty substrate hypothesis” posits that ketone bodies are more energy efficient compared with other cardiac oxidative substrates such as fatty acids. This work aimed to study whether a KD with presumed increased myocardial ketone body utilization reduces cardiac fatty acid uptake and oxidation, resulting in decreased myocardial oxygen consumption (MVO2).
Methods
This randomized controlled crossover trial examined 11 individuals with overweight or obesity on two occasions: (1) after a KD and (2) after a standard diet. Myocardial free fatty acid (FFA) oxidation, uptake, and esterification rate were measured using dynamic [11C]palmitate positron emission tomography (PET)/computed tomography, whereas MVO2 and myocardial external efficiency (MEE) were measured using dynamic [11C]acetate PET.
Results
The KD increased plasma β-hydroxybutyrate, reduced myocardial FFA oxidation (p < 0.01) and uptake (p = 0.03), and increased FFA esterification (p = 0.03). No changes were observed in MVO2 (p = 0.2) or MEE (p = 0.87).
Conclusions
A KD significantly reduced myocardial FFA uptake and oxidation, presumably by increasing ketone body oxidation. However, this change in cardiac substrate utilization did not improve MVO2, speaking against the thrifty substrate hypothesis.
Study Importance
What is already known?
- A ketogenic diet (KD) is recognized for its capacity to induce weight loss, attributed initially to glycogen store depletion and later to increased satiety, reduced appetite, and presumably increased lipid utilization.
- It is unknown to what extent organs rebalance oxidation of lipids during the KD and whether this may have advantageous effects beyond weight loss.
- According to the “thrifty substrate hypothesis,” oxidation of ketone bodies could be oxygen efficient and thus cardioprotective.
What does this study add?
- The KD reduced myocardial fatty acid oxidation and uptake by 25%, indicating increased ketone body oxidation.
- Importantly, this modification in cardiac substrate usage did not have an impact on cardiac energetics, challenging the notion of the thrifty substrate hypothesis.
How might these results change the direction of research or the focus of clinical practice?
- The beneficial effects of the KD are probably not caused by changes in myocardial substrate use but rather by weight loss and as yet unknown metabolic adaptations in other organs and tissues.
INTRODUCTION
A ketogenic diet (KD) typically requires a carbohydrate intake under 5% to 10% of energy intake, resulting in decreased insulin levels, increased lipolysis, elevated levels of circulating free fatty acids (FFA), and subsequently, increased conversion of FFA into ketone bodies in the liver. Depending on the proportion of carbohydrates, lipids, and protein, KDs may result in circulating ketone body levels ranging from 0.5 to values exceeding 5 mM [(1)]. KDs have been used to treat drug-resistant epilepsy in children [(2)], brain oncology [(3)], Alzheimer's disease [(4)], and type 2 diabetes (T2D) [(5)]. Recently, KDs have also gained more widespread popularity as a way to sustain ketosis, which has been postulated to have numerous benefits for overall health and cardiac function in particular. Thus, it has been hypothesized that the cardioprotective effects of sodium-glucose cotransporter 2 (SGLT2) inhibition are caused less by the lowering of glucose and more by the moderate ketosis that patients treated with SGLT2 inhibitors usually exhibit [(6)]. According to the “thrifty substrate hypothesis,” generating ATP from ketone bodies requires less oxygen than generating the same amount of ATP from fatty acid oxidation [(7)]. As a result, ketone bodies may function as a fuel that conserves oxygen in tissues that is exposed to periodic high-energy demands and reduced blood flow. This adaptive response to an apparently more energetically efficient substrate could potentially explain why patients with heart failure (HF) gradually transition from relying on fatty acids to using ketone bodies for myocardial fuel [(8, 9)]. Therefore, it is possible that the decrease in myocardial fatty acid utilization and subsequent increase in ketone body utilization observed in patients with HF [(10, 11)] and diabetes [(12)] is not a maladaptive pathophysiological process but merely reflects a substrate hierarchy with ketone bodies as a preferred cardiac fuel over fatty acids or glucose.
We have previously demonstrated that an exogenous infusion of ketone bodies results in a halving of myocardial glucose uptake even in the presence of hyperinsulinemia [(13)] and that a modest SGLT2 inhibition induced increase in ketone bodies (to ~0.3 mM) in patients with T2D and reduced postabsorptive myocardial glucose uptake [(14)]. In both studies, it was inferred that the increased levels of circulating ketone bodies also resulted in an increase in cardiac ketone body uptake. However, in the SGLT2 study, we failed to observe a significant decrease in myocardial fatty acid metabolism or oxygen consumption, which would appear to challenge the thrifty substrate hypothesis. However, the ketosis achieved by SGLT2 inhibition is usually in the range from 0.3 to 0.7 mM [(15)], which is modest compared with the ketosis that can be achieved by a KD, prolonged fasting or intake of exogenous ketone supplements.
Therefore, the primary aim of this study was to investigate whether a physiological and more pronounced ketosis (~1 mM) obtained by ketogenic dieting results in decreased myocardial fatty acid consumption and supports the existence of a myocardial substrate hierarchy. As a secondary objective, our aim was to examine whether transitioning from fatty acids to ketone bodies as the primary cardiac fuel was linked to enhanced cardiac energetics and function, as indicated by a reduction in myocardial oxygen consumption (MVO2), an increase in myocardial external efficiency (MEE) or an improved myocardial flow reserve (MFR).
METHODS
Trial design and participants
The study was a randomized controlled crossover trial consisting of two periods with a 3-week KD followed by a 3-week standard diet (SDD) or vice versa with a 1-week washout period in between the two periods. Both diet periods were concluded with a positron emission tomography (PET) study day during which myocardial FFA metabolism was measured by [11C]palmitate PET/computed tomography (CT), MFR by [15O]H2O PET/CT, and myocardial oxygen consumption MVO2 and MEE by [11C]acetate PET/CT. All participants underwent screening before inclusion, which involved collection of demographic data, clinical history, height, weight, blood pressure, pulse, electrocardiogram (ECG), blood tests, and a whole-body DXA scan (Horizon, Hologic, and Discovery). Informed written consent was obtained from participants who met the inclusion criteria. The study protocol was approved by The Central Denmark Region Committees on Health Research Ethics (1-10-72-232-18) and was conducted in accordance with the principles of the Declaration of Helsinki.
Inclusion criteria were (1) age 50 to 70 years and (2) BMI 28 to 40 kg/m2. Exclusion criteria were (1) pharmacological treatment that could affect outcome measures such as insulin, antidiabetics, statins or β-blockers; (2) HbA1c >48 mmol/mol; (3) impaired cardiac function; (4) impaired renal function (estimated glomerular filtration rate <60 mL/min); (5) liver disease (alanine aminotransferase >3 times of the upper limit of 70 U/L); (6) active or prior cancer; (7) anemia (hemoglobin <6.5 mmol/L), (8) blood donations within the last 3 months; (9) participation in other studies involving radioactive isotopes within the last 6 months; and (10) alcohol abuse.
Dieting periods
All participants received an individualized KD plan adjusted for calorie intake based on sex, weight, and approximate activity level. Each diet plan included options for every meal, ensuring a macronutrient distribution of 5 E% carbohydrates, 20 E% protein, and 75 E% fat. These diet plans were created by a dietitian using Vitakost Pro, a professional dietary tool that uses the Danish Food database. A low glycemic index source of carbohydrate was recommended during the KD. Fat intake ranged from 200 to 300 g, with an even distribution between saturated and monounsaturated fatty acids. Participants were responsible for buying ingredients, preparing meals, and adhering to the diet in their usual environment. During the SDD, participants were instructed to eat with a macronutrient distribution following the Nordic Nutritional Recommendations (45–60 E% carbohydrates, 10–20 E% protein, 25–40 E% fat). The participants were randomly assigned in a 1:1 ratio to receive either KD or an SDD in the first study period. To ensure compliance, participants were instructed to measure their -hydroxybutyrate (OHB) levels using a flash system (Freestyle Libre; Abbott, Witney, UK) every morning (at 7 am) and evening (at 7 pm) throughout both diet periods. They were required to provide daily reports of the results and their dietary progress. Participants who failed to increase their OHB levels to 0.3 mmol/L during the first week of the KD would be excluded. They were instructed to maintain their usual activities to ensure comparable activity levels during both periods. After each period, participants were examined at the Department of Nuclear Medicine and PET Center, Aarhus University Hospital, Aarhus N, Denmark, after an overnight fast. Participants were instructed to abstain from alcohol consumption during the KD and from strenuous physical activity for 72 h before each study day.
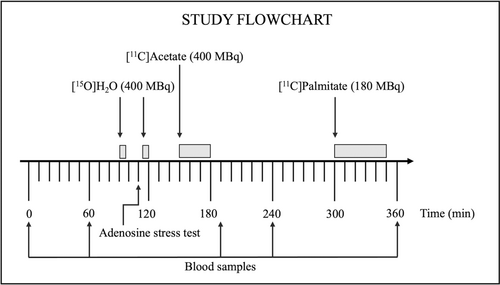
PET study day
Dynamic cardiac PET/CT scans were performed using a Biograph Vision 600 PET/CT system (Siemens, Erlangen, Germany). The duration of the PET/CT was planned to allow for radiotracer decay (Figure 1). The PET scanning protocols can be found in the online Supporting Information. In short, myocardial blood flow (MBF) during rest and hyperemia, the latter obtained by infusing the vasodilator adenosine, was measured with [15O]H2O PET. Afterwards, [11C]acetate PET was performed to measure MVO2 and MEE, and lastly, myocardial FFA metabolism was measured with [11C]palmitate PET.
PET image analysis
Left ventricular mass (LVmass) and forward cardiac output (FCO) were obtained from the PET data set, whereas mean arterial pressure was measured manually. Rest and stress MBF were measured by [15O]H2O and analyzed using a previously described method allowing for highly automated calculation of parametric MBF imaging [(20)]. In brief, parametric MBF images were generated using cluster analysis and implementation of a basis function method of the single-tissue model with additional right ventricle spillover correction. All parametric images were automatically segmented according to the 17-segment model advocated by the American Heart Association [(21)].
Blood samples
Blood samples for measurement of glucose, OHB, FFA, lactate, and insulin were collected four times (t = 0, 60, 190, 240, and 360 min) during the study day. Total cholesterol, high-density lipoprotein, low-density lipoprotein, triglyceride, hemoglobin, and erythropoietin were measured at baseline (t = 0 min). Glucose was analyzed after sampling using YSI glucose analyzer (YSI Life Sciences, Yellow Springs, Ohio). The OHB samples were stored at −20°C, and other samples were stored at −80°C until batch analysis. After the study day, measurements of OHB concentrations were quantified in plasma using hydrophilic interaction liquid chromatography–tandem mass spectrometry (HILIC-MS/MS) [(22)]. Serum FFA concentrations were analyzed with an enzymatic colorimetric method assay for nonesterified fatty acids (NEFA)-HR (Wako Chemicals Europe GmbH, Neuss, Germany), lactate concentrations with immobilized enzyme biosensor technology (YSI 2300 model Stat Plus; YSI Life Sciences), and insulin with Insulin ELISA (Mercodia AB, Uppsala, Sweden).
Statistics
Statistical analyses and graphs were generated using Stata 14 (College Station, Texas) and GraphPad Prism 9 (San Diego, California). Data presented in the text are expressed as mean ± standard deviation (SD) or median with 95% confidence interval (CI). Data in the graphs are displayed as mean ± SD or median (interquartile range [IQR]). To evaluate normal distribution, Q–Q plots were examined. Data were assessed by performing paired t tests or Wilcoxon signed rank tests comparing the effects of KD with the effects of SDD. A repeated measures mixed model with time, treatment, and order as fixed factors and participant ID as random effect was used to compare changes in hormone and substrate concentrations during the study day (baseline to 240 min). The model's assumptions were validated by examining scatter- and Q–Q plots of the predicted versus fitted residuals. When required, data were logarithmically transformed. Interaction between time and treatment are shown as “interaction”; main effects are shown as “treatment,” “time,” or “order,” followed by the corresponding p value. A p value of <0.05 was deemed significant. The primary endpoint was the MFAO rate measured with the tracer [11C]palmitate. To detect a clinically relevant reduction in FFA oxidation of 20%, a prestudy power calculation resulted in a study sample size of n = 10. The remaining outcomes were established as secondary measures in our research protocol.
RESULTS
Participants
A total of 12 participants were recruited between August 1, 2021, and August 31, 2022. One participant had a severe bout of migraine on the first study day and was excluded from the study. One participant received an antihypertensive drug (losartan), and one participant received an anticoagulant (rivaroxaban). Both medications were considered not to impact the results. In total, 11 participants (six women and five men) with a mean age of 56.6 ± 5.8 years (range 50–68 years) and a mean BMI of 32.5 ± 4.1 kg/m2 (range 28.2–40.6 kg/m2) were included. All participants achieved a D-OHB level of 0.3 mM within the first week and maintained levels above 0.5 throughout the 3 weeks (Figure 2). As a result, no participants were excluded. The adenosine stress test was discontinued in two participants due to intolerable side effects, and only nine full data sets of [15O]H2O PET scans were available for analysis. In one participant, synthesis of [11C]palmitate failed, and 10 fully completed scans were therefore available. The participant profile at screening and after the two diets is presented in Table 1. No differences in heart rate or blood pressure were observed during the study day (Figure S1).

| Screening | KD | SDD | p value | |
|---|---|---|---|---|
| Age (y) | 56.6 5.8 | |||
| HbA1c (mmol/L) | 36.7 6.2 | |||
| Weight (kg) | 96.4 12.1 | 93.7 12.2 | 95.9 12.1 | < 0.05 |
| BMI (kg/m2) | 32.5 4.1 | 31.6 4.5 | 32.3 4.3 | 0.02 |
| Fat mass (kg) | 36.3 9.2 | 35.1 9.8 | 36.2 9.5 | < 0.05 |
| Lean mass (g) | 57.9 10.6 | 56.5 10.1 | 57.9 10.2 | < 0.05 |
| Fat percent (%) | 37.6 7.9 | 37.2 8.4 | 37.5 7.9 | 0.45 |
| Cholesterol (mmol/L) | 5.8 (5.0–6.4) | 5.3 (3.9–5.8) | 5.3 (4.3–6.3) | 0.60 |
| HDL (mmol/L) | 1.5 0.3 | 1.3 0.4 | 1.4 0.42 | 0.12 |
| LDL (mmol/L) | 3.4 0.8 | 3.7 1.1 | 3.3 0.7 | 0.26 |
| Triglyceride (mmol/L) | 1.6 0.8 | 0.9 0.2 | 1.4 0.7 | 0.03 |
| Hemoglobin (mmol/L) | 8.8 0.7 | 8.4 0.7 | 8.3 0.8 | 0.40 |
| Erythropoietin (U/L) | 10.3 5.7 | 8.6 2.7 | 10.3 4.7 | 0.23 |
- Note: Comparison is made between the two diet periods. P values were calculated using paired samples t test and Wilcoxon rank sum test (cholesterol). Values are shown as mean SD or median (95% CI).
- Abbreviations: HDL, high-density lipoprotein; KD, ketogenic diet; LDL, low-density lipoprotein; SDD, standard diet.
Metabolic variables
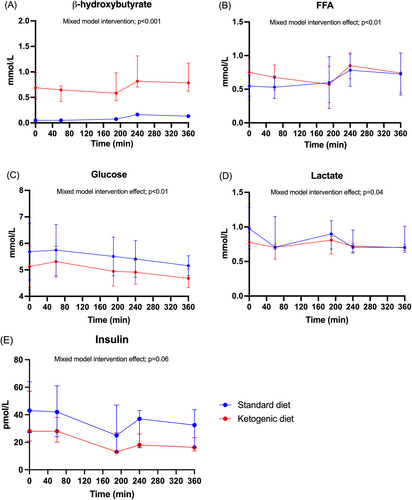
At the time of the [11C]palmitate PET/CT, plasma OHB concentrations were consistently higher during the KD compared with the SDD (1123 911 vs. 170 65 mol/L, p < 0.01) and increased during the study day with both interventions (intervention: p < 0.001; time effect: p < 0.001; order: 0.6; interaction: p = 0.02) (Figure 3A). Plasma FFA concentrations were higher during the KD compared with the SDD (0.9 ± 0.2 vs. 0.8 ± 0.2 mmol/L) and increased during the study day with both interventions (intervention: p < 0.01; time effect: p < 0.01; order: p = 0.01; interaction: p = 0.2) (Figure 3B). FFA concentrations were lower during the SDD for participants who completed the KD first (Figure S2), which is the opposite of what would have been expected if there had been a carry-over effect. Plasma glucose was consistently lower during the KD compared with the SDD (4.9 ± 0.5 vs. 5.4 ± 0.7 mmol/L) and remained stable throughout the study day with both interventions (intervention: p < 0.01; time effect: p = 0.01; order: p = 0.06; interaction: p = 1.0) (Figure 3C). Plasma lactate concentrations were lower during the KD compared with the SDD (intervention: p = 0.04; time: p = 0.05; order: p = 0.2; interaction: p = 0.8) (Figure 3D). Plasma insulin concentrations were similar on the KD and SDD and decreased during the study day with both interventions (intervention: p = 0.06; time effect: p < 0.01; order: p = 0.01; interaction: p = 0.9) (Figure 3E). Insulin concentrations were also lower during the SDD for participants who completed the KD first (Figure S2).
Myocardial [11C]palmitate metabolism
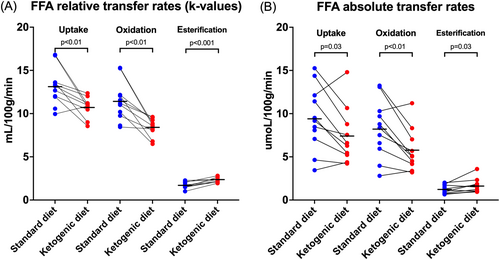
The KD reduced the relative MFAO rate by 26.3% (8.4 1.1 vs. 11.4 2.3 mL/100 g/min, p < 0.01) and the relative MFAU rate by 18.3% (10.7 1.2 vs. 13.1 2.2 m/100 g/min, p < 0.01), whereas it increased the relative MFAE rate by 34.9% (2.3 0.3 vs. 1.7 0.4 mL/100 g/min, p < 0.001) (Figure 4A). Similarly, the KD reduced the absolute MFAO rate by 29.7% (5.8 2.5 vs. 8.2 3.3 mol/100 g/min, p < 0.01) and the absolute MFAU rate by 21.5% (7.4 3.3 vs. 9.4 3.7 mol/100 g/min, p = 0.03) and increased the absolute MFAE rate by 34.7% (1.6 0.8 vs. 1.2 0.5 mol/100 g/min, p = 0.03) (Figure 4B).
MVO2, MEE, left ventricular mass, and ejection fraction
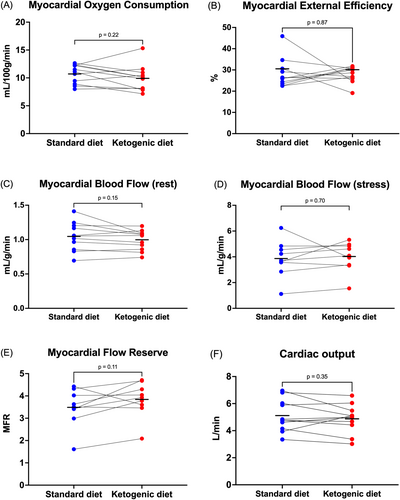
The KD did not significantly change MVO2 (10.0 2.3 vs. 10.7 1.7 mL/100 g/min, p = 0.20) or MEE (27.9% 3.8% vs. 28.3% 6.9%, p = 0.87) (Figure 5A,B) and did not significantly affect left ventricular mass (111.7 3.1 vs. 114.1 30.8 g, p = 0.68) or ejection fraction (EF) (65.8% 5.9% vs. 66.0% 5.8%, p = 0.88).
MBF
The KD did not significantly affect resting MBF (1.0 0.1 vs. 1.15 0.2 mL/g/min, p = 0.15), stress MBF (4.0 1.2 vs. 3.9 1.4 mL/g/min, p = 0.70), MFR (3.8 0.8 vs. 3.5 0.8, p = 0.11), or cardiac output (CO) (4.9 1.0 vs. 5.1 1.2 L/min, p = 0.35) (Figure 5C–F). Because cardiac work affects MBF, we also compared MBF at rest normalized to the rate pressure product (RPP) (10,000/(pulse × systolic blood pressure)). Again, no difference in RPP-normalized MBF at rest was observed (1.3 0.2 vs. 1.4 0.2 mL/g/min, p = 0.55).
DISCUSSION
It has recently been debated whether substituting glucose and fatty acids for ketone bodies as a cardiac substrate improves cardiac energetics, lowers oxygen consumption, or increases perfusion. The notion of ketone bodies as a favorable “thrifty” substrate has been driven by observations of decreased cardiac mortality and morbidity in patients treated with SGLT2 inhibitors experiencing mild ketosis, as well as cross-sectional observations of increased cardiac ketone body consumption and decreased FFA oxidation in patients with HF [(8)]. However, there is no consensus that reducing cardiac FFA oxidation is a beneficial adaptive process because metabolism of FFA yields large amounts of calories necessary to maintain cardiac function, especially in patients with insulin resistance. Thus, the aim of this study was to determine if endogenous ketosis in individuals who are healthy but have overweight obtained by a 3-week KD shifts a substantial amount of cardiac energy consumption from fatty acids towards ketone bodies and if this metabolic shift alters MVO2 and MEE. Our main finding was that the KD resulted in a significant decrease in MFAO and MFAU despite increased circulating FFA, presumably due to increased cardiac ketone body uptake. However, this shift in cardiac energy substrates during sustained ketosis was not accompanied by detectable changes in MVO2, MEE, or MBF. We also observed a discrete but significant increase in myocardial FFA channeled towards esterification and storage, a potentially harmful effect of the KD.
By design, the very high-fat, low-carbohydrate KD used in the current study resulted in increased levels of circulating lipids in the form of FFA. Because cardiac FFA availability is generally thought to be the main driver of MFAU [(23)] and healthy individuals derive more than 70% of cardiac energy needs from lipids [(8, 24, 25)], increased MFAU and MFAO should be expected if the heart prioritizes FFA as a source of energy regardless of the presence of other substrates. However, this was not the case in the present study. In fact, MFAO decreased from 8.21 to 5.77 μmol/100 g/min or ~30%, a substantial decrease in FFA–derived energy. Cardiac workload did not differ between the two diets. Consequently, the decrease in energy derived from FFA must have been counterbalanced by other substrates. Both plasma glucose and lactate decreased during the KD, leaving only increased availability of ketone bodies to compensate the energy gap. In line with this, ketone body levels increased on average more than five-fold during ketogenic dieting and reached levels significantly above those observed during SGLT2 inhibition [(14)]. Considering that ketone bodies are metabolized in a concentration-dependent manner in the brain [(26)], it is plausible that a similar pattern exists for the heart. Thus, in the presence of an ample supply of both fatty acids and ketone bodies, our present data show that the healthy heart appears to prioritize the latter for uptake and oxidation during a KD. We have previously demonstrated that OHB are also preferred by the heart over glucose in normal volunteers even during conditions of hyperinsulinemia [(13)]. Thus, in the hierarchy of cardiac energy substrates, ketone bodies appear to reside on top.
However, whether oxidizing ketone bodies at the expense of fatty acids and glucose is physiologically advantageous or simply reflects the omnivorous and metabolically flexible nature of the heart is not entirely clear; results from both previous studies and the current one appear to conflict on this point, but our study was done in people with normal cardiac function.
First, it has been demonstrated in cross-sectional studies that the failing heart increasingly uses ketone bodies as a source of energy [(11, 27)]. During HF, ATP production by glucose oxidation decreases, whereas the less-efficient energy production by glycolysis increases [(28)]. Moreover, FFA oxidation decreases in tandem with the increased glycolysis in patients with ischemic HF [(10)]. Murashige et al. demonstrated that the failing heart compensates for this loss of glucose and fatty acids as sources of energy by increasing myocardial ketone body oxidation up to 16% of total cardiac ATP generation [(8)]. This process may be adaptive as reflected by the increased expression of ketolytic enzymes BDH1 and SCOT in the failing heart [(9)]. In summary, these observations demonstrate that the failing heart is capable of switching from fatty acids to ketone bodies as a source of energy, but they do not show whether this metabolic switch per se is associated with beneficial cardiac effects. In fact, several drug regimens have been developed specifically to not decrease but rather increase MFAO [(29, 30)].
Second, from animal studies, it has been hypothesized that ketone bodies may not only substitute for fatty acids calorie by calorie but that generating ATP from ketone bodies requires less oxygen than generating the same amount of ATP from FFA oxidation [(31, 32)]. This notion was postulated for application in humans as well, and the “thrifty substrate hypothesis” was put forward as an explanation to the striking reduction in cardiac risk during mild ketosis induced by SGLT2 inhibition [(6)]. We, therefore, investigated whether substituting fatty acids by ketone bodies affected cardiac oxygen in vivo by employing [11C]acetate PET, which is a robust method to assess both MVO2 and MEE [(33, 34)]. MEE is a measure of cardiac efficiency to generate external work for a given consumption of oxygen and takes into account both how much oxygen is used and the conditions under which oxygen consumption is measured [(35)]. In our present study, neither MEE nor MVO2 was affected by the KD, and switching from fatty acids to ketone bodies did not appear to confer any benefits in terms of cardiac energetics, at least in healthy individuals who have overweight. However, even if all fatty acids were replaced by ketone bodies as a source of myocardial energy, this would only reduce oxygen consumption by ~5% as calculated by the equations given as the foundation for the thrifty substrate hypothesis [(6)]. Our study was clearly not powered to detect such subtle changes, even though they may be physiologically relevant. Whether ketone bodies measurably impact MEE in patients with HF cannot be inferred from this study.
Third, we investigated whether sustained ketosis would impact myocardial perfusion in a manner similar to what we have observed during infusions of exogenous ketone salts [(13, 36)], wherein both perfusion and CO increased in patients with HF and healthy volunteers. In the current study, no effect was observed in either parameter. It is possible that a higher concentration of OHB may be needed to observe an increase in MBF in healthy individuals. Furthermore, chronic elevation of ketone bodies may trigger regulatory mechanisms that counteract their putative vasodilatory effects. Such an effect would be analogous to the development of tolerance within 12–24 h that occurs with prolonged treatment with nitrates [(37)].
The potential adverse cardiovascular effects of a KD should be acknowledged. These are both related to processes in the circulation and in the heart. During the initial weeks of the KD, circulating low-density lipoprotein cholesterol levels may increase substantially [(38)], adding to the risks of acute ischemic events. Endothelial dysfunction may also be exacerbated within the first few days of the diet [(39)]. In the heart, we observed a 25% increase in FFA esterification, which may have several adverse effects. Increased MFAE may lead to overproduction of reactive oxygen species (ROS) beyond the body's scavenging capacity, causing mitochondrial damage [(40, 41)]. Furthermore, accumulation of lipids in the heart has been shown to precipitate the development of both metabolic and contractile dysfunction [(42)]. Conversely, ketone body oxidation has been found to reduce oxidative stress and the production of ROS by inhibiting histone deacetylase [(43, 44)]. It is uncertain whether this increase in ketone body oxidation might offset some of the negative effects of increased lipid storage in the heart during a KD. Moreover, there may be variations in the cardiovascular risks associated with a KD between individuals with no underlying health conditions and those with existing cardiac diseases.
The current study was powered to evaluate changes in cardiac FFA metabolism, which may have limited our ability to detect more subtle changes in secondary endpoints. Second, no measurements of ketone body, glucose, or lactate oxidation in the heart were performed. However, the heart's utilization of glucose and lactate is typically low [(8)], and this study observed a decrease in circulating levels of both substrates. Therefore, it is unlikely that the decrease in MFAO is attributable to displacement by glucose and lactate but rather by ketone bodies during a KD. Third, the participants lost around 2 kg including 1 kg of fat mass during the KD, which may have confounded the results. Fourth, because we employed a crossover design, there is a risk of a carry-over effect. An order effect was observed for FFA and insulin concentrations, but the effect on FFA was opposite to what would be expected if there were a carry-over effect. Therefore, only insulin concentrations exhibited a carry-over effect, which may be attributed to chance or the small number of participants in each order, as FFA and OHB levels were not higher in those who followed the SDD as the second diet. Finally, including HF patients would potentially have produced significant findings across all PET measures; however, it is difficult to put this subgroup on a KD, owing to their cardiovascular risks. Thus, it would be reasonable to first observe the side effects, risks, and outcomes associated with a KD and the involved methods in a healthy population before conducting similar studies in patients with ischemic HF.
CONCLUSION
Three weeks on a KD resulted in significantly reduced MFAO and presumably increased ketone body oxidation. However, this change in cardiac substrate preference did not result in any significant changes in myocardial oxygen consumption or perfusion. These results go against the “thrifty substrate hypothesis” and indicate that the observed cardioprotective effect associated with increased ketone body levels during SGLT2 inhibition may not solely be attributed to changes in substrate utilization.
AUTHOR CONTRIBUTIONS
Conceptualization: Thien Vinh Luong, Katrine Meyer Lauritsen, Lars Christian Gormsen, Esben Søndergaard, Niels Møller, Lars Poulsen Tolbod, Stephen C. Cunnane, EC. Methodology: All authors. Validation: Thien Vinh Luong, Lars Christian Gormsen, Esben Søndergaard, Niels Møller, Lars Poulsen Tolbod. Formal analysis: Thien Vinh Luong, Lars Christian Gormsen, Esben Søndergaard, Lars Poulsen Tolbod. Investigation: Thien Vinh Luong, Mette Glavind Bülow Pedersen, Katrine Meyer Lauritsen, Mette Louise Gram Kjærulff, Lars Christian Gormsen, Esben Søndergaard. Resources: Thien Vinh Luong, Lars Christian Gormsen, Esben Søndergaard, Niels Møller, Katrine Meyer Lauritsen, Lars Poulsen Tolbod, Mette Glavind Bülow Pedersen, Caroline Bruun Abild. Writing – Original draft: Thien Vinh Luong, Lars Christian Gormsen, Esben Søndergaard. Writing – Review & Editing: All authors.
FUNDING INFORMATION
The work was supported by The Novo Nordisk Foundation. The funding was awarded to Lars Christian Gormsen (NF18OC0052953).
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT05012748.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.